How Ethyl Acetate Facilitates Efficient Solvent Recycling?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Recycling Background and Objectives
Ethyl acetate, a versatile organic compound, has gained significant attention in the realm of solvent recycling due to its unique properties and widespread industrial applications. The evolution of ethyl acetate recycling technology can be traced back to the early 20th century when the chemical industry began to recognize the importance of sustainable practices and resource conservation.
Initially, the recovery of ethyl acetate was primarily driven by economic considerations, as the compound's value and increasing demand made recycling an attractive option for manufacturers. Over time, environmental concerns and stricter regulations on waste disposal further propelled the development of more efficient recycling methods.
The technological progression in ethyl acetate recycling has been marked by several key milestones. Early techniques relied on simple distillation processes, which were energy-intensive and often resulted in significant product loss. As understanding of chemical separation techniques advanced, more sophisticated methods such as azeotropic distillation and membrane separation were introduced, greatly improving recovery rates and product purity.
In recent years, the focus has shifted towards developing more sustainable and energy-efficient recycling processes. This has led to the exploration of novel technologies such as pervaporation, adsorption, and reactive distillation, which promise higher efficiency and reduced environmental impact.
The primary objective of ethyl acetate recycling is to maximize the recovery of the solvent while minimizing energy consumption and operational costs. This involves developing processes that can effectively separate ethyl acetate from various mixtures, including water and other organic compounds, with high purity and yield.
Another critical goal is to enhance the sustainability of industrial processes that utilize ethyl acetate. By implementing efficient recycling systems, companies can significantly reduce their raw material consumption, waste generation, and overall environmental footprint. This aligns with global efforts to promote circular economy principles and reduce dependence on fossil-based resources.
Furthermore, the development of ethyl acetate recycling technologies aims to address specific challenges in different industries. For instance, in the pharmaceutical sector, there is a growing need for recycling methods that can handle complex mixtures while meeting stringent purity requirements. In the packaging industry, the focus is on developing closed-loop systems that can efficiently recover ethyl acetate from printing and laminating processes.
As we look towards the future, the objectives of ethyl acetate recycling are expanding to include the integration of smart technologies and process intensification. This involves the development of automated systems that can optimize recycling parameters in real-time, as well as the design of compact, modular units that can be easily scaled and adapted to various industrial settings.
Initially, the recovery of ethyl acetate was primarily driven by economic considerations, as the compound's value and increasing demand made recycling an attractive option for manufacturers. Over time, environmental concerns and stricter regulations on waste disposal further propelled the development of more efficient recycling methods.
The technological progression in ethyl acetate recycling has been marked by several key milestones. Early techniques relied on simple distillation processes, which were energy-intensive and often resulted in significant product loss. As understanding of chemical separation techniques advanced, more sophisticated methods such as azeotropic distillation and membrane separation were introduced, greatly improving recovery rates and product purity.
In recent years, the focus has shifted towards developing more sustainable and energy-efficient recycling processes. This has led to the exploration of novel technologies such as pervaporation, adsorption, and reactive distillation, which promise higher efficiency and reduced environmental impact.
The primary objective of ethyl acetate recycling is to maximize the recovery of the solvent while minimizing energy consumption and operational costs. This involves developing processes that can effectively separate ethyl acetate from various mixtures, including water and other organic compounds, with high purity and yield.
Another critical goal is to enhance the sustainability of industrial processes that utilize ethyl acetate. By implementing efficient recycling systems, companies can significantly reduce their raw material consumption, waste generation, and overall environmental footprint. This aligns with global efforts to promote circular economy principles and reduce dependence on fossil-based resources.
Furthermore, the development of ethyl acetate recycling technologies aims to address specific challenges in different industries. For instance, in the pharmaceutical sector, there is a growing need for recycling methods that can handle complex mixtures while meeting stringent purity requirements. In the packaging industry, the focus is on developing closed-loop systems that can efficiently recover ethyl acetate from printing and laminating processes.
As we look towards the future, the objectives of ethyl acetate recycling are expanding to include the integration of smart technologies and process intensification. This involves the development of automated systems that can optimize recycling parameters in real-time, as well as the design of compact, modular units that can be easily scaled and adapted to various industrial settings.
Market Demand for Sustainable Solvent Solutions
The global market for sustainable solvent solutions has been experiencing significant growth in recent years, driven by increasing environmental concerns and stringent regulations. Ethyl acetate, as a versatile and relatively eco-friendly solvent, has emerged as a key player in this market, particularly in the context of efficient solvent recycling.
The demand for ethyl acetate in solvent recycling applications is primarily fueled by industries such as pharmaceuticals, paints and coatings, adhesives, and electronics. These sectors are increasingly seeking ways to reduce their environmental footprint and operational costs through improved solvent management practices. The ability of ethyl acetate to facilitate efficient solvent recycling aligns well with these industry needs.
In the pharmaceutical industry, where solvent use is extensive and regulatory scrutiny is high, there is a growing emphasis on green chemistry principles. Ethyl acetate's low toxicity and high recovery rate make it an attractive option for solvent recycling in drug manufacturing processes. Similarly, the paints and coatings industry, facing pressure to reduce volatile organic compound (VOC) emissions, is turning to recyclable solvents like ethyl acetate to meet environmental standards while maintaining product quality.
The electronics industry, particularly in the production of printed circuit boards and semiconductor cleaning, is another significant market for ethyl acetate-based solvent recycling solutions. The need for high-purity solvents in these applications, coupled with the economic benefits of recycling, drives the demand for efficient recovery systems.
Market analysts project a steady growth in the demand for sustainable solvent solutions, with ethyl acetate playing a crucial role. This growth is supported by the increasing adoption of circular economy principles across industries and the push towards more sustainable manufacturing practices. The market is also seeing a rise in demand for closed-loop solvent recycling systems, where ethyl acetate's properties make it a preferred choice.
Geographically, the market for ethyl acetate in solvent recycling applications is showing strong growth in regions with strict environmental regulations, such as Europe and North America. However, emerging economies in Asia-Pacific are also witnessing increased demand, driven by rapid industrialization and growing awareness of sustainable practices.
The market demand is further bolstered by technological advancements in solvent recycling processes, which are enhancing the efficiency and cost-effectiveness of ethyl acetate recovery. These innovations are making solvent recycling more accessible to small and medium-sized enterprises, thus expanding the market base.
The demand for ethyl acetate in solvent recycling applications is primarily fueled by industries such as pharmaceuticals, paints and coatings, adhesives, and electronics. These sectors are increasingly seeking ways to reduce their environmental footprint and operational costs through improved solvent management practices. The ability of ethyl acetate to facilitate efficient solvent recycling aligns well with these industry needs.
In the pharmaceutical industry, where solvent use is extensive and regulatory scrutiny is high, there is a growing emphasis on green chemistry principles. Ethyl acetate's low toxicity and high recovery rate make it an attractive option for solvent recycling in drug manufacturing processes. Similarly, the paints and coatings industry, facing pressure to reduce volatile organic compound (VOC) emissions, is turning to recyclable solvents like ethyl acetate to meet environmental standards while maintaining product quality.
The electronics industry, particularly in the production of printed circuit boards and semiconductor cleaning, is another significant market for ethyl acetate-based solvent recycling solutions. The need for high-purity solvents in these applications, coupled with the economic benefits of recycling, drives the demand for efficient recovery systems.
Market analysts project a steady growth in the demand for sustainable solvent solutions, with ethyl acetate playing a crucial role. This growth is supported by the increasing adoption of circular economy principles across industries and the push towards more sustainable manufacturing practices. The market is also seeing a rise in demand for closed-loop solvent recycling systems, where ethyl acetate's properties make it a preferred choice.
Geographically, the market for ethyl acetate in solvent recycling applications is showing strong growth in regions with strict environmental regulations, such as Europe and North America. However, emerging economies in Asia-Pacific are also witnessing increased demand, driven by rapid industrialization and growing awareness of sustainable practices.
The market demand is further bolstered by technological advancements in solvent recycling processes, which are enhancing the efficiency and cost-effectiveness of ethyl acetate recovery. These innovations are making solvent recycling more accessible to small and medium-sized enterprises, thus expanding the market base.
Current Challenges in Ethyl Acetate Recovery
The recovery of ethyl acetate presents several significant challenges in the realm of solvent recycling. One of the primary obstacles is the formation of azeotropes, particularly with water. Ethyl acetate forms a low-boiling azeotrope with water at 70.4°C, making conventional distillation methods ineffective for complete separation. This azeotropic behavior necessitates more complex and energy-intensive separation techniques, such as azeotropic distillation or extractive distillation.
Another challenge lies in the potential degradation of ethyl acetate during the recovery process. Exposure to high temperatures or acidic conditions can lead to hydrolysis, resulting in the formation of ethanol and acetic acid. This degradation not only reduces the yield of recovered ethyl acetate but also introduces impurities that may affect the quality of the recycled solvent.
The presence of other organic compounds in the waste stream further complicates the recovery process. Industrial processes often involve multiple solvents or generate by-products that can interfere with ethyl acetate separation. These contaminants may have similar boiling points or form additional azeotropes, requiring more sophisticated purification steps.
Energy efficiency remains a critical concern in ethyl acetate recovery. Traditional distillation methods consume substantial amounts of energy, particularly when dealing with azeotropic mixtures. The need for multiple distillation stages or the use of entrainers in azeotropic distillation further increases energy requirements, potentially offsetting the economic and environmental benefits of solvent recycling.
Membrane-based separation technologies, while promising, face limitations in ethyl acetate recovery. Issues such as membrane fouling, limited selectivity, and reduced flux over time pose challenges to their widespread adoption. Additionally, the development of membranes with high selectivity for ethyl acetate while maintaining adequate permeability remains an ongoing research challenge.
The scale-up of laboratory-proven recovery methods to industrial-scale operations presents its own set of challenges. Factors such as heat transfer efficiency, mixing dynamics, and equipment design must be carefully considered to maintain separation efficiency at larger scales. Furthermore, the economic viability of implementing advanced recovery technologies on an industrial scale often requires significant capital investment, which can be a barrier for smaller operations.
Regulatory compliance and product purity specifications add another layer of complexity to ethyl acetate recovery. Stringent quality standards for recycled solvents, particularly in industries such as pharmaceuticals and electronics, necessitate highly efficient purification processes. Meeting these standards while maintaining cost-effectiveness remains a balancing act for many manufacturers.
Another challenge lies in the potential degradation of ethyl acetate during the recovery process. Exposure to high temperatures or acidic conditions can lead to hydrolysis, resulting in the formation of ethanol and acetic acid. This degradation not only reduces the yield of recovered ethyl acetate but also introduces impurities that may affect the quality of the recycled solvent.
The presence of other organic compounds in the waste stream further complicates the recovery process. Industrial processes often involve multiple solvents or generate by-products that can interfere with ethyl acetate separation. These contaminants may have similar boiling points or form additional azeotropes, requiring more sophisticated purification steps.
Energy efficiency remains a critical concern in ethyl acetate recovery. Traditional distillation methods consume substantial amounts of energy, particularly when dealing with azeotropic mixtures. The need for multiple distillation stages or the use of entrainers in azeotropic distillation further increases energy requirements, potentially offsetting the economic and environmental benefits of solvent recycling.
Membrane-based separation technologies, while promising, face limitations in ethyl acetate recovery. Issues such as membrane fouling, limited selectivity, and reduced flux over time pose challenges to their widespread adoption. Additionally, the development of membranes with high selectivity for ethyl acetate while maintaining adequate permeability remains an ongoing research challenge.
The scale-up of laboratory-proven recovery methods to industrial-scale operations presents its own set of challenges. Factors such as heat transfer efficiency, mixing dynamics, and equipment design must be carefully considered to maintain separation efficiency at larger scales. Furthermore, the economic viability of implementing advanced recovery technologies on an industrial scale often requires significant capital investment, which can be a barrier for smaller operations.
Regulatory compliance and product purity specifications add another layer of complexity to ethyl acetate recovery. Stringent quality standards for recycled solvents, particularly in industries such as pharmaceuticals and electronics, necessitate highly efficient purification processes. Meeting these standards while maintaining cost-effectiveness remains a balancing act for many manufacturers.
Existing Ethyl Acetate Recycling Methods
01 Ethyl acetate production methods
Various methods for producing ethyl acetate efficiently are described, including esterification of ethanol and acetic acid, dehydrogenation of ethanol, and oxidative esterification. These processes aim to improve yield, reduce energy consumption, and minimize byproduct formation.- Ethyl acetate production methods: Various methods for producing ethyl acetate efficiently are described, including esterification of ethanol and acetic acid, dehydrogenation of ethanol, and oxidation of ethanol. These processes aim to improve yield and reduce energy consumption in ethyl acetate production.
- Purification and separation techniques: Efficient purification and separation methods for ethyl acetate are discussed, including distillation, extraction, and membrane separation. These techniques aim to increase the purity of ethyl acetate and improve overall process efficiency.
- Catalysts for ethyl acetate synthesis: Various catalysts are explored to enhance the efficiency of ethyl acetate production. These include heterogeneous catalysts, enzyme catalysts, and novel catalyst formulations designed to improve reaction rates and selectivity.
- Process optimization and energy efficiency: Strategies for optimizing ethyl acetate production processes are presented, focusing on energy efficiency, heat integration, and process intensification. These approaches aim to reduce operating costs and environmental impact while maintaining high product quality.
- Applications and utilization efficiency: Various applications of ethyl acetate are explored, along with methods to improve its utilization efficiency in different industries. This includes its use as a solvent, in chemical synthesis, and in the production of coatings and adhesives.
02 Purification and separation techniques
Efficient purification and separation methods for ethyl acetate are discussed, including distillation, extraction, and membrane separation. These techniques focus on improving product purity, reducing energy consumption, and increasing overall process efficiency.Expand Specific Solutions03 Catalysts for ethyl acetate synthesis
Various catalysts are explored to enhance the efficiency of ethyl acetate production. These include heterogeneous catalysts, enzyme catalysts, and novel catalyst formulations designed to improve reaction rates, selectivity, and catalyst longevity.Expand Specific Solutions04 Process optimization and energy efficiency
Strategies for optimizing ethyl acetate production processes are presented, focusing on energy efficiency, heat integration, and process intensification. These approaches aim to reduce operating costs and environmental impact while maintaining high product quality.Expand Specific Solutions05 Applications and utilization of ethyl acetate
Various applications and efficient utilization methods for ethyl acetate are discussed, including its use as a solvent, in chemical synthesis, and in the production of coatings and adhesives. The focus is on maximizing the effectiveness of ethyl acetate in different industrial processes.Expand Specific Solutions
Key Players in Solvent Recycling Industry
The ethyl acetate solvent recycling market is in a growth phase, driven by increasing environmental regulations and sustainability initiatives. The market size is expanding due to rising demand in various industries, including pharmaceuticals, paints, and coatings. Technologically, the field is advancing, with companies like Viridis Chemical LLC and Wacker Chemie AG leading innovations in bio-based ethyl acetate production and recycling processes. Major players such as China Petroleum & Chemical Corp. and Celanese International Corp. are investing in efficient recycling technologies. Universities like the University of Campinas and South China University of Technology are contributing to research and development, enhancing the technical maturity of ethyl acetate recycling methods.
Viridis Chemical LLC
Technical Solution: Viridis Chemical LLC has developed an innovative approach to ethyl acetate recycling, focusing on sustainable production methods. Their process involves the use of advanced distillation techniques and catalytic conversion to efficiently separate and purify ethyl acetate from waste streams. The company employs a closed-loop system that minimizes energy consumption and reduces environmental impact. Their technology allows for the recovery of up to 99% of ethyl acetate from industrial waste streams[1], significantly reducing the need for new production and associated carbon emissions. Additionally, Viridis has implemented a proprietary membrane separation technology that enhances the purity of recovered ethyl acetate, making it suitable for high-grade applications in pharmaceuticals and electronics[2].
Strengths: High recovery rate, closed-loop system, and enhanced purity of recycled product. Weaknesses: Potentially high initial investment costs and limited scalability for smaller operations.
Wacker Chemie AG
Technical Solution: Wacker Chemie AG has developed a cutting-edge solvent recycling system specifically designed for ethyl acetate. Their approach utilizes a combination of advanced distillation columns and molecular sieves to achieve high-purity solvent recovery. The process incorporates a multi-stage distillation system that effectively separates ethyl acetate from other solvents and impurities. Wacker's technology also features an innovative heat integration system that reduces energy consumption by up to 30% compared to conventional recycling methods[3]. Furthermore, the company has implemented an automated control system that optimizes the recycling process in real-time, ensuring consistent quality and maximizing efficiency. Wacker's ethyl acetate recycling technology can handle large volumes, with capacities ranging from 1,000 to 10,000 tons per year[4].
Strengths: Energy-efficient process, high-capacity handling, and consistent high-quality output. Weaknesses: May require significant space for installation and potentially high initial capital investment.
Innovative Approaches in Ethyl Acetate Recovery
Ethyl acetate production and purification
PatentPendingUS20250179003A1
Innovation
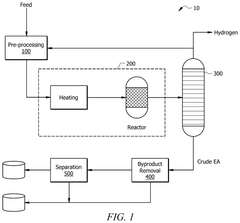
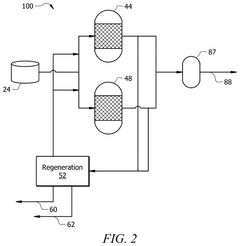
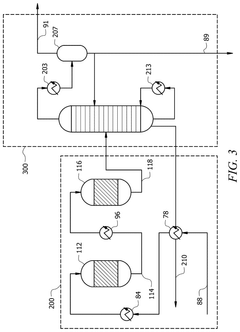
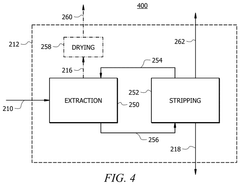
- A method involving the use of a solvent with an extracting agent and a defoamer to purify an ethyl acetate stream. The solvent contacts an inlet stream containing ethyl acetate and impurities, transferring impurities into the solvent to form an extract and a purified product. The extract is then separated from the purified product, and the impurities are removed using a defoamer to prevent foam formation, resulting in a regenerated solvent that is recycled.
Compositions and methods of isolating policosanol and sterols
PatentPendingEP4491605A1
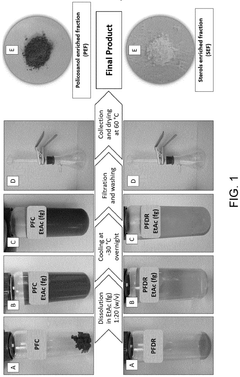
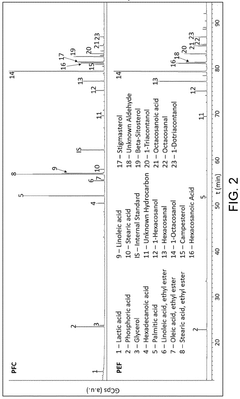
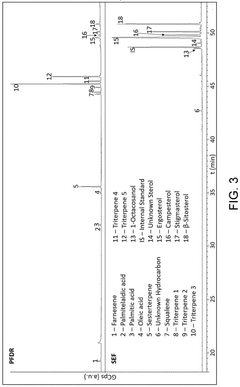
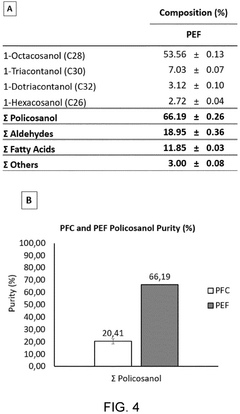
Innovation
- A winterization process using a single food-grade solvent, ethyl acetate, to dissolve and cool lipid extracts from sugarcane processing and fermentation waste streams, allowing policosanol and sterols to precipitate and be isolated through filtration, resulting in a cost-effective and sustainable method.
Environmental Impact of Ethyl Acetate Recycling
The recycling of ethyl acetate has significant environmental implications, both positive and negative. On the positive side, efficient solvent recycling reduces the overall consumption of raw materials and energy required for ethyl acetate production. This leads to a decrease in greenhouse gas emissions and helps conserve natural resources. Additionally, recycling minimizes the amount of waste sent to landfills or incineration facilities, reducing the environmental burden associated with disposal.
However, the recycling process itself can have environmental impacts. The distillation and purification steps involved in ethyl acetate recycling require energy, typically in the form of heat. This energy consumption contributes to carbon emissions if fossil fuels are used as the primary energy source. Furthermore, the process may generate small amounts of waste or byproducts that require proper handling and disposal.
Water usage is another environmental consideration in ethyl acetate recycling. Some recycling methods involve water-based extraction or washing steps, which can lead to increased water consumption and potential wastewater generation. Proper treatment of this wastewater is essential to prevent contamination of local water sources.
The transportation of used ethyl acetate to recycling facilities also has environmental implications. The carbon footprint associated with transportation can be significant, especially if recycling facilities are located far from the point of use. However, this impact is generally outweighed by the benefits of recycling compared to producing new ethyl acetate.
From a lifecycle perspective, recycling ethyl acetate typically results in a net positive environmental impact. Studies have shown that the energy and resources saved by recycling outweigh the environmental costs of the recycling process itself. This is particularly true when considering the avoided impacts of producing virgin ethyl acetate and managing waste from single-use solvents.
The environmental benefits of ethyl acetate recycling can be further enhanced through the use of renewable energy sources in the recycling process and the optimization of transportation logistics. Additionally, advancements in recycling technologies, such as more efficient distillation methods or novel separation techniques, can further reduce the environmental footprint of the recycling process.
In conclusion, while ethyl acetate recycling does have some environmental impacts, particularly related to energy use and potential emissions, the overall effect is generally positive when compared to single-use scenarios. The key to maximizing the environmental benefits lies in continually improving recycling technologies and implementing best practices in process efficiency and waste management.
However, the recycling process itself can have environmental impacts. The distillation and purification steps involved in ethyl acetate recycling require energy, typically in the form of heat. This energy consumption contributes to carbon emissions if fossil fuels are used as the primary energy source. Furthermore, the process may generate small amounts of waste or byproducts that require proper handling and disposal.
Water usage is another environmental consideration in ethyl acetate recycling. Some recycling methods involve water-based extraction or washing steps, which can lead to increased water consumption and potential wastewater generation. Proper treatment of this wastewater is essential to prevent contamination of local water sources.
The transportation of used ethyl acetate to recycling facilities also has environmental implications. The carbon footprint associated with transportation can be significant, especially if recycling facilities are located far from the point of use. However, this impact is generally outweighed by the benefits of recycling compared to producing new ethyl acetate.
From a lifecycle perspective, recycling ethyl acetate typically results in a net positive environmental impact. Studies have shown that the energy and resources saved by recycling outweigh the environmental costs of the recycling process itself. This is particularly true when considering the avoided impacts of producing virgin ethyl acetate and managing waste from single-use solvents.
The environmental benefits of ethyl acetate recycling can be further enhanced through the use of renewable energy sources in the recycling process and the optimization of transportation logistics. Additionally, advancements in recycling technologies, such as more efficient distillation methods or novel separation techniques, can further reduce the environmental footprint of the recycling process.
In conclusion, while ethyl acetate recycling does have some environmental impacts, particularly related to energy use and potential emissions, the overall effect is generally positive when compared to single-use scenarios. The key to maximizing the environmental benefits lies in continually improving recycling technologies and implementing best practices in process efficiency and waste management.
Economic Feasibility of Recycling Processes
The economic feasibility of recycling ethyl acetate is a critical consideration for industries seeking to implement sustainable solvent management practices. The process of recycling ethyl acetate can offer significant cost savings and environmental benefits, but it requires careful analysis of various economic factors.
One of the primary economic advantages of recycling ethyl acetate is the reduction in raw material costs. By recovering and reusing the solvent, companies can substantially decrease their reliance on purchasing new ethyl acetate. This can lead to considerable savings, especially for industries that consume large quantities of the solvent in their production processes.
The initial investment in recycling equipment and infrastructure is a key economic consideration. While the upfront costs can be substantial, including distillation units, storage tanks, and associated piping systems, these expenses are often offset by long-term savings. The payback period for such investments typically ranges from one to three years, depending on the scale of operations and the efficiency of the recycling process.
Energy consumption is another crucial factor in the economic equation of ethyl acetate recycling. The distillation process used to purify the recovered solvent requires significant energy input. However, advancements in energy-efficient distillation technologies have helped to reduce these costs. Additionally, the energy required for recycling is generally lower than that needed for the production of new ethyl acetate, contributing to overall energy savings.
Labor costs associated with operating and maintaining the recycling system must also be factored into the economic analysis. While these costs can be significant, they are often outweighed by the savings in raw material purchases and waste disposal fees. Furthermore, the implementation of automated recycling systems can help minimize labor requirements and associated costs.
The market value of recycled ethyl acetate is an important consideration. In many cases, properly recycled ethyl acetate can be sold or reused at a value comparable to virgin solvent, provided it meets the required purity standards. This creates potential revenue streams or cost offsets for companies engaging in recycling practices.
Regulatory compliance and environmental considerations also play a role in the economic feasibility of ethyl acetate recycling. While compliance with environmental regulations may incur additional costs, it can also lead to avoided penalties and improved corporate image. Moreover, companies that demonstrate commitment to sustainable practices may gain competitive advantages in markets that prioritize environmental responsibility.
The scale of operations significantly impacts the economic viability of ethyl acetate recycling. Larger-scale operations generally benefit from economies of scale, with lower per-unit costs for recycling. However, even smaller operations can achieve economic benefits through careful process optimization and potentially by partnering with specialized recycling facilities.
One of the primary economic advantages of recycling ethyl acetate is the reduction in raw material costs. By recovering and reusing the solvent, companies can substantially decrease their reliance on purchasing new ethyl acetate. This can lead to considerable savings, especially for industries that consume large quantities of the solvent in their production processes.
The initial investment in recycling equipment and infrastructure is a key economic consideration. While the upfront costs can be substantial, including distillation units, storage tanks, and associated piping systems, these expenses are often offset by long-term savings. The payback period for such investments typically ranges from one to three years, depending on the scale of operations and the efficiency of the recycling process.
Energy consumption is another crucial factor in the economic equation of ethyl acetate recycling. The distillation process used to purify the recovered solvent requires significant energy input. However, advancements in energy-efficient distillation technologies have helped to reduce these costs. Additionally, the energy required for recycling is generally lower than that needed for the production of new ethyl acetate, contributing to overall energy savings.
Labor costs associated with operating and maintaining the recycling system must also be factored into the economic analysis. While these costs can be significant, they are often outweighed by the savings in raw material purchases and waste disposal fees. Furthermore, the implementation of automated recycling systems can help minimize labor requirements and associated costs.
The market value of recycled ethyl acetate is an important consideration. In many cases, properly recycled ethyl acetate can be sold or reused at a value comparable to virgin solvent, provided it meets the required purity standards. This creates potential revenue streams or cost offsets for companies engaging in recycling practices.
Regulatory compliance and environmental considerations also play a role in the economic feasibility of ethyl acetate recycling. While compliance with environmental regulations may incur additional costs, it can also lead to avoided penalties and improved corporate image. Moreover, companies that demonstrate commitment to sustainable practices may gain competitive advantages in markets that prioritize environmental responsibility.
The scale of operations significantly impacts the economic viability of ethyl acetate recycling. Larger-scale operations generally benefit from economies of scale, with lower per-unit costs for recycling. However, even smaller operations can achieve economic benefits through careful process optimization and potentially by partnering with specialized recycling facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!