How Ethyl Acetate Enhances Vinyl Acetate Production?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate in VAM Production: Background and Objectives
Vinyl acetate monomer (VAM) production has been a cornerstone of the chemical industry for decades, with applications spanning from adhesives to paints and textiles. The traditional process for VAM synthesis involves the reaction of ethylene and acetic acid with oxygen over a palladium-based catalyst. However, recent advancements have highlighted the potential of ethyl acetate as a key enhancer in this production process, marking a significant shift in the industry's approach to VAM manufacturing.
The integration of ethyl acetate into VAM production stems from the need to improve process efficiency, reduce environmental impact, and enhance overall product quality. Ethyl acetate, a versatile organic compound, has demonstrated remarkable capabilities in optimizing the reaction conditions and increasing yield rates. This development represents a convergence of chemical engineering principles and innovative thinking, aimed at addressing the growing global demand for VAM while simultaneously meeting stringent environmental regulations.
The evolution of VAM production techniques can be traced back to the 1920s, with significant improvements occurring in the latter half of the 20th century. The introduction of ethyl acetate as a process enhancer is part of this ongoing evolution, reflecting the industry's commitment to continuous improvement and technological advancement. This latest innovation builds upon decades of research and development in catalysis, reaction kinetics, and process optimization.
As we delve into the role of ethyl acetate in VAM production, it is crucial to understand the underlying chemical mechanisms and the potential benefits this approach offers. The primary objectives of incorporating ethyl acetate into the production process include increasing reaction rates, improving selectivity, and reducing energy consumption. These goals align with broader industry trends towards sustainability and efficiency, making the study of ethyl acetate's role in VAM production a topic of significant interest and importance.
The exploration of ethyl acetate's enhancing effects on VAM production is not merely an academic exercise but a response to real-world industrial challenges. As global demand for VAM continues to rise, driven by growth in end-use industries such as construction, automotive, and packaging, manufacturers are under pressure to innovate and optimize their processes. The potential of ethyl acetate to address these challenges makes it a focal point for research and development efforts across the chemical industry.
In the following sections, we will examine the technical aspects of ethyl acetate's role in VAM production, analyze its market implications, and explore the future prospects of this innovative approach. By understanding the background and objectives of this technological advancement, we lay the groundwork for a comprehensive evaluation of its potential to reshape the landscape of VAM production in the years to come.
The integration of ethyl acetate into VAM production stems from the need to improve process efficiency, reduce environmental impact, and enhance overall product quality. Ethyl acetate, a versatile organic compound, has demonstrated remarkable capabilities in optimizing the reaction conditions and increasing yield rates. This development represents a convergence of chemical engineering principles and innovative thinking, aimed at addressing the growing global demand for VAM while simultaneously meeting stringent environmental regulations.
The evolution of VAM production techniques can be traced back to the 1920s, with significant improvements occurring in the latter half of the 20th century. The introduction of ethyl acetate as a process enhancer is part of this ongoing evolution, reflecting the industry's commitment to continuous improvement and technological advancement. This latest innovation builds upon decades of research and development in catalysis, reaction kinetics, and process optimization.
As we delve into the role of ethyl acetate in VAM production, it is crucial to understand the underlying chemical mechanisms and the potential benefits this approach offers. The primary objectives of incorporating ethyl acetate into the production process include increasing reaction rates, improving selectivity, and reducing energy consumption. These goals align with broader industry trends towards sustainability and efficiency, making the study of ethyl acetate's role in VAM production a topic of significant interest and importance.
The exploration of ethyl acetate's enhancing effects on VAM production is not merely an academic exercise but a response to real-world industrial challenges. As global demand for VAM continues to rise, driven by growth in end-use industries such as construction, automotive, and packaging, manufacturers are under pressure to innovate and optimize their processes. The potential of ethyl acetate to address these challenges makes it a focal point for research and development efforts across the chemical industry.
In the following sections, we will examine the technical aspects of ethyl acetate's role in VAM production, analyze its market implications, and explore the future prospects of this innovative approach. By understanding the background and objectives of this technological advancement, we lay the groundwork for a comprehensive evaluation of its potential to reshape the landscape of VAM production in the years to come.
Market Analysis for Enhanced VAM Production
The global market for vinyl acetate monomer (VAM) has been experiencing steady growth, driven by increasing demand in various end-use industries such as adhesives, paints, coatings, and textiles. The enhancement of VAM production through the use of ethyl acetate presents a significant opportunity for market expansion and improved efficiency.
The current VAM market is characterized by a strong demand-supply dynamic, with major consumers located in Asia-Pacific, North America, and Europe. China, in particular, has emerged as a key player, both in terms of production and consumption. The growing construction and automotive industries in developing economies have been major contributors to the increased demand for VAM-based products.
The introduction of ethyl acetate as an enhancer in VAM production has the potential to reshape market dynamics. This innovation addresses several key market needs, including improved production efficiency, reduced environmental impact, and enhanced product quality. As sustainability becomes increasingly important in chemical manufacturing, processes that offer better resource utilization and lower emissions are gaining traction among both producers and consumers.
From a market perspective, the enhanced VAM production method using ethyl acetate could lead to a more competitive landscape. Producers who adopt this technology may gain a significant advantage in terms of cost reduction and product differentiation. This could potentially lead to a shift in market shares and the emergence of new players who are quick to implement the improved production process.
The market for VAM is closely tied to economic growth and industrial development. As such, the enhanced production method could have far-reaching effects on various sectors. For instance, the construction industry, which relies heavily on VAM-based adhesives and coatings, could benefit from more cost-effective and higher-quality products. Similarly, the packaging industry, another major consumer of VAM derivatives, could see improvements in product performance and sustainability profiles.
Looking at market trends, there is a growing emphasis on bio-based and sustainable chemical production. The use of ethyl acetate in VAM production aligns well with this trend, potentially opening up new market segments for "greener" VAM products. This could lead to premium pricing opportunities and expanded market reach, particularly in regions with stringent environmental regulations.
The global nature of the VAM market means that any significant production enhancement could have ripple effects across supply chains and trade patterns. Regions with access to abundant ethyl acetate supplies may see increased investment in VAM production facilities, potentially altering the current geographical distribution of production capacities.
The current VAM market is characterized by a strong demand-supply dynamic, with major consumers located in Asia-Pacific, North America, and Europe. China, in particular, has emerged as a key player, both in terms of production and consumption. The growing construction and automotive industries in developing economies have been major contributors to the increased demand for VAM-based products.
The introduction of ethyl acetate as an enhancer in VAM production has the potential to reshape market dynamics. This innovation addresses several key market needs, including improved production efficiency, reduced environmental impact, and enhanced product quality. As sustainability becomes increasingly important in chemical manufacturing, processes that offer better resource utilization and lower emissions are gaining traction among both producers and consumers.
From a market perspective, the enhanced VAM production method using ethyl acetate could lead to a more competitive landscape. Producers who adopt this technology may gain a significant advantage in terms of cost reduction and product differentiation. This could potentially lead to a shift in market shares and the emergence of new players who are quick to implement the improved production process.
The market for VAM is closely tied to economic growth and industrial development. As such, the enhanced production method could have far-reaching effects on various sectors. For instance, the construction industry, which relies heavily on VAM-based adhesives and coatings, could benefit from more cost-effective and higher-quality products. Similarly, the packaging industry, another major consumer of VAM derivatives, could see improvements in product performance and sustainability profiles.
Looking at market trends, there is a growing emphasis on bio-based and sustainable chemical production. The use of ethyl acetate in VAM production aligns well with this trend, potentially opening up new market segments for "greener" VAM products. This could lead to premium pricing opportunities and expanded market reach, particularly in regions with stringent environmental regulations.
The global nature of the VAM market means that any significant production enhancement could have ripple effects across supply chains and trade patterns. Regions with access to abundant ethyl acetate supplies may see increased investment in VAM production facilities, potentially altering the current geographical distribution of production capacities.
Current Challenges in VAM Synthesis
The synthesis of vinyl acetate monomer (VAM) faces several significant challenges that hinder its efficiency and sustainability. One of the primary issues is the low conversion rate of ethylene and acetic acid, the main reactants in VAM production. This limitation necessitates the use of excess reactants, leading to increased production costs and waste generation.
Another major challenge is the catalyst deactivation during the VAM synthesis process. The palladium-based catalysts used in the reaction are prone to sintering and coking, which reduces their effectiveness over time. This results in decreased productivity and increased downtime for catalyst regeneration or replacement, impacting the overall economics of the process.
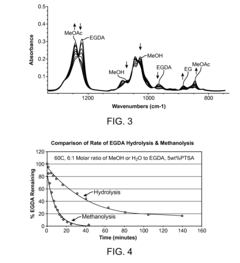
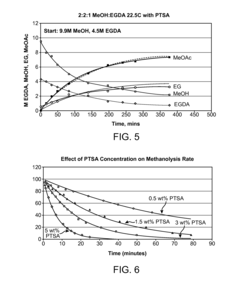
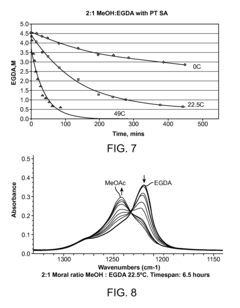
The selectivity of the reaction also poses a significant challenge. Side reactions, such as the formation of carbon dioxide and ethyl acetate, compete with the desired VAM production. These unwanted by-products not only reduce the yield of VAM but also complicate the separation and purification processes, adding to the overall production costs.
Energy efficiency is another critical concern in VAM synthesis. The reaction requires high temperatures and pressures, leading to substantial energy consumption. This not only increases production costs but also contributes to the carbon footprint of the process, raising environmental concerns in an increasingly sustainability-conscious industry.
The corrosive nature of acetic acid, a key reactant in VAM synthesis, presents material challenges. It necessitates the use of specialized, corrosion-resistant equipment, which increases capital and maintenance costs. Additionally, the handling and storage of large quantities of acetic acid pose safety risks that must be carefully managed.
Furthermore, the volatility of raw material prices, particularly ethylene and acetic acid, introduces economic uncertainties in VAM production. These price fluctuations can significantly impact the profitability of VAM synthesis, making it challenging for producers to maintain consistent profit margins.
Lastly, stringent environmental regulations pose ongoing challenges for VAM producers. The need to reduce emissions, particularly volatile organic compounds (VOCs) and carbon dioxide, requires continuous innovation in process design and pollution control technologies. This regulatory pressure drives the need for more sustainable production methods, which often come with their own technical and economic challenges.
Another major challenge is the catalyst deactivation during the VAM synthesis process. The palladium-based catalysts used in the reaction are prone to sintering and coking, which reduces their effectiveness over time. This results in decreased productivity and increased downtime for catalyst regeneration or replacement, impacting the overall economics of the process.
The selectivity of the reaction also poses a significant challenge. Side reactions, such as the formation of carbon dioxide and ethyl acetate, compete with the desired VAM production. These unwanted by-products not only reduce the yield of VAM but also complicate the separation and purification processes, adding to the overall production costs.
Energy efficiency is another critical concern in VAM synthesis. The reaction requires high temperatures and pressures, leading to substantial energy consumption. This not only increases production costs but also contributes to the carbon footprint of the process, raising environmental concerns in an increasingly sustainability-conscious industry.
The corrosive nature of acetic acid, a key reactant in VAM synthesis, presents material challenges. It necessitates the use of specialized, corrosion-resistant equipment, which increases capital and maintenance costs. Additionally, the handling and storage of large quantities of acetic acid pose safety risks that must be carefully managed.
Furthermore, the volatility of raw material prices, particularly ethylene and acetic acid, introduces economic uncertainties in VAM production. These price fluctuations can significantly impact the profitability of VAM synthesis, making it challenging for producers to maintain consistent profit margins.
Lastly, stringent environmental regulations pose ongoing challenges for VAM producers. The need to reduce emissions, particularly volatile organic compounds (VOCs) and carbon dioxide, requires continuous innovation in process design and pollution control technologies. This regulatory pressure drives the need for more sustainable production methods, which often come with their own technical and economic challenges.
Existing Ethyl Acetate Integration Methods
01 Improved catalytic processes
Enhancement of ethyl acetate production through advanced catalytic processes. This includes the development of novel catalysts, optimization of reaction conditions, and the use of more efficient catalytic systems to increase yield and selectivity in the esterification process.- Improved catalytic processes: Enhancing ethyl acetate production through advanced catalytic processes, including the use of novel catalysts or optimized reaction conditions. These improvements can lead to increased yield, selectivity, and efficiency in the production process.
- Reactor design and optimization: Developing innovative reactor designs or optimizing existing reactors to improve ethyl acetate production. This may involve modifications to reactor geometry, flow patterns, or heat transfer mechanisms to enhance reaction kinetics and overall productivity.
- Feedstock purification and preparation: Implementing advanced techniques for purifying and preparing feedstocks used in ethyl acetate production. This can include methods for removing impurities, adjusting feedstock composition, or pre-treating raw materials to improve reaction efficiency and product quality.
- Process intensification and integration: Applying process intensification techniques and integrating various unit operations to enhance ethyl acetate production. This may involve combining reaction and separation steps, implementing novel heat integration strategies, or utilizing advanced process control systems to optimize overall production efficiency.
- Separation and purification techniques: Developing improved separation and purification methods for ethyl acetate production. This can include advanced distillation techniques, membrane-based separations, or novel extraction processes to increase product purity and recovery rates while reducing energy consumption.
02 Continuous flow reactors
Implementation of continuous flow reactors for ethyl acetate production. These systems offer advantages such as improved heat and mass transfer, better process control, and increased productivity compared to batch reactors. Continuous flow technology can lead to higher yields and more efficient use of raw materials.Expand Specific Solutions03 Reactive distillation
Utilization of reactive distillation techniques to enhance ethyl acetate production. This process combines reaction and separation in a single unit operation, leading to improved conversion rates, reduced energy consumption, and increased product purity. It can also help overcome equilibrium limitations in the esterification reaction.Expand Specific Solutions04 Membrane separation technology
Application of membrane separation technology in ethyl acetate production processes. This includes the use of pervaporation membranes for product purification and water removal, as well as membrane reactors for process intensification. These technologies can lead to improved product quality and reduced energy consumption.Expand Specific Solutions05 Process integration and optimization
Implementation of advanced process integration and optimization techniques to enhance ethyl acetate production. This involves the use of computer-aided process design, heat integration, and pinch analysis to minimize energy consumption and maximize resource utilization. It also includes the development of more efficient separation and purification methods.Expand Specific Solutions
Key Players in VAM and Ethyl Acetate Industry
The production of vinyl acetate using ethyl acetate is an emerging technology in the chemical industry, currently in the early stages of development. The market for this process is growing but still relatively small compared to traditional methods. Technologically, it's in the early maturity phase, with companies like Celanese International Corp., LyondellBasell Acetyls LLC, and Wacker Chemie AG leading research and development efforts. These firms are investing in pilot plants and process optimization to improve efficiency and reduce costs. While the technology shows promise for enhancing vinyl acetate production, it has not yet achieved widespread commercial adoption, indicating potential for further innovation and market expansion in the coming years.
Celanese International Corp.
Technical Solution: Celanese has developed an advanced process for enhancing vinyl acetate production using ethyl acetate. Their method involves a two-stage reaction system where ethyl acetate is first converted to acetic acid and ethylene in a hydrolysis reactor. The acetic acid is then reacted with additional ethylene and oxygen over a palladium-based catalyst to produce vinyl acetate[1]. This process achieves higher conversion rates and selectivity compared to traditional methods. Celanese has also implemented a novel heat integration system that recovers energy from the exothermic reactions, significantly improving overall energy efficiency[3]. The company has further optimized catalyst formulations to extend catalyst life and reduce precious metal usage[5].
Strengths: High conversion rates, improved energy efficiency, and reduced catalyst costs. Weaknesses: Complex two-stage process may require higher initial capital investment.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a proprietary technology for enhancing vinyl acetate production using ethyl acetate as a co-feedstock. Their process involves a single-stage fluidized bed reactor where ethyl acetate, ethylene, and oxygen are simultaneously fed over a specially designed catalyst[2]. This approach allows for direct conversion of ethyl acetate to vinyl acetate, bypassing the need for separate hydrolysis. Sinopec's catalyst formulation incorporates promoters that enhance selectivity towards vinyl acetate formation while minimizing unwanted side reactions[4]. The company has also implemented advanced process control systems to optimize reaction conditions in real-time, ensuring consistent product quality and maximizing yield[6].
Strengths: Simplified single-stage process, high selectivity, and real-time optimization. Weaknesses: May require more precise control of reaction conditions to maintain performance.
Core Innovations in Ethyl Acetate-Enhanced VAM Synthesis
Vinyl acetate production process
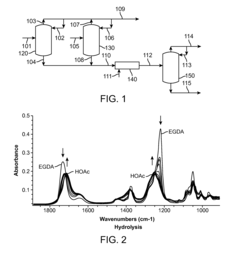
PatentWO2011071652A1
Innovation
- A process involving reactive distillation of ethylene glycol diacetate in the presence of a hydrolysis catalyst to recover acetic acid, which is then recycled, optimizing the use of catalysts and improving acetic acid recovery.
Vinyl acetate production process
PatentInactiveUS20120264970A1
Innovation
- A process involving the methanolysis of ethylene glycol diacetate in a reactive distillation system to recover methyl acetate, which is then recycled as a feedstock to an acetic acid plant, improving overall yield and eliminating the need for energy-intensive acetic acid and water separation.
Environmental Impact of Ethyl Acetate in VAM Production
The use of ethyl acetate in vinyl acetate monomer (VAM) production has significant environmental implications that warrant careful consideration. While ethyl acetate enhances the efficiency of VAM production, its environmental impact must be thoroughly assessed to ensure sustainable manufacturing practices.
One of the primary environmental concerns associated with ethyl acetate in VAM production is its potential for air pollution. As a volatile organic compound (VOC), ethyl acetate can contribute to the formation of ground-level ozone and smog when released into the atmosphere. This can have detrimental effects on air quality, particularly in urban and industrial areas where VAM production facilities are often located.
Water pollution is another critical environmental issue to consider. Ethyl acetate, if not properly managed, can contaminate water sources through spills, leaks, or improper disposal. This contamination can harm aquatic ecosystems and potentially impact human health if it enters drinking water supplies. Implementing robust wastewater treatment systems and spill prevention measures is essential to mitigate these risks.
The production and use of ethyl acetate in VAM manufacturing also contribute to greenhouse gas emissions, both directly through the production process and indirectly through energy consumption. As climate change concerns intensify, reducing the carbon footprint of industrial processes, including VAM production, becomes increasingly important.
Waste management is a crucial aspect of addressing the environmental impact of ethyl acetate in VAM production. Proper handling, storage, and disposal of ethyl acetate and related waste products are necessary to prevent soil contamination and minimize the overall environmental footprint of the manufacturing process.
However, it is important to note that the use of ethyl acetate in VAM production can also have positive environmental implications. By enhancing production efficiency, it may lead to reduced energy consumption and resource utilization per unit of VAM produced. This improved efficiency can potentially offset some of the negative environmental impacts associated with its use.
To address these environmental challenges, industry stakeholders are exploring various mitigation strategies. These include the development of closed-loop systems to minimize ethyl acetate emissions, the implementation of advanced air and water treatment technologies, and the exploration of more environmentally friendly alternatives or process modifications.
Regulatory compliance and adherence to environmental standards play a crucial role in managing the environmental impact of ethyl acetate in VAM production. Manufacturers must navigate an increasingly complex landscape of environmental regulations, which vary across different regions and jurisdictions.
In conclusion, while ethyl acetate enhances VAM production efficiency, its environmental impact requires careful management and ongoing research to develop more sustainable production methods. Balancing the benefits of improved production with environmental stewardship remains a key challenge for the industry.
One of the primary environmental concerns associated with ethyl acetate in VAM production is its potential for air pollution. As a volatile organic compound (VOC), ethyl acetate can contribute to the formation of ground-level ozone and smog when released into the atmosphere. This can have detrimental effects on air quality, particularly in urban and industrial areas where VAM production facilities are often located.
Water pollution is another critical environmental issue to consider. Ethyl acetate, if not properly managed, can contaminate water sources through spills, leaks, or improper disposal. This contamination can harm aquatic ecosystems and potentially impact human health if it enters drinking water supplies. Implementing robust wastewater treatment systems and spill prevention measures is essential to mitigate these risks.
The production and use of ethyl acetate in VAM manufacturing also contribute to greenhouse gas emissions, both directly through the production process and indirectly through energy consumption. As climate change concerns intensify, reducing the carbon footprint of industrial processes, including VAM production, becomes increasingly important.
Waste management is a crucial aspect of addressing the environmental impact of ethyl acetate in VAM production. Proper handling, storage, and disposal of ethyl acetate and related waste products are necessary to prevent soil contamination and minimize the overall environmental footprint of the manufacturing process.
However, it is important to note that the use of ethyl acetate in VAM production can also have positive environmental implications. By enhancing production efficiency, it may lead to reduced energy consumption and resource utilization per unit of VAM produced. This improved efficiency can potentially offset some of the negative environmental impacts associated with its use.
To address these environmental challenges, industry stakeholders are exploring various mitigation strategies. These include the development of closed-loop systems to minimize ethyl acetate emissions, the implementation of advanced air and water treatment technologies, and the exploration of more environmentally friendly alternatives or process modifications.
Regulatory compliance and adherence to environmental standards play a crucial role in managing the environmental impact of ethyl acetate in VAM production. Manufacturers must navigate an increasingly complex landscape of environmental regulations, which vary across different regions and jurisdictions.
In conclusion, while ethyl acetate enhances VAM production efficiency, its environmental impact requires careful management and ongoing research to develop more sustainable production methods. Balancing the benefits of improved production with environmental stewardship remains a key challenge for the industry.
Economic Feasibility of Ethyl Acetate Integration
The integration of ethyl acetate in vinyl acetate production presents a compelling economic opportunity for manufacturers. By utilizing ethyl acetate as a co-reactant or solvent in the process, companies can potentially reduce production costs and improve overall efficiency. The economic feasibility of this integration hinges on several key factors.
Firstly, the cost of raw materials plays a crucial role. Ethyl acetate is generally less expensive than traditional reactants used in vinyl acetate production, such as acetic acid. This cost differential can lead to significant savings in material expenses, especially for large-scale operations. However, the price volatility of ethyl acetate in the global market must be considered when evaluating long-term economic benefits.
The capital investment required for retrofitting existing production facilities or constructing new ones optimized for ethyl acetate integration is another critical economic consideration. While initial costs may be substantial, the potential for increased production capacity and improved product quality could justify the investment over time. Companies must conduct thorough cost-benefit analyses to determine the payback period and return on investment.
Energy consumption is a significant factor in the economic feasibility assessment. Ethyl acetate integration may lead to reduced energy requirements in certain process steps, such as distillation or purification. This energy efficiency can translate to lower operational costs and improved sustainability metrics, which are increasingly important in today's market.
The potential for increased production yield is a key economic driver. If ethyl acetate integration can enhance the conversion rate of raw materials to vinyl acetate or reduce byproduct formation, it could significantly boost overall production efficiency. This increased yield can lead to higher revenues and improved profit margins for manufacturers.
Market demand for higher purity vinyl acetate products may also influence the economic viability of ethyl acetate integration. If the process results in a superior quality product, manufacturers may command premium prices or gain market share, further enhancing the economic benefits of the technology.
Regulatory considerations and compliance costs must be factored into the economic assessment. While ethyl acetate is generally considered less hazardous than some traditional solvents, any changes in production processes may require new permits or safety measures, potentially impacting the overall economic feasibility.
In conclusion, the economic feasibility of ethyl acetate integration in vinyl acetate production is multifaceted. While it offers potential cost savings and efficiency improvements, careful analysis of market conditions, capital requirements, and operational impacts is essential for manufacturers to make informed decisions about adopting this technology.
Firstly, the cost of raw materials plays a crucial role. Ethyl acetate is generally less expensive than traditional reactants used in vinyl acetate production, such as acetic acid. This cost differential can lead to significant savings in material expenses, especially for large-scale operations. However, the price volatility of ethyl acetate in the global market must be considered when evaluating long-term economic benefits.
The capital investment required for retrofitting existing production facilities or constructing new ones optimized for ethyl acetate integration is another critical economic consideration. While initial costs may be substantial, the potential for increased production capacity and improved product quality could justify the investment over time. Companies must conduct thorough cost-benefit analyses to determine the payback period and return on investment.
Energy consumption is a significant factor in the economic feasibility assessment. Ethyl acetate integration may lead to reduced energy requirements in certain process steps, such as distillation or purification. This energy efficiency can translate to lower operational costs and improved sustainability metrics, which are increasingly important in today's market.
The potential for increased production yield is a key economic driver. If ethyl acetate integration can enhance the conversion rate of raw materials to vinyl acetate or reduce byproduct formation, it could significantly boost overall production efficiency. This increased yield can lead to higher revenues and improved profit margins for manufacturers.
Market demand for higher purity vinyl acetate products may also influence the economic viability of ethyl acetate integration. If the process results in a superior quality product, manufacturers may command premium prices or gain market share, further enhancing the economic benefits of the technology.
Regulatory considerations and compliance costs must be factored into the economic assessment. While ethyl acetate is generally considered less hazardous than some traditional solvents, any changes in production processes may require new permits or safety measures, potentially impacting the overall economic feasibility.
In conclusion, the economic feasibility of ethyl acetate integration in vinyl acetate production is multifaceted. While it offers potential cost savings and efficiency improvements, careful analysis of market conditions, capital requirements, and operational impacts is essential for manufacturers to make informed decisions about adopting this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!