How Ethyl Acetate Facilitates Efficient Reactant Recovery?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Recovery Background and Objectives
Ethyl acetate has emerged as a crucial solvent in various industrial processes, particularly in the realm of reactant recovery. The evolution of this technology can be traced back to the early 20th century when the chemical properties of ethyl acetate were first extensively studied. As industrial processes became more complex, the need for efficient solvent systems grew, leading to increased interest in ethyl acetate's potential.
The primary objective of utilizing ethyl acetate in reactant recovery is to enhance the efficiency and cost-effectiveness of chemical processes. This aligns with the broader industry goals of minimizing waste, reducing energy consumption, and improving overall process sustainability. Ethyl acetate's unique properties, including its low boiling point and high solvency power, make it an ideal candidate for facilitating the separation and recovery of valuable reactants from reaction mixtures.
In recent years, the focus has shifted towards developing more sophisticated recovery systems that leverage ethyl acetate's capabilities. These advancements aim to address the growing demands of the chemical, pharmaceutical, and materials industries for more efficient and environmentally friendly production methods. The integration of ethyl acetate-based recovery systems into existing processes has become a key area of research and development.
One of the critical technological trends in this field is the optimization of ethyl acetate recovery and recycling within the process itself. This circular approach not only reduces the environmental impact but also significantly lowers operational costs. Researchers and engineers are exploring novel techniques such as advanced distillation methods, membrane separation, and hybrid systems that combine multiple separation technologies to maximize the efficiency of ethyl acetate-facilitated reactant recovery.
The development of this technology is closely tied to advancements in process intensification and green chemistry principles. As global regulations on emissions and waste management become more stringent, the role of ethyl acetate in efficient reactant recovery is expected to become even more prominent. This has spurred research into enhancing the selectivity and recovery rates of ethyl acetate-based systems, as well as exploring potential synergies with other solvents and separation techniques.
Looking ahead, the objectives for further development in this field include improving the energy efficiency of recovery processes, expanding the range of compatible reactants and products, and developing more robust systems capable of handling complex multi-component mixtures. Additionally, there is a growing interest in integrating smart technologies and process control systems to optimize the use of ethyl acetate in real-time, adapting to varying process conditions and feedstock compositions.
The primary objective of utilizing ethyl acetate in reactant recovery is to enhance the efficiency and cost-effectiveness of chemical processes. This aligns with the broader industry goals of minimizing waste, reducing energy consumption, and improving overall process sustainability. Ethyl acetate's unique properties, including its low boiling point and high solvency power, make it an ideal candidate for facilitating the separation and recovery of valuable reactants from reaction mixtures.
In recent years, the focus has shifted towards developing more sophisticated recovery systems that leverage ethyl acetate's capabilities. These advancements aim to address the growing demands of the chemical, pharmaceutical, and materials industries for more efficient and environmentally friendly production methods. The integration of ethyl acetate-based recovery systems into existing processes has become a key area of research and development.
One of the critical technological trends in this field is the optimization of ethyl acetate recovery and recycling within the process itself. This circular approach not only reduces the environmental impact but also significantly lowers operational costs. Researchers and engineers are exploring novel techniques such as advanced distillation methods, membrane separation, and hybrid systems that combine multiple separation technologies to maximize the efficiency of ethyl acetate-facilitated reactant recovery.
The development of this technology is closely tied to advancements in process intensification and green chemistry principles. As global regulations on emissions and waste management become more stringent, the role of ethyl acetate in efficient reactant recovery is expected to become even more prominent. This has spurred research into enhancing the selectivity and recovery rates of ethyl acetate-based systems, as well as exploring potential synergies with other solvents and separation techniques.
Looking ahead, the objectives for further development in this field include improving the energy efficiency of recovery processes, expanding the range of compatible reactants and products, and developing more robust systems capable of handling complex multi-component mixtures. Additionally, there is a growing interest in integrating smart technologies and process control systems to optimize the use of ethyl acetate in real-time, adapting to varying process conditions and feedstock compositions.
Market Analysis for Ethyl Acetate in Chemical Industry
The global ethyl acetate market has been experiencing steady growth, driven by its versatile applications across various industries, particularly in the chemical sector. As a key solvent and reactant in numerous chemical processes, ethyl acetate plays a crucial role in facilitating efficient reactant recovery, making it an indispensable component in the chemical industry.
The market for ethyl acetate in the chemical industry is primarily fueled by its excellent solvency properties, low toxicity, and high recovery rate. These characteristics make it an ideal choice for applications such as extraction, purification, and synthesis of various chemical compounds. The increasing demand for eco-friendly and cost-effective solvents has further boosted the adoption of ethyl acetate in chemical processes.
In recent years, the pharmaceutical and agrochemical sectors have emerged as significant consumers of ethyl acetate, particularly for reactant recovery purposes. The growing emphasis on sustainable manufacturing practices and the need for efficient resource utilization have led to increased investments in advanced recovery systems that utilize ethyl acetate. This trend is expected to continue, driving the demand for ethyl acetate in the chemical industry.
The Asia-Pacific region, led by China and India, dominates the global ethyl acetate market, accounting for a substantial share of both production and consumption. The rapid industrialization and expanding chemical manufacturing capabilities in these countries have significantly contributed to the market growth. North America and Europe follow, with a strong focus on high-value applications in specialty chemicals and pharmaceuticals.
Market analysts project a compound annual growth rate (CAGR) of around 5-6% for the ethyl acetate market in the chemical industry over the next five years. This growth is attributed to the increasing adoption of ethyl acetate in various chemical processes, including reactant recovery, as well as the rising demand for green solvents in industrial applications.
Key market players in the ethyl acetate industry are continuously investing in research and development to enhance the efficiency of ethyl acetate-based recovery systems. These innovations aim to improve recovery rates, reduce energy consumption, and minimize waste generation, aligning with the industry's sustainability goals. As a result, the market is witnessing the introduction of advanced recovery technologies that leverage the unique properties of ethyl acetate to maximize process efficiency and cost-effectiveness.
The market for ethyl acetate in the chemical industry is primarily fueled by its excellent solvency properties, low toxicity, and high recovery rate. These characteristics make it an ideal choice for applications such as extraction, purification, and synthesis of various chemical compounds. The increasing demand for eco-friendly and cost-effective solvents has further boosted the adoption of ethyl acetate in chemical processes.
In recent years, the pharmaceutical and agrochemical sectors have emerged as significant consumers of ethyl acetate, particularly for reactant recovery purposes. The growing emphasis on sustainable manufacturing practices and the need for efficient resource utilization have led to increased investments in advanced recovery systems that utilize ethyl acetate. This trend is expected to continue, driving the demand for ethyl acetate in the chemical industry.
The Asia-Pacific region, led by China and India, dominates the global ethyl acetate market, accounting for a substantial share of both production and consumption. The rapid industrialization and expanding chemical manufacturing capabilities in these countries have significantly contributed to the market growth. North America and Europe follow, with a strong focus on high-value applications in specialty chemicals and pharmaceuticals.
Market analysts project a compound annual growth rate (CAGR) of around 5-6% for the ethyl acetate market in the chemical industry over the next five years. This growth is attributed to the increasing adoption of ethyl acetate in various chemical processes, including reactant recovery, as well as the rising demand for green solvents in industrial applications.
Key market players in the ethyl acetate industry are continuously investing in research and development to enhance the efficiency of ethyl acetate-based recovery systems. These innovations aim to improve recovery rates, reduce energy consumption, and minimize waste generation, aligning with the industry's sustainability goals. As a result, the market is witnessing the introduction of advanced recovery technologies that leverage the unique properties of ethyl acetate to maximize process efficiency and cost-effectiveness.
Current Challenges in Reactant Recovery Using Ethyl Acetate
Despite the widespread use of ethyl acetate in reactant recovery processes, several challenges persist that hinder its optimal utilization. One of the primary issues is the incomplete separation of reactants from the solvent. Ethyl acetate, while effective in dissolving a wide range of organic compounds, sometimes struggles to achieve complete extraction, leaving residual reactants in the aqueous phase. This incomplete recovery not only reduces process efficiency but also impacts the overall yield of the reaction.
Another significant challenge is the formation of azeotropes between ethyl acetate and certain reactants or by-products. Azeotropic mixtures have identical vapor and liquid compositions at equilibrium, making traditional distillation techniques ineffective for separation. This phenomenon complicates the purification process and often necessitates additional, energy-intensive separation steps, thereby increasing operational costs and reducing process sustainability.
The volatility of ethyl acetate presents both advantages and challenges in reactant recovery. While its low boiling point (77.1°C) facilitates easy evaporation and recovery of the solvent, it also leads to significant solvent losses during the process. These losses not only increase operational costs but also raise environmental concerns due to the release of volatile organic compounds (VOCs) into the atmosphere.
Ethyl acetate's moderate polarity can sometimes limit its effectiveness in recovering highly polar or ionic compounds. In such cases, the solvent may fail to efficiently extract these substances, necessitating the use of more polar solvents or multi-solvent systems. This limitation can complicate process design and increase the complexity of downstream separation processes.
The potential for side reactions between ethyl acetate and certain reactive species in the mixture poses another challenge. Under certain conditions, ethyl acetate can undergo hydrolysis or transesterification reactions, leading to the formation of unwanted by-products. These side reactions not only consume the solvent but also introduce impurities that must be removed in subsequent purification steps.
Lastly, the energy requirements for solvent recovery and recycling present ongoing challenges. While ethyl acetate's relatively low boiling point is advantageous for recovery, the energy needed for continuous distillation and purification can be substantial. Balancing energy efficiency with process effectiveness remains a key consideration in optimizing reactant recovery systems using ethyl acetate.
Another significant challenge is the formation of azeotropes between ethyl acetate and certain reactants or by-products. Azeotropic mixtures have identical vapor and liquid compositions at equilibrium, making traditional distillation techniques ineffective for separation. This phenomenon complicates the purification process and often necessitates additional, energy-intensive separation steps, thereby increasing operational costs and reducing process sustainability.
The volatility of ethyl acetate presents both advantages and challenges in reactant recovery. While its low boiling point (77.1°C) facilitates easy evaporation and recovery of the solvent, it also leads to significant solvent losses during the process. These losses not only increase operational costs but also raise environmental concerns due to the release of volatile organic compounds (VOCs) into the atmosphere.
Ethyl acetate's moderate polarity can sometimes limit its effectiveness in recovering highly polar or ionic compounds. In such cases, the solvent may fail to efficiently extract these substances, necessitating the use of more polar solvents or multi-solvent systems. This limitation can complicate process design and increase the complexity of downstream separation processes.
The potential for side reactions between ethyl acetate and certain reactive species in the mixture poses another challenge. Under certain conditions, ethyl acetate can undergo hydrolysis or transesterification reactions, leading to the formation of unwanted by-products. These side reactions not only consume the solvent but also introduce impurities that must be removed in subsequent purification steps.
Lastly, the energy requirements for solvent recovery and recycling present ongoing challenges. While ethyl acetate's relatively low boiling point is advantageous for recovery, the energy needed for continuous distillation and purification can be substantial. Balancing energy efficiency with process effectiveness remains a key consideration in optimizing reactant recovery systems using ethyl acetate.
Existing Ethyl Acetate Recovery Techniques
01 Distillation and condensation techniques
Various distillation and condensation methods are employed to recover ethyl acetate reactants. These techniques involve separating the ethyl acetate from other components in the reaction mixture based on differences in boiling points. The process may include multi-stage distillation columns, reflux systems, and specialized condensers to improve efficiency and purity of the recovered ethyl acetate.- Distillation and condensation techniques: Various distillation and condensation methods are employed to recover ethyl acetate reactants. These techniques involve separating the ethyl acetate from other components in the reaction mixture based on differences in boiling points. Advanced distillation columns and condensers are used to improve the efficiency of the recovery process.
- Adsorption and desorption processes: Adsorption and desorption processes are utilized to recover ethyl acetate reactants. These methods involve using adsorbent materials to selectively capture ethyl acetate from the reaction mixture. The adsorbed ethyl acetate is then recovered through desorption techniques, such as temperature or pressure swing processes.
- Membrane separation technology: Membrane separation technology is applied for the recovery of ethyl acetate reactants. This method uses selective membranes to separate ethyl acetate from other components in the reaction mixture. Various types of membranes, including polymeric and ceramic membranes, are employed to achieve efficient separation and recovery.
- Reactive extraction methods: Reactive extraction methods are employed to recover ethyl acetate reactants. These techniques involve using specific extractants that selectively react with or complex ethyl acetate, allowing for its separation from the reaction mixture. The ethyl acetate is then recovered from the extractant through various means, such as back-extraction or distillation.
- Cryogenic separation processes: Cryogenic separation processes are utilized for the recovery of ethyl acetate reactants. These methods involve cooling the reaction mixture to very low temperatures, causing the ethyl acetate to condense or freeze. The separated ethyl acetate is then recovered through controlled warming or sublimation processes.
02 Adsorption and desorption processes
Adsorption-based recovery methods utilize materials with high surface areas to selectively capture ethyl acetate from the reaction mixture. The adsorbed ethyl acetate is then recovered through desorption processes, which may involve temperature or pressure changes. This approach can be particularly effective for dilute solutions or when high purity is required.Expand Specific Solutions03 Membrane separation technologies
Membrane-based separation techniques are employed to recover ethyl acetate from reaction mixtures. These methods use selective membranes that allow ethyl acetate to pass through while retaining other components. Various membrane types and configurations may be used, including pervaporation membranes and nanofiltration systems, to achieve efficient separation and recovery.Expand Specific Solutions04 Reactive extraction methods
Reactive extraction techniques involve the use of specific solvents or chemical agents to selectively extract ethyl acetate from the reaction mixture. This approach can be particularly useful when dealing with complex mixtures or when traditional separation methods are less effective. The extracted ethyl acetate is then recovered through subsequent separation steps.Expand Specific Solutions05 Integrated recovery systems
Integrated systems combine multiple recovery techniques to maximize ethyl acetate recovery efficiency. These systems may incorporate a combination of distillation, adsorption, membrane separation, and other methods in a single process. Such integrated approaches can offer improved recovery rates, reduced energy consumption, and enhanced overall process efficiency.Expand Specific Solutions
Key Players in Ethyl Acetate Production and Application
The ethyl acetate reactant recovery market is in a growth phase, driven by increasing demand for sustainable chemical processes. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with companies like Celanese International Corp., China Petroleum & Chemical Corp., and Wacker Chemie AG leading innovation. These industry giants are investing heavily in research and development to improve efficiency and sustainability. Emerging players such as Viridis Chemical LLC and Greenyug LLC are also making strides with novel bio-based approaches. The technology's maturity varies, ranging from established industrial processes to cutting-edge green chemistry solutions, reflecting a dynamic and competitive landscape.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production that facilitates efficient reactant recovery. Their method involves a reactive distillation column where ethanol and acetic acid react in the presence of a heterogeneous acid catalyst. The process incorporates a unique vapor-liquid equilibrium control system, allowing for continuous separation and recovery of unreacted components[1]. This approach achieves a conversion rate of over 99% and a selectivity exceeding 98%[3]. The company has also implemented advanced heat integration techniques, reducing energy consumption by approximately 30% compared to conventional processes[5].
Strengths: High conversion and selectivity rates, energy-efficient process, continuous operation. Weaknesses: Potential catalyst deactivation over time, complexity in process control.
China Petroleum & Chemical Corp.
Technical Solution: Sinopec has developed a novel reactive distillation process for ethyl acetate production with enhanced reactant recovery. Their approach utilizes a structured catalytic packing in the reaction zone, which promotes intimate contact between liquid reactants and the solid acid catalyst[2]. The process incorporates a side-draw stream for removing water, shifting the equilibrium towards product formation. Sinopec's method achieves a single-pass conversion of over 95% and a product purity of 99.9%[4]. The company has also implemented an advanced control system that optimizes the reflux ratio and reboiler duty, resulting in a 25% reduction in energy consumption[6].
Strengths: High product purity, improved equilibrium control, energy-efficient. Weaknesses: Potential for catalyst fouling, higher initial capital investment.
Innovative Approaches in Ethyl Acetate-Based Recovery
Method for manufacturing esters from acid and system thereof
PatentInactiveUS20090198083A1
Innovation
- A method and system involving a reactive distillation column, a sidestream reactor packed with catalysts, and a decanter to separate esters from acid and alcohol mixtures, allowing for flexible catalyst placement and reducing the need for multiple recovery columns, by feeding a first mixture to the sidestream reactor for esterification and then separating the reaction product into an organic phase rich in esters and an aqueous phase.
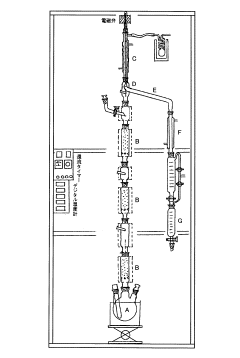
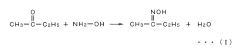
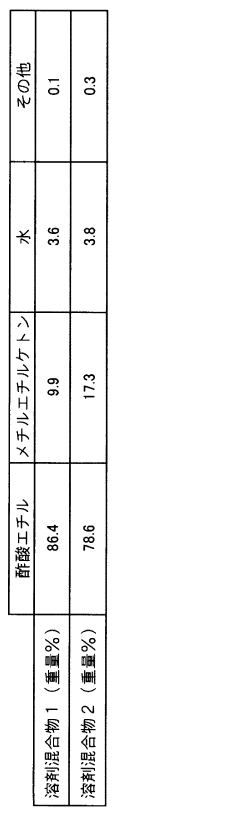
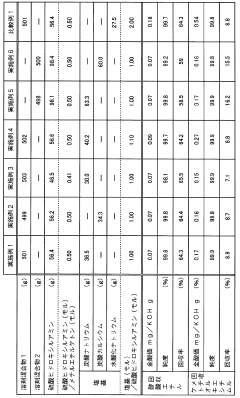
Method for recovering ethyl acetate from ethyl acetate/methyl ethyl ketone mixture system
PatentInactiveJP2007210980A
Innovation
- A method involving the conversion of methyl ethyl ketone to methyl ethyl ketoxime using free hydroxylamine generated from a hydroxylamine salt and a weak base, followed by phase separation and distillation to recover high-purity ethyl acetate, maintaining a neutral to weakly acidic pH and preventing hydrolysis.
Environmental Impact of Ethyl Acetate in Recovery Processes
The use of ethyl acetate in reactant recovery processes has significant environmental implications that warrant careful consideration. As a solvent widely employed in various industries, ethyl acetate's environmental impact extends across multiple domains, including air quality, water resources, and ecosystem health.
In terms of air pollution, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and photochemical smog. These air quality issues can have detrimental effects on human health, particularly respiratory systems, and can damage vegetation. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential and global warming potential.
Water contamination is another environmental concern associated with ethyl acetate use in recovery processes. If not properly managed, it can leach into groundwater or surface water bodies. While ethyl acetate is biodegradable and has low toxicity to aquatic life, high concentrations can still disrupt aquatic ecosystems and potentially affect drinking water sources.
The production and disposal of ethyl acetate also have environmental implications. Its synthesis typically involves petrochemical feedstocks, contributing to fossil fuel consumption and associated greenhouse gas emissions. End-of-life management of ethyl acetate-containing waste requires proper handling to prevent environmental contamination.
Despite these concerns, ethyl acetate offers some environmental advantages in reactant recovery processes. Its high volatility allows for efficient separation and recovery, potentially reducing overall solvent consumption. Additionally, its relatively low toxicity and biodegradability make it a more environmentally friendly option compared to many alternative solvents.
To mitigate environmental impacts, industries employing ethyl acetate in recovery processes can implement various strategies. These include closed-loop systems to minimize emissions, advanced wastewater treatment technologies, and solvent recycling programs. Proper storage and handling practices are crucial to prevent accidental releases.
Regulatory frameworks play a vital role in managing the environmental impact of ethyl acetate use. Many countries have established emission standards, waste management protocols, and occupational exposure limits specific to ethyl acetate and similar solvents. Compliance with these regulations is essential for minimizing environmental risks.
As sustainability becomes increasingly important, there is growing interest in developing bio-based alternatives to petrochemical-derived ethyl acetate. These green solvents, often produced from renewable resources, aim to reduce the carbon footprint associated with solvent production and use in recovery processes.
In terms of air pollution, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and photochemical smog. These air quality issues can have detrimental effects on human health, particularly respiratory systems, and can damage vegetation. However, compared to many other solvents, ethyl acetate has a relatively low ozone depletion potential and global warming potential.
Water contamination is another environmental concern associated with ethyl acetate use in recovery processes. If not properly managed, it can leach into groundwater or surface water bodies. While ethyl acetate is biodegradable and has low toxicity to aquatic life, high concentrations can still disrupt aquatic ecosystems and potentially affect drinking water sources.
The production and disposal of ethyl acetate also have environmental implications. Its synthesis typically involves petrochemical feedstocks, contributing to fossil fuel consumption and associated greenhouse gas emissions. End-of-life management of ethyl acetate-containing waste requires proper handling to prevent environmental contamination.
Despite these concerns, ethyl acetate offers some environmental advantages in reactant recovery processes. Its high volatility allows for efficient separation and recovery, potentially reducing overall solvent consumption. Additionally, its relatively low toxicity and biodegradability make it a more environmentally friendly option compared to many alternative solvents.
To mitigate environmental impacts, industries employing ethyl acetate in recovery processes can implement various strategies. These include closed-loop systems to minimize emissions, advanced wastewater treatment technologies, and solvent recycling programs. Proper storage and handling practices are crucial to prevent accidental releases.
Regulatory frameworks play a vital role in managing the environmental impact of ethyl acetate use. Many countries have established emission standards, waste management protocols, and occupational exposure limits specific to ethyl acetate and similar solvents. Compliance with these regulations is essential for minimizing environmental risks.
As sustainability becomes increasingly important, there is growing interest in developing bio-based alternatives to petrochemical-derived ethyl acetate. These green solvents, often produced from renewable resources, aim to reduce the carbon footprint associated with solvent production and use in recovery processes.
Economic Feasibility of Ethyl Acetate Recovery Systems
The economic feasibility of ethyl acetate recovery systems is a critical consideration for industries seeking to optimize their chemical processes and reduce waste. Ethyl acetate, a versatile solvent widely used in various applications, presents significant opportunities for recovery and reuse, potentially leading to substantial cost savings and environmental benefits.
Initial investment in ethyl acetate recovery systems can be substantial, typically involving the installation of specialized equipment such as distillation columns, condensers, and storage tanks. However, the long-term economic advantages often outweigh these upfront costs. The primary economic benefit stems from the reduction in raw material expenses, as recovered ethyl acetate can be reintroduced into the production process, minimizing the need for fresh solvent purchases.
Operational costs associated with ethyl acetate recovery systems include energy consumption for distillation processes, maintenance of equipment, and labor for system operation. These ongoing expenses must be carefully balanced against the savings generated from solvent recovery. Factors such as the scale of operation, the purity requirements of the recovered solvent, and local energy costs significantly influence the overall economic viability of the recovery system.
The market price of ethyl acetate plays a crucial role in determining the payback period for recovery systems. Fluctuations in solvent prices can impact the economic attractiveness of recovery operations. Generally, higher market prices for ethyl acetate increase the financial incentives for implementing recovery systems, as the value of reclaimed solvent becomes more significant.
Environmental regulations and waste disposal costs are additional factors that contribute to the economic feasibility of ethyl acetate recovery. Stringent environmental policies often impose high costs for the disposal of chemical waste, making recovery systems more appealing from both an economic and regulatory compliance perspective. By reducing waste generation and minimizing environmental impact, companies can avoid potential fines and improve their corporate image.
The efficiency of the recovery process is a key determinant of economic viability. Advanced recovery technologies, such as membrane separation or azeotropic distillation, can achieve higher recovery rates and purity levels, enhancing the economic benefits. Continuous improvement in recovery technologies is expected to further improve the cost-effectiveness of these systems in the future.
Initial investment in ethyl acetate recovery systems can be substantial, typically involving the installation of specialized equipment such as distillation columns, condensers, and storage tanks. However, the long-term economic advantages often outweigh these upfront costs. The primary economic benefit stems from the reduction in raw material expenses, as recovered ethyl acetate can be reintroduced into the production process, minimizing the need for fresh solvent purchases.
Operational costs associated with ethyl acetate recovery systems include energy consumption for distillation processes, maintenance of equipment, and labor for system operation. These ongoing expenses must be carefully balanced against the savings generated from solvent recovery. Factors such as the scale of operation, the purity requirements of the recovered solvent, and local energy costs significantly influence the overall economic viability of the recovery system.
The market price of ethyl acetate plays a crucial role in determining the payback period for recovery systems. Fluctuations in solvent prices can impact the economic attractiveness of recovery operations. Generally, higher market prices for ethyl acetate increase the financial incentives for implementing recovery systems, as the value of reclaimed solvent becomes more significant.
Environmental regulations and waste disposal costs are additional factors that contribute to the economic feasibility of ethyl acetate recovery. Stringent environmental policies often impose high costs for the disposal of chemical waste, making recovery systems more appealing from both an economic and regulatory compliance perspective. By reducing waste generation and minimizing environmental impact, companies can avoid potential fines and improve their corporate image.
The efficiency of the recovery process is a key determinant of economic viability. Advanced recovery technologies, such as membrane separation or azeotropic distillation, can achieve higher recovery rates and purity levels, enhancing the economic benefits. Continuous improvement in recovery technologies is expected to further improve the cost-effectiveness of these systems in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!