How Ethyl Acetate Promotes Resource Optimization?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Background
Ethyl acetate, a versatile organic compound with the chemical formula CH3COOC2H5, has gained significant attention in recent years due to its potential in promoting resource optimization across various industries. This colorless liquid, characterized by its fruity odor, is produced through the esterification of ethanol and acetic acid. Its widespread use can be attributed to its unique properties, including low toxicity, high solvency power, and relatively low cost of production.
The history of ethyl acetate dates back to the early 19th century when it was first synthesized in laboratory settings. However, its industrial production and application did not gain momentum until the mid-20th century. Initially, ethyl acetate was primarily used as a solvent in the production of paints, coatings, and adhesives. Over time, its applications expanded to include use in the food industry as a flavoring agent and in the pharmaceutical sector for drug formulation.
In recent decades, the role of ethyl acetate in resource optimization has become increasingly prominent. This shift can be attributed to growing environmental concerns and the need for sustainable industrial practices. Ethyl acetate's biodegradability and low environmental impact have made it an attractive alternative to more harmful solvents, aligning with global efforts to reduce the carbon footprint of industrial processes.
The chemical's ability to dissolve a wide range of substances has led to its adoption in various extraction and purification processes. In the pharmaceutical industry, ethyl acetate is used in the extraction of active pharmaceutical ingredients, enabling more efficient and cost-effective drug production. Similarly, in the food industry, it plays a crucial role in the extraction of natural flavors and fragrances, reducing the need for synthetic alternatives.
Furthermore, ethyl acetate's role in resource optimization extends to waste management and recycling processes. Its effectiveness in removing impurities from recycled materials has contributed to improved quality of recycled products, particularly in the plastics and paper industries. This application not only enhances the efficiency of recycling processes but also promotes the circular economy by increasing the value and usability of recycled materials.
The growing interest in bio-based chemicals has also positioned ethyl acetate as a key player in the transition towards more sustainable industrial practices. Research into the production of ethyl acetate from renewable resources, such as biomass-derived ethanol and acetic acid, is gaining traction. This bio-based approach not only reduces dependence on fossil fuels but also offers a pathway to carbon-neutral production methods, further enhancing the compound's role in resource optimization.
The history of ethyl acetate dates back to the early 19th century when it was first synthesized in laboratory settings. However, its industrial production and application did not gain momentum until the mid-20th century. Initially, ethyl acetate was primarily used as a solvent in the production of paints, coatings, and adhesives. Over time, its applications expanded to include use in the food industry as a flavoring agent and in the pharmaceutical sector for drug formulation.
In recent decades, the role of ethyl acetate in resource optimization has become increasingly prominent. This shift can be attributed to growing environmental concerns and the need for sustainable industrial practices. Ethyl acetate's biodegradability and low environmental impact have made it an attractive alternative to more harmful solvents, aligning with global efforts to reduce the carbon footprint of industrial processes.
The chemical's ability to dissolve a wide range of substances has led to its adoption in various extraction and purification processes. In the pharmaceutical industry, ethyl acetate is used in the extraction of active pharmaceutical ingredients, enabling more efficient and cost-effective drug production. Similarly, in the food industry, it plays a crucial role in the extraction of natural flavors and fragrances, reducing the need for synthetic alternatives.
Furthermore, ethyl acetate's role in resource optimization extends to waste management and recycling processes. Its effectiveness in removing impurities from recycled materials has contributed to improved quality of recycled products, particularly in the plastics and paper industries. This application not only enhances the efficiency of recycling processes but also promotes the circular economy by increasing the value and usability of recycled materials.
The growing interest in bio-based chemicals has also positioned ethyl acetate as a key player in the transition towards more sustainable industrial practices. Research into the production of ethyl acetate from renewable resources, such as biomass-derived ethanol and acetic acid, is gaining traction. This bio-based approach not only reduces dependence on fossil fuels but also offers a pathway to carbon-neutral production methods, further enhancing the compound's role in resource optimization.
Market Analysis
The market for ethyl acetate as a resource optimization agent has shown significant growth in recent years, driven by increasing demand for sustainable and efficient industrial processes. This versatile organic compound, known for its low toxicity and high solvency, has found applications across various sectors, particularly in coatings, adhesives, and pharmaceutical industries.
In the coatings industry, ethyl acetate has emerged as a preferred solvent due to its rapid evaporation rate and ability to dissolve a wide range of resins. This property has led to improved efficiency in paint and coating applications, reducing drying times and enhancing overall productivity. The automotive and construction sectors, in particular, have been key drivers of this demand, as they seek more environmentally friendly and cost-effective coating solutions.
The adhesives market has also witnessed a surge in ethyl acetate usage, especially in the production of flexible packaging materials. Its excellent solvent properties enable manufacturers to create high-performance adhesives that meet stringent environmental regulations while maintaining strong bonding capabilities. This has been particularly beneficial in the food packaging industry, where safety and sustainability are paramount concerns.
In the pharmaceutical sector, ethyl acetate plays a crucial role in the extraction and purification of active pharmaceutical ingredients (APIs). Its ability to selectively dissolve certain compounds while leaving others undissolved has made it an invaluable tool in drug manufacturing processes. This application has contributed significantly to resource optimization by improving yields and reducing waste in pharmaceutical production.
The global push towards sustainability has further bolstered the market for ethyl acetate. As industries seek to reduce their environmental footprint, the use of ethyl acetate as a replacement for more harmful solvents has gained traction. Its biodegradability and lower toxicity compared to alternatives have positioned it as a key component in green chemistry initiatives.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, driven by rapid industrialization and the growth of manufacturing sectors in countries like China and India. North America and Europe follow closely, with increasing adoption in high-tech industries and stringent environmental regulations fueling demand.
Looking ahead, the market for ethyl acetate in resource optimization is expected to continue its upward trajectory. Innovations in production methods, such as bio-based ethyl acetate derived from renewable resources, are likely to open new avenues for growth and further enhance its appeal as a sustainable solution. As industries continue to prioritize efficiency and environmental responsibility, ethyl acetate's role in promoting resource optimization is set to become even more prominent in the coming years.
In the coatings industry, ethyl acetate has emerged as a preferred solvent due to its rapid evaporation rate and ability to dissolve a wide range of resins. This property has led to improved efficiency in paint and coating applications, reducing drying times and enhancing overall productivity. The automotive and construction sectors, in particular, have been key drivers of this demand, as they seek more environmentally friendly and cost-effective coating solutions.
The adhesives market has also witnessed a surge in ethyl acetate usage, especially in the production of flexible packaging materials. Its excellent solvent properties enable manufacturers to create high-performance adhesives that meet stringent environmental regulations while maintaining strong bonding capabilities. This has been particularly beneficial in the food packaging industry, where safety and sustainability are paramount concerns.
In the pharmaceutical sector, ethyl acetate plays a crucial role in the extraction and purification of active pharmaceutical ingredients (APIs). Its ability to selectively dissolve certain compounds while leaving others undissolved has made it an invaluable tool in drug manufacturing processes. This application has contributed significantly to resource optimization by improving yields and reducing waste in pharmaceutical production.
The global push towards sustainability has further bolstered the market for ethyl acetate. As industries seek to reduce their environmental footprint, the use of ethyl acetate as a replacement for more harmful solvents has gained traction. Its biodegradability and lower toxicity compared to alternatives have positioned it as a key component in green chemistry initiatives.
Geographically, Asia-Pacific has emerged as the largest market for ethyl acetate, driven by rapid industrialization and the growth of manufacturing sectors in countries like China and India. North America and Europe follow closely, with increasing adoption in high-tech industries and stringent environmental regulations fueling demand.
Looking ahead, the market for ethyl acetate in resource optimization is expected to continue its upward trajectory. Innovations in production methods, such as bio-based ethyl acetate derived from renewable resources, are likely to open new avenues for growth and further enhance its appeal as a sustainable solution. As industries continue to prioritize efficiency and environmental responsibility, ethyl acetate's role in promoting resource optimization is set to become even more prominent in the coming years.
Technical Challenges
The use of ethyl acetate in resource optimization faces several technical challenges that require innovative solutions. One of the primary obstacles is the energy-intensive nature of ethyl acetate production, which often involves high-temperature reactions and complex separation processes. This energy consumption not only increases production costs but also contributes to a larger carbon footprint, contradicting the goal of resource optimization.
Another significant challenge lies in the purification of ethyl acetate. Traditional methods often result in the formation of azeotropes, making it difficult to achieve high purity levels without extensive energy input. This issue is particularly problematic in industries requiring high-grade ethyl acetate, such as pharmaceuticals and electronics manufacturing.
The volatility of ethyl acetate presents additional hurdles in its handling and storage. Its low boiling point and high vapor pressure necessitate specialized containment systems to prevent losses through evaporation. This not only impacts the efficiency of resource utilization but also raises safety concerns in industrial settings.
Furthermore, the sourcing of raw materials for ethyl acetate production poses sustainability challenges. The primary feedstocks, ethanol and acetic acid, are often derived from fossil fuels or food crops, raising questions about long-term availability and environmental impact. Developing renewable sources for these precursors remains a critical technical challenge in promoting resource optimization.
The recovery and recycling of ethyl acetate from industrial processes present another set of technical difficulties. Many current recycling methods are inefficient, leading to significant material losses and increased waste generation. Improving recovery rates while maintaining product quality is essential for closing the loop in ethyl acetate utilization.
Additionally, the optimization of reaction kinetics and catalysis in ethyl acetate production remains an ongoing challenge. Enhancing reaction efficiency and selectivity could significantly reduce energy consumption and improve resource utilization. However, developing catalysts that are both highly active and stable under industrial conditions is a complex task requiring further research and development.
Lastly, the integration of ethyl acetate production and utilization into broader circular economy models presents systemic challenges. This includes developing compatible process technologies, establishing efficient logistics for material flows, and creating markets for recycled or bio-based ethyl acetate. Overcoming these challenges requires not only technical innovations but also shifts in industrial practices and regulatory frameworks.
Another significant challenge lies in the purification of ethyl acetate. Traditional methods often result in the formation of azeotropes, making it difficult to achieve high purity levels without extensive energy input. This issue is particularly problematic in industries requiring high-grade ethyl acetate, such as pharmaceuticals and electronics manufacturing.
The volatility of ethyl acetate presents additional hurdles in its handling and storage. Its low boiling point and high vapor pressure necessitate specialized containment systems to prevent losses through evaporation. This not only impacts the efficiency of resource utilization but also raises safety concerns in industrial settings.
Furthermore, the sourcing of raw materials for ethyl acetate production poses sustainability challenges. The primary feedstocks, ethanol and acetic acid, are often derived from fossil fuels or food crops, raising questions about long-term availability and environmental impact. Developing renewable sources for these precursors remains a critical technical challenge in promoting resource optimization.
The recovery and recycling of ethyl acetate from industrial processes present another set of technical difficulties. Many current recycling methods are inefficient, leading to significant material losses and increased waste generation. Improving recovery rates while maintaining product quality is essential for closing the loop in ethyl acetate utilization.
Additionally, the optimization of reaction kinetics and catalysis in ethyl acetate production remains an ongoing challenge. Enhancing reaction efficiency and selectivity could significantly reduce energy consumption and improve resource utilization. However, developing catalysts that are both highly active and stable under industrial conditions is a complex task requiring further research and development.
Lastly, the integration of ethyl acetate production and utilization into broader circular economy models presents systemic challenges. This includes developing compatible process technologies, establishing efficient logistics for material flows, and creating markets for recycled or bio-based ethyl acetate. Overcoming these challenges requires not only technical innovations but also shifts in industrial practices and regulatory frameworks.
Current EA Solutions
01 Process optimization for ethyl acetate production
Optimization of the ethyl acetate production process involves improving reaction conditions, catalysts, and separation techniques. This can include adjusting temperature and pressure parameters, selecting more efficient catalysts, and implementing advanced distillation methods to enhance yield and purity while reducing energy consumption and waste.- Process optimization for ethyl acetate production: Optimization of the ethyl acetate production process involves improving reaction conditions, catalysts, and separation techniques. This can include adjusting temperature and pressure parameters, selecting more efficient catalysts, and implementing advanced distillation methods to enhance yield and purity while reducing energy consumption.
- Resource allocation and scheduling in ethyl acetate manufacturing: Efficient resource allocation and scheduling are crucial for optimizing ethyl acetate production. This involves implementing advanced algorithms and software systems to manage raw material inventory, production equipment utilization, and workforce scheduling. Such systems can help minimize waste, reduce downtime, and improve overall production efficiency.
- Energy efficiency improvements in ethyl acetate production: Enhancing energy efficiency in ethyl acetate production focuses on reducing overall energy consumption. This can be achieved through heat integration, waste heat recovery, and the implementation of more energy-efficient equipment. Advanced process control systems can also be utilized to optimize energy use throughout the production process.
- Supply chain optimization for ethyl acetate manufacturing: Optimizing the supply chain for ethyl acetate production involves improving logistics, inventory management, and demand forecasting. This can include implementing just-in-time delivery systems, optimizing transportation routes, and utilizing advanced analytics to predict market demand and adjust production accordingly.
- Quality control and process monitoring in ethyl acetate production: Implementing advanced quality control and process monitoring systems is essential for optimizing ethyl acetate production. This can involve the use of real-time sensors, spectroscopic analysis, and machine learning algorithms to detect and correct process deviations quickly. Such systems can help maintain product quality while minimizing waste and improving overall resource utilization.
02 Resource allocation and scheduling in ethyl acetate manufacturing
Efficient resource allocation and scheduling are crucial for optimizing ethyl acetate production. This involves implementing advanced algorithms and software systems to manage raw materials, equipment utilization, and production schedules. Such systems can help minimize downtime, reduce waste, and improve overall production efficiency.Expand Specific Solutions03 Energy efficiency improvements in ethyl acetate production
Enhancing energy efficiency in ethyl acetate production focuses on reducing energy consumption throughout the manufacturing process. This can include implementing heat integration systems, utilizing renewable energy sources, and optimizing equipment performance to minimize energy losses and reduce the overall carbon footprint of the production process.Expand Specific Solutions04 Waste reduction and recycling in ethyl acetate manufacturing
Minimizing waste and implementing effective recycling strategies are essential for optimizing ethyl acetate production. This involves developing closed-loop systems, recovering and reusing solvents, and implementing advanced purification techniques to maximize resource utilization and reduce environmental impact.Expand Specific Solutions05 Supply chain optimization for ethyl acetate production
Optimizing the supply chain for ethyl acetate production involves improving logistics, inventory management, and demand forecasting. This can include implementing real-time tracking systems, developing predictive models for market demand, and establishing strategic partnerships with suppliers and distributors to ensure a steady and cost-effective flow of raw materials and finished products.Expand Specific Solutions
Key Industry Players
The ethyl acetate market is in a mature growth stage, characterized by steady demand and established production processes. The global market size is estimated to be around $3-4 billion, with moderate annual growth. Technologically, ethyl acetate production is well-developed, with major players like Celanese, BASF, and Sinopec Group employing efficient processes. However, there's ongoing innovation in resource optimization and sustainability. Companies such as Nantong Baichuan New Material and Jiangsu Baichuan High-Tech New Materials are exploring novel catalysts and green chemistry approaches to improve yield and reduce environmental impact. The competitive landscape is diverse, with both large chemical conglomerates and specialized manufacturers vying for market share.
Celanese International Corp.
Technical Solution: Celanese has developed an innovative process for ethyl acetate production that promotes resource optimization. Their method utilizes a reactive distillation technology, which combines reaction and separation in a single unit operation[1]. This approach significantly reduces energy consumption and raw material usage compared to conventional processes. The company has also implemented advanced catalysts that improve selectivity and yield, further enhancing resource efficiency[3]. Additionally, Celanese has integrated heat recovery systems and process intensification techniques to minimize waste and maximize product output[5].
Strengths: Reduced energy consumption, improved raw material utilization, and increased process efficiency. Weaknesses: Potentially higher initial capital investment and complexity in process control.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to ethyl acetate production that focuses on resource optimization. Their method employs a proprietary catalyst system that enables direct synthesis from ethanol and acetic acid under milder conditions[2]. This process reduces energy requirements and improves atom economy. Sinopec has also implemented advanced separation technologies, such as membrane-based systems, to enhance product purity and reduce solvent consumption[4]. Furthermore, the company has integrated their ethyl acetate production with existing refinery operations, allowing for efficient use of byproducts and waste streams as feedstock[6].
Strengths: Improved energy efficiency, better integration with existing operations, and reduced environmental impact. Weaknesses: Potential limitations in scalability and dependence on specific feedstock availability.
Innovative EA Research
Process of low energy consumption for preparing a carboxylic acid ester
PatentInactiveEP2686292A1
Innovation
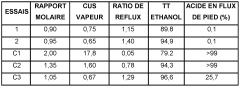
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and a reflux ratio between 1.0 and 1.5, which allows for simultaneous reaction and separation in multiple zones, reducing energy costs and acetic acid content.
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
Environmental Impact
The use of ethyl acetate in resource optimization processes has significant environmental implications that warrant careful consideration. As a solvent widely used in various industries, ethyl acetate's impact on the environment is multifaceted and extends throughout its lifecycle.
One of the primary environmental benefits of ethyl acetate is its relatively low toxicity compared to other organic solvents. It is biodegradable and does not persist in the environment for extended periods, reducing long-term ecological risks. Additionally, ethyl acetate has a lower ozone depletion potential than many chlorinated solvents, contributing to the protection of the Earth's ozone layer.
However, the production and use of ethyl acetate are not without environmental concerns. The manufacturing process typically involves the esterification of ethanol and acetic acid, which requires energy input and may result in greenhouse gas emissions. The extraction of raw materials for ethyl acetate production, particularly if derived from fossil fuels, can lead to habitat disruption and contribute to carbon footprint.
In terms of air quality, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can participate in photochemical reactions, potentially contributing to the formation of ground-level ozone and smog. This aspect necessitates proper handling and emission control measures in industrial settings to minimize air pollution.
Water pollution is another environmental consideration. Although ethyl acetate has limited water solubility, improper disposal or accidental spills can lead to contamination of water bodies. This may affect aquatic ecosystems and potentially enter the food chain, highlighting the importance of responsible waste management practices.
On the positive side, the use of ethyl acetate in resource optimization can indirectly benefit the environment by improving process efficiencies. By enhancing extraction and separation processes, it can reduce overall energy consumption and waste generation in various industries. This optimization can lead to a decrease in the environmental footprint of industrial operations.
Furthermore, the recyclability of ethyl acetate presents an opportunity for sustainable practices. Proper recovery and reuse systems can significantly reduce the need for fresh solvent production, thereby conserving resources and minimizing waste. This circular approach aligns with principles of green chemistry and sustainable industrial practices.
In conclusion, while ethyl acetate offers certain environmental advantages in resource optimization, its use must be carefully managed to mitigate potential negative impacts. Balancing its benefits with responsible handling, emission control, and recycling practices is crucial for ensuring that its application in resource optimization truly contributes to environmental sustainability.
One of the primary environmental benefits of ethyl acetate is its relatively low toxicity compared to other organic solvents. It is biodegradable and does not persist in the environment for extended periods, reducing long-term ecological risks. Additionally, ethyl acetate has a lower ozone depletion potential than many chlorinated solvents, contributing to the protection of the Earth's ozone layer.
However, the production and use of ethyl acetate are not without environmental concerns. The manufacturing process typically involves the esterification of ethanol and acetic acid, which requires energy input and may result in greenhouse gas emissions. The extraction of raw materials for ethyl acetate production, particularly if derived from fossil fuels, can lead to habitat disruption and contribute to carbon footprint.
In terms of air quality, ethyl acetate is classified as a volatile organic compound (VOC). When released into the atmosphere, it can participate in photochemical reactions, potentially contributing to the formation of ground-level ozone and smog. This aspect necessitates proper handling and emission control measures in industrial settings to minimize air pollution.
Water pollution is another environmental consideration. Although ethyl acetate has limited water solubility, improper disposal or accidental spills can lead to contamination of water bodies. This may affect aquatic ecosystems and potentially enter the food chain, highlighting the importance of responsible waste management practices.
On the positive side, the use of ethyl acetate in resource optimization can indirectly benefit the environment by improving process efficiencies. By enhancing extraction and separation processes, it can reduce overall energy consumption and waste generation in various industries. This optimization can lead to a decrease in the environmental footprint of industrial operations.
Furthermore, the recyclability of ethyl acetate presents an opportunity for sustainable practices. Proper recovery and reuse systems can significantly reduce the need for fresh solvent production, thereby conserving resources and minimizing waste. This circular approach aligns with principles of green chemistry and sustainable industrial practices.
In conclusion, while ethyl acetate offers certain environmental advantages in resource optimization, its use must be carefully managed to mitigate potential negative impacts. Balancing its benefits with responsible handling, emission control, and recycling practices is crucial for ensuring that its application in resource optimization truly contributes to environmental sustainability.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in its application for resource optimization. Governments and international bodies have established comprehensive guidelines to ensure the safe and responsible use of this chemical compound across various industries.
At the global level, organizations such as the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) provide overarching recommendations for the handling and disposal of ethyl acetate. These guidelines serve as a foundation for national and regional regulations, promoting consistency in safety standards and environmental protection measures.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The agency has set specific exposure limits and handling protocols to minimize potential health risks associated with its use. Additionally, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for workers in industries that utilize ethyl acetate.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which governs the production, import, and use of chemical substances, including ethyl acetate. This comprehensive framework ensures that manufacturers and importers assess and manage the risks associated with the compound throughout its lifecycle.
In the context of resource optimization, regulatory bodies have increasingly focused on promoting sustainable practices in ethyl acetate production and usage. This includes incentives for developing more efficient manufacturing processes, implementing closed-loop recycling systems, and exploring bio-based alternatives.
Many countries have also introduced regulations to encourage the recovery and reuse of ethyl acetate in industrial processes. For instance, some jurisdictions offer tax incentives or grants to companies that invest in solvent recovery technologies, thereby reducing waste and optimizing resource utilization.
The regulatory landscape also addresses the transportation and storage of ethyl acetate. International agreements, such as the United Nations Recommendations on the Transport of Dangerous Goods, provide standardized guidelines for the safe movement of this chemical across borders. These regulations ensure that ethyl acetate is properly packaged, labeled, and handled during transit, minimizing the risk of accidents and environmental contamination.
As the focus on sustainability intensifies, regulatory bodies are increasingly emphasizing life cycle assessments for chemicals like ethyl acetate. This approach considers the environmental impact of the compound from production to disposal, encouraging manufacturers to adopt more eco-friendly practices throughout the supply chain.
At the global level, organizations such as the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) provide overarching recommendations for the handling and disposal of ethyl acetate. These guidelines serve as a foundation for national and regional regulations, promoting consistency in safety standards and environmental protection measures.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The agency has set specific exposure limits and handling protocols to minimize potential health risks associated with its use. Additionally, the Occupational Safety and Health Administration (OSHA) has established permissible exposure limits (PELs) for workers in industries that utilize ethyl acetate.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which governs the production, import, and use of chemical substances, including ethyl acetate. This comprehensive framework ensures that manufacturers and importers assess and manage the risks associated with the compound throughout its lifecycle.
In the context of resource optimization, regulatory bodies have increasingly focused on promoting sustainable practices in ethyl acetate production and usage. This includes incentives for developing more efficient manufacturing processes, implementing closed-loop recycling systems, and exploring bio-based alternatives.
Many countries have also introduced regulations to encourage the recovery and reuse of ethyl acetate in industrial processes. For instance, some jurisdictions offer tax incentives or grants to companies that invest in solvent recovery technologies, thereby reducing waste and optimizing resource utilization.
The regulatory landscape also addresses the transportation and storage of ethyl acetate. International agreements, such as the United Nations Recommendations on the Transport of Dangerous Goods, provide standardized guidelines for the safe movement of this chemical across borders. These regulations ensure that ethyl acetate is properly packaged, labeled, and handled during transit, minimizing the risk of accidents and environmental contamination.
As the focus on sustainability intensifies, regulatory bodies are increasingly emphasizing life cycle assessments for chemicals like ethyl acetate. This approach considers the environmental impact of the compound from production to disposal, encouraging manufacturers to adopt more eco-friendly practices throughout the supply chain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!