How Ethyl Acetate Factors into Zero-Waste Productions?
JUN 27, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate in Zero-Waste: Background and Objectives
Ethyl acetate has emerged as a crucial component in the pursuit of zero-waste production systems, marking a significant shift in industrial practices towards sustainability. This versatile organic compound, with its unique chemical properties, has become a focal point for researchers and manufacturers alike in their quest to minimize environmental impact and maximize resource efficiency.
The concept of zero-waste production has gained traction in recent years as industries face mounting pressure to reduce their ecological footprint. This paradigm shift aims to create closed-loop systems where all materials are reused, recycled, or composted, effectively eliminating the notion of waste. Within this context, ethyl acetate's role has evolved from a mere solvent to a key player in sustainable manufacturing processes.
Historically, ethyl acetate has been widely used in various industries, including pharmaceuticals, cosmetics, and food production, primarily as a solvent and flavoring agent. However, its potential in zero-waste applications has only recently been fully recognized. The compound's low toxicity, high solvency power, and relatively easy recovery have positioned it as an ideal candidate for sustainable production methods.
The evolution of ethyl acetate's application in zero-waste production can be traced through several key technological advancements. Initially, the focus was on improving recovery and recycling techniques to minimize solvent loss. This led to the development of more efficient distillation and membrane separation technologies, significantly reducing the environmental impact of ethyl acetate use.
As the zero-waste movement gained momentum, researchers began exploring novel applications for ethyl acetate beyond its traditional roles. One notable development has been its use in bio-based production processes, where ethyl acetate serves as both a solvent and a platform chemical for the synthesis of various compounds. This approach aligns perfectly with the principles of green chemistry and circular economy.
The objectives of incorporating ethyl acetate into zero-waste productions are multifaceted. Primarily, it aims to reduce the overall environmental impact of industrial processes by minimizing waste generation and resource consumption. Additionally, there is a strong focus on improving the economic viability of sustainable practices, as efficient use and recovery of ethyl acetate can lead to significant cost savings.
Looking forward, the integration of ethyl acetate into zero-waste production systems presents several challenges and opportunities. Researchers are actively working on developing more sustainable methods for ethyl acetate production, exploring bio-based feedstocks and enzymatic processes. Furthermore, there is a growing interest in creating novel materials and products that leverage ethyl acetate's unique properties while adhering to zero-waste principles.
As industries continue to evolve towards more sustainable practices, the role of ethyl acetate in zero-waste production is expected to expand further. This trajectory not only promises environmental benefits but also opens up new avenues for innovation and economic growth in the realm of green technology.
The concept of zero-waste production has gained traction in recent years as industries face mounting pressure to reduce their ecological footprint. This paradigm shift aims to create closed-loop systems where all materials are reused, recycled, or composted, effectively eliminating the notion of waste. Within this context, ethyl acetate's role has evolved from a mere solvent to a key player in sustainable manufacturing processes.
Historically, ethyl acetate has been widely used in various industries, including pharmaceuticals, cosmetics, and food production, primarily as a solvent and flavoring agent. However, its potential in zero-waste applications has only recently been fully recognized. The compound's low toxicity, high solvency power, and relatively easy recovery have positioned it as an ideal candidate for sustainable production methods.
The evolution of ethyl acetate's application in zero-waste production can be traced through several key technological advancements. Initially, the focus was on improving recovery and recycling techniques to minimize solvent loss. This led to the development of more efficient distillation and membrane separation technologies, significantly reducing the environmental impact of ethyl acetate use.
As the zero-waste movement gained momentum, researchers began exploring novel applications for ethyl acetate beyond its traditional roles. One notable development has been its use in bio-based production processes, where ethyl acetate serves as both a solvent and a platform chemical for the synthesis of various compounds. This approach aligns perfectly with the principles of green chemistry and circular economy.
The objectives of incorporating ethyl acetate into zero-waste productions are multifaceted. Primarily, it aims to reduce the overall environmental impact of industrial processes by minimizing waste generation and resource consumption. Additionally, there is a strong focus on improving the economic viability of sustainable practices, as efficient use and recovery of ethyl acetate can lead to significant cost savings.
Looking forward, the integration of ethyl acetate into zero-waste production systems presents several challenges and opportunities. Researchers are actively working on developing more sustainable methods for ethyl acetate production, exploring bio-based feedstocks and enzymatic processes. Furthermore, there is a growing interest in creating novel materials and products that leverage ethyl acetate's unique properties while adhering to zero-waste principles.
As industries continue to evolve towards more sustainable practices, the role of ethyl acetate in zero-waste production is expected to expand further. This trajectory not only promises environmental benefits but also opens up new avenues for innovation and economic growth in the realm of green technology.
Market Demand for Sustainable Solvent Solutions
The market demand for sustainable solvent solutions has been steadily increasing in recent years, driven by growing environmental concerns and stringent regulations. Ethyl acetate, a versatile and relatively eco-friendly solvent, has emerged as a key player in this shift towards more sustainable industrial practices. Its role in zero-waste productions is particularly significant, as it offers a balance between performance and environmental impact.
In the chemical industry, there is a strong push for greener alternatives to traditional solvents. Many companies are actively seeking ways to reduce their carbon footprint and minimize waste generation. Ethyl acetate fits well into this paradigm, as it can be produced from renewable resources and is biodegradable. This aligns with the circular economy principles that many industries are striving to adopt.
The paint and coatings sector has shown considerable interest in ethyl acetate as a replacement for more harmful solvents. Its low toxicity and quick evaporation rate make it an attractive option for manufacturers looking to comply with volatile organic compound (VOC) regulations while maintaining product quality. The automotive and construction industries, in particular, have been driving this demand as they seek to meet increasingly strict environmental standards.
In the pharmaceutical industry, the demand for ethyl acetate in zero-waste productions is also on the rise. Its use in drug synthesis and as an extraction solvent in the production of natural compounds has increased, partly due to its lower environmental impact compared to alternatives. The industry's focus on green chemistry principles has led to a reevaluation of solvent choices, with ethyl acetate often emerging as a preferred option.
The electronics sector presents another growing market for ethyl acetate in sustainable manufacturing processes. Its effectiveness in cleaning and degreasing electronic components, coupled with its lower toxicity, makes it an attractive choice for companies looking to improve their environmental credentials without compromising on performance.
Consumer awareness of environmental issues has also played a role in driving market demand. Products that use ethyl acetate as a solvent can be marketed as more environmentally friendly, appealing to eco-conscious consumers. This has led to increased adoption in consumer goods such as nail polish removers and household cleaners.
Despite the growing demand, challenges remain in fully integrating ethyl acetate into zero-waste productions. The cost of transitioning to new solvent systems and potential process adjustments can be significant. Additionally, while ethyl acetate is more environmentally friendly than many alternatives, it is not without its own environmental considerations, particularly in terms of production methods and energy consumption.
In the chemical industry, there is a strong push for greener alternatives to traditional solvents. Many companies are actively seeking ways to reduce their carbon footprint and minimize waste generation. Ethyl acetate fits well into this paradigm, as it can be produced from renewable resources and is biodegradable. This aligns with the circular economy principles that many industries are striving to adopt.
The paint and coatings sector has shown considerable interest in ethyl acetate as a replacement for more harmful solvents. Its low toxicity and quick evaporation rate make it an attractive option for manufacturers looking to comply with volatile organic compound (VOC) regulations while maintaining product quality. The automotive and construction industries, in particular, have been driving this demand as they seek to meet increasingly strict environmental standards.
In the pharmaceutical industry, the demand for ethyl acetate in zero-waste productions is also on the rise. Its use in drug synthesis and as an extraction solvent in the production of natural compounds has increased, partly due to its lower environmental impact compared to alternatives. The industry's focus on green chemistry principles has led to a reevaluation of solvent choices, with ethyl acetate often emerging as a preferred option.
The electronics sector presents another growing market for ethyl acetate in sustainable manufacturing processes. Its effectiveness in cleaning and degreasing electronic components, coupled with its lower toxicity, makes it an attractive choice for companies looking to improve their environmental credentials without compromising on performance.
Consumer awareness of environmental issues has also played a role in driving market demand. Products that use ethyl acetate as a solvent can be marketed as more environmentally friendly, appealing to eco-conscious consumers. This has led to increased adoption in consumer goods such as nail polish removers and household cleaners.
Despite the growing demand, challenges remain in fully integrating ethyl acetate into zero-waste productions. The cost of transitioning to new solvent systems and potential process adjustments can be significant. Additionally, while ethyl acetate is more environmentally friendly than many alternatives, it is not without its own environmental considerations, particularly in terms of production methods and energy consumption.
Current State and Challenges in Ethyl Acetate Recycling
Ethyl acetate recycling has gained significant attention in recent years as industries strive towards zero-waste production. The current state of ethyl acetate recycling is characterized by a mix of established methods and emerging technologies, each presenting its own set of challenges.
Traditional distillation remains the most widely used method for ethyl acetate recovery. This process involves heating the solvent mixture to separate components based on their boiling points. While effective, distillation is energy-intensive and can lead to product degradation at high temperatures. Improvements in distillation column design and the implementation of heat integration systems have somewhat mitigated these issues, but energy consumption remains a significant challenge.
Membrane separation technologies have emerged as a promising alternative to distillation. These processes use selective membranes to separate ethyl acetate from other components in the waste stream. Pervaporation, in particular, has shown potential for ethyl acetate recovery. However, challenges persist in membrane stability and selectivity, especially when dealing with complex mixtures encountered in industrial settings.
Adsorption-based methods, using materials such as activated carbon or zeolites, offer another approach to ethyl acetate recycling. These techniques can be effective for low concentration streams but face limitations in capacity and regeneration efficiency. Research is ongoing to develop novel adsorbents with improved selectivity and regeneration characteristics.
A major challenge across all recycling methods is dealing with impurities and contaminants in the waste stream. These can include reaction by-products, unreacted raw materials, and other solvents. The presence of azeotropes, particularly the ethyl acetate-water azeotrope, further complicates separation processes. Developing robust purification strategies that can handle diverse and variable waste compositions remains a key focus area.
Scale-up and integration of recycling processes into existing production lines present additional challenges. Many promising lab-scale technologies struggle to maintain performance at industrial scales. Furthermore, retrofitting existing facilities with recycling capabilities often requires significant capital investment and process modifications.
Economic viability remains a critical factor in the adoption of ethyl acetate recycling technologies. While the environmental benefits are clear, the cost of recycling must be competitive with the price of fresh solvent for widespread implementation. This balance is influenced by factors such as energy costs, regulatory pressures, and market demand for sustainable practices.
Regulatory compliance and product quality assurance add another layer of complexity to ethyl acetate recycling efforts. Stringent standards for recycled solvents, particularly in industries like pharmaceuticals and food packaging, necessitate robust quality control measures and may limit the applicability of some recycling technologies.
Traditional distillation remains the most widely used method for ethyl acetate recovery. This process involves heating the solvent mixture to separate components based on their boiling points. While effective, distillation is energy-intensive and can lead to product degradation at high temperatures. Improvements in distillation column design and the implementation of heat integration systems have somewhat mitigated these issues, but energy consumption remains a significant challenge.
Membrane separation technologies have emerged as a promising alternative to distillation. These processes use selective membranes to separate ethyl acetate from other components in the waste stream. Pervaporation, in particular, has shown potential for ethyl acetate recovery. However, challenges persist in membrane stability and selectivity, especially when dealing with complex mixtures encountered in industrial settings.
Adsorption-based methods, using materials such as activated carbon or zeolites, offer another approach to ethyl acetate recycling. These techniques can be effective for low concentration streams but face limitations in capacity and regeneration efficiency. Research is ongoing to develop novel adsorbents with improved selectivity and regeneration characteristics.
A major challenge across all recycling methods is dealing with impurities and contaminants in the waste stream. These can include reaction by-products, unreacted raw materials, and other solvents. The presence of azeotropes, particularly the ethyl acetate-water azeotrope, further complicates separation processes. Developing robust purification strategies that can handle diverse and variable waste compositions remains a key focus area.
Scale-up and integration of recycling processes into existing production lines present additional challenges. Many promising lab-scale technologies struggle to maintain performance at industrial scales. Furthermore, retrofitting existing facilities with recycling capabilities often requires significant capital investment and process modifications.
Economic viability remains a critical factor in the adoption of ethyl acetate recycling technologies. While the environmental benefits are clear, the cost of recycling must be competitive with the price of fresh solvent for widespread implementation. This balance is influenced by factors such as energy costs, regulatory pressures, and market demand for sustainable practices.
Regulatory compliance and product quality assurance add another layer of complexity to ethyl acetate recycling efforts. Stringent standards for recycled solvents, particularly in industries like pharmaceuticals and food packaging, necessitate robust quality control measures and may limit the applicability of some recycling technologies.
Existing Zero-Waste Solutions for Ethyl Acetate
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The processes aim to optimize the production of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described. These include esterification processes, distillation techniques, and the use of specific catalysts to improve yield and purity. The production methods aim to optimize the synthesis of ethyl acetate from ethanol and acetic acid or other precursors.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. The versatility of ethyl acetate in different chemical reactions and industrial processes is highlighted.
- Ethyl acetate in extraction and separation processes: Ethyl acetate is employed in extraction and separation processes for various compounds. Its properties make it suitable for liquid-liquid extraction, azeotropic distillation, and other separation techniques. The use of ethyl acetate in these processes can improve efficiency and yield in chemical separations.
- Environmental and safety considerations for ethyl acetate: The environmental impact and safety aspects of ethyl acetate production and use are addressed. This includes methods for reducing emissions, improving process safety, and developing more sustainable production routes. Considerations for handling, storage, and disposal of ethyl acetate are also discussed.
- Novel derivatives and formulations of ethyl acetate: Research into novel derivatives and formulations of ethyl acetate is presented. This includes the development of new compounds based on ethyl acetate, as well as innovative formulations for specific applications. The aim is to expand the utility of ethyl acetate and its derivatives in various industries.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in various chemical processes as a solvent, reactant, or intermediate. It finds applications in the production of pharmaceuticals, polymers, and other organic compounds. The versatility of ethyl acetate in different chemical reactions and industrial processes is highlighted.Expand Specific Solutions03 Ethyl acetate in extraction and separation processes
Ethyl acetate is employed in extraction and separation processes for various compounds. Its use as a solvent in liquid-liquid extraction, chromatography, and other separation techniques is described. The effectiveness of ethyl acetate in isolating specific compounds from complex mixtures is emphasized.Expand Specific Solutions04 Ethyl acetate in formulations and compositions
The incorporation of ethyl acetate in various formulations and compositions is discussed. Its use as a solvent or carrier in paints, coatings, adhesives, and other products is described. The role of ethyl acetate in enhancing the properties and performance of these formulations is highlighted.Expand Specific Solutions05 Recovery and recycling of ethyl acetate
Methods for recovering and recycling ethyl acetate from industrial processes are presented. These include techniques for separating ethyl acetate from waste streams, purifying recovered ethyl acetate, and reusing it in various applications. The environmental and economic benefits of ethyl acetate recovery are emphasized.Expand Specific Solutions
Key Players in Sustainable Solvent Industry
The ethyl acetate market is in a mature growth phase, characterized by established production processes and widespread industrial applications. The global market size is estimated to be over $3 billion, with steady growth projected due to increasing demand in various sectors like coatings, adhesives, and pharmaceuticals. Technologically, the field is evolving towards more sustainable production methods. Companies like Wacker Chemie AG, Celanese International Corp., and Eastman Chemical Co. are at the forefront, developing bio-based ethyl acetate and zero-waste production techniques. Emerging players such as LanzaTech NZ, Inc. and Viridis Chemical LLC are introducing innovative biotechnology approaches, signaling a shift towards greener manufacturing processes in this mature industry.
Wacker Chemie AG
Technical Solution: Wacker Chemie has developed an integrated acetic acid and ethyl acetate production process that significantly contributes to zero-waste goals. Their approach utilizes a unique catalytic system that enables the direct conversion of ethylene and acetic acid to ethyl acetate in a single step, bypassing the need for intermediate ethanol production[1]. This streamlined process reduces energy consumption and minimizes byproduct formation. Wacker has also implemented an advanced distillation system that achieves high-purity ethyl acetate while recovering and recycling unreacted materials[2]. The company has further enhanced sustainability by incorporating biomass-derived acetic acid into their feedstock, reducing the carbon footprint of the process. Additionally, Wacker has developed a novel membrane technology for product purification that reduces solvent usage and waste generation compared to traditional methods[3].
Strengths: Efficient one-step process, advanced distillation and membrane technologies, and use of bio-based feedstocks. Weaknesses: Dependence on ethylene availability and potential sensitivity to feedstock price fluctuations.
Celanese International Corp.
Technical Solution: Celanese has made significant strides in zero-waste ethyl acetate production through their "TCX® Technology". This innovative process utilizes a highly selective carbonylation catalyst that converts ethanol directly to ethyl acetate with exceptional yield and selectivity[1]. The technology operates in a continuous flow system, minimizing waste generation and energy consumption. Celanese has also implemented a sophisticated solvent recovery system that recycles nearly all process solvents, dramatically reducing waste output[2]. Furthermore, the company has developed a bio-ethanol integration strategy, allowing for the use of renewable ethanol sources in their production process. Celanese has also pioneered an advanced process control system that optimizes reaction conditions in real-time, ensuring maximum efficiency and minimal waste generation[3]. The company's commitment to sustainability is further demonstrated by their implementation of a zero-liquid discharge system at several production facilities.
Strengths: Highly selective catalytic process, efficient solvent recovery, bio-ethanol integration, and advanced process control. Weaknesses: Potential reliance on specific catalyst technology and need for consistent bio-ethanol supply.
Innovations in Ethyl Acetate Recovery and Reuse
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
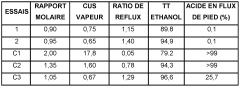
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
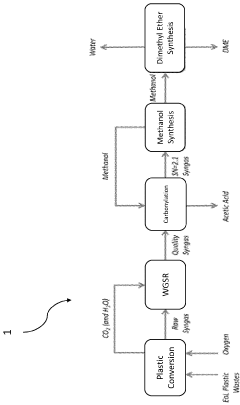
Zero-emission self-sustainable process for producing chemicals from organic sources
PatentWO2023166438A1
Innovation
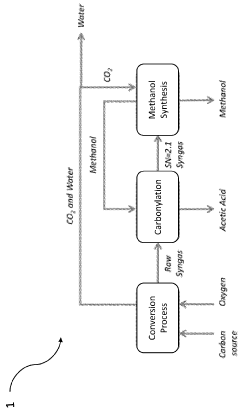
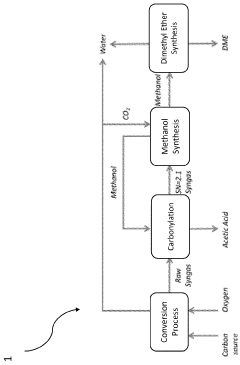
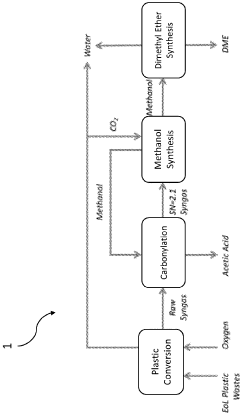
- A process that converts plastic waste into syngas, carbon dioxide, and water, then produces high-value chemicals like acetic acid and methanol with minimal emissions, using a combination of gasification, steam reforming, and water-gas shift reactions, which can be reused to sustainably fuel aquatic vehicles and finance cleanup operations.
Environmental Impact Assessment of Ethyl Acetate Use
The environmental impact assessment of ethyl acetate use in zero-waste productions is a critical aspect of sustainable manufacturing practices. Ethyl acetate, a widely used solvent in various industries, plays a significant role in the transition towards more environmentally friendly production processes.
One of the primary environmental benefits of ethyl acetate is its biodegradability. Unlike many other solvents, ethyl acetate breaks down relatively quickly in the environment, reducing its long-term impact on ecosystems. This characteristic makes it an attractive option for industries aiming to minimize their ecological footprint and align with zero-waste principles.
However, the production and use of ethyl acetate still have environmental implications that must be carefully considered. The manufacturing process of ethyl acetate typically involves the reaction of ethanol with acetic acid, both of which are derived from petrochemical sources. This reliance on fossil fuels contributes to greenhouse gas emissions and resource depletion, potentially offsetting some of the environmental benefits of its use in zero-waste productions.
In terms of air quality, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and smog, which have adverse effects on human health and the environment. Proper handling, storage, and emission control measures are essential to mitigate these impacts in industrial settings.
Water pollution is another concern associated with ethyl acetate use. Although it has low water solubility, improper disposal or accidental spills can lead to contamination of water bodies. This can affect aquatic ecosystems and potentially enter the food chain. Implementing robust waste management and spill prevention protocols is crucial for minimizing these risks in zero-waste production systems.
On the positive side, ethyl acetate's relatively low toxicity compared to many other industrial solvents makes it a safer choice for workers and surrounding communities. Its use can help reduce occupational health risks and the potential for environmental accidents, aligning with the broader goals of sustainable and responsible manufacturing.
In the context of zero-waste productions, the recyclability of ethyl acetate is a significant advantage. Advanced recovery and purification techniques allow for the efficient recapture and reuse of ethyl acetate within closed-loop systems. This not only reduces waste but also decreases the need for continuous production of new solvent, further minimizing the overall environmental impact.
As industries strive for more sustainable practices, the life cycle assessment of ethyl acetate becomes increasingly important. Evaluating its environmental impact from production to disposal, including energy consumption, emissions, and resource utilization, provides a comprehensive understanding of its role in zero-waste strategies. This holistic approach enables manufacturers to make informed decisions about solvent selection and process optimization, balancing environmental considerations with production efficiency and product quality.
One of the primary environmental benefits of ethyl acetate is its biodegradability. Unlike many other solvents, ethyl acetate breaks down relatively quickly in the environment, reducing its long-term impact on ecosystems. This characteristic makes it an attractive option for industries aiming to minimize their ecological footprint and align with zero-waste principles.
However, the production and use of ethyl acetate still have environmental implications that must be carefully considered. The manufacturing process of ethyl acetate typically involves the reaction of ethanol with acetic acid, both of which are derived from petrochemical sources. This reliance on fossil fuels contributes to greenhouse gas emissions and resource depletion, potentially offsetting some of the environmental benefits of its use in zero-waste productions.
In terms of air quality, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can contribute to the formation of ground-level ozone and smog, which have adverse effects on human health and the environment. Proper handling, storage, and emission control measures are essential to mitigate these impacts in industrial settings.
Water pollution is another concern associated with ethyl acetate use. Although it has low water solubility, improper disposal or accidental spills can lead to contamination of water bodies. This can affect aquatic ecosystems and potentially enter the food chain. Implementing robust waste management and spill prevention protocols is crucial for minimizing these risks in zero-waste production systems.
On the positive side, ethyl acetate's relatively low toxicity compared to many other industrial solvents makes it a safer choice for workers and surrounding communities. Its use can help reduce occupational health risks and the potential for environmental accidents, aligning with the broader goals of sustainable and responsible manufacturing.
In the context of zero-waste productions, the recyclability of ethyl acetate is a significant advantage. Advanced recovery and purification techniques allow for the efficient recapture and reuse of ethyl acetate within closed-loop systems. This not only reduces waste but also decreases the need for continuous production of new solvent, further minimizing the overall environmental impact.
As industries strive for more sustainable practices, the life cycle assessment of ethyl acetate becomes increasingly important. Evaluating its environmental impact from production to disposal, including energy consumption, emissions, and resource utilization, provides a comprehensive understanding of its role in zero-waste strategies. This holistic approach enables manufacturers to make informed decisions about solvent selection and process optimization, balancing environmental considerations with production efficiency and product quality.
Regulatory Framework for Solvent Management in Industry
The regulatory framework for solvent management in industry plays a crucial role in ensuring the responsible use of ethyl acetate in zero-waste productions. Governments and international organizations have established comprehensive guidelines and regulations to address the environmental and health concerns associated with solvent use, including ethyl acetate.
At the international level, the United Nations Environment Programme (UNEP) has developed the Strategic Approach to International Chemicals Management (SAICM), which provides a policy framework for promoting chemical safety around the world. This framework includes provisions for the safe management of solvents, including ethyl acetate, throughout their lifecycle.
In the United States, the Environmental Protection Agency (EPA) regulates solvent use under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The EPA has established emission standards for volatile organic compounds (VOCs), including ethyl acetate, and requires industries to implement best available control technologies (BACT) to minimize emissions.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to register chemical substances, including solvents like ethyl acetate. REACH also mandates the assessment of potential risks associated with these substances and the implementation of appropriate risk management measures.
Many countries have adopted occupational health and safety regulations that set exposure limits for workers handling solvents. For example, the Occupational Safety and Health Administration (OSHA) in the United States has established permissible exposure limits (PELs) for ethyl acetate and other solvents to protect workers from potential health hazards.
In the context of zero-waste productions, regulatory frameworks increasingly emphasize the principles of circular economy and waste reduction. The European Union's Circular Economy Action Plan, for instance, promotes the development of sustainable chemical processes and the use of safer alternatives to traditional solvents.
To comply with these regulations, industries using ethyl acetate in their processes must implement robust solvent management systems. This includes proper storage and handling procedures, emission control technologies, and waste reduction strategies. Many regulatory frameworks also require companies to maintain detailed records of solvent use and disposal, as well as to report emissions to relevant authorities.
As the focus on sustainability and zero-waste production intensifies, regulatory bodies are likely to introduce more stringent requirements for solvent management. This may include incentives for the development and adoption of green solvents, as well as stricter limits on emissions and waste generation. Industries utilizing ethyl acetate will need to stay abreast of these evolving regulations and adapt their processes accordingly to ensure compliance and environmental responsibility.
At the international level, the United Nations Environment Programme (UNEP) has developed the Strategic Approach to International Chemicals Management (SAICM), which provides a policy framework for promoting chemical safety around the world. This framework includes provisions for the safe management of solvents, including ethyl acetate, throughout their lifecycle.
In the United States, the Environmental Protection Agency (EPA) regulates solvent use under the Toxic Substances Control Act (TSCA) and the Clean Air Act. The EPA has established emission standards for volatile organic compounds (VOCs), including ethyl acetate, and requires industries to implement best available control technologies (BACT) to minimize emissions.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which requires manufacturers and importers to register chemical substances, including solvents like ethyl acetate. REACH also mandates the assessment of potential risks associated with these substances and the implementation of appropriate risk management measures.
Many countries have adopted occupational health and safety regulations that set exposure limits for workers handling solvents. For example, the Occupational Safety and Health Administration (OSHA) in the United States has established permissible exposure limits (PELs) for ethyl acetate and other solvents to protect workers from potential health hazards.
In the context of zero-waste productions, regulatory frameworks increasingly emphasize the principles of circular economy and waste reduction. The European Union's Circular Economy Action Plan, for instance, promotes the development of sustainable chemical processes and the use of safer alternatives to traditional solvents.
To comply with these regulations, industries using ethyl acetate in their processes must implement robust solvent management systems. This includes proper storage and handling procedures, emission control technologies, and waste reduction strategies. Many regulatory frameworks also require companies to maintain detailed records of solvent use and disposal, as well as to report emissions to relevant authorities.
As the focus on sustainability and zero-waste production intensifies, regulatory bodies are likely to introduce more stringent requirements for solvent management. This may include incentives for the development and adoption of green solvents, as well as stricter limits on emissions and waste generation. Industries utilizing ethyl acetate will need to stay abreast of these evolving regulations and adapt their processes accordingly to ensure compliance and environmental responsibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!