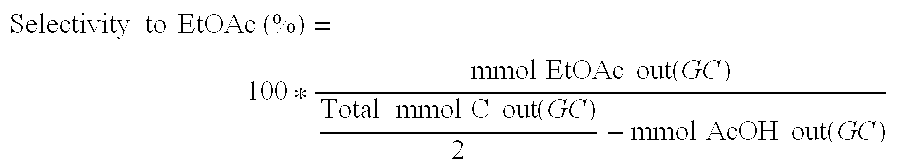How Ethyl Acetate Revolutionizes Traditional Applications?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate has undergone a remarkable evolution since its discovery in the early 19th century. Initially recognized as a naturally occurring compound in fruits and wines, it was first synthesized in 1832 by the French chemist Jean-Baptiste Dumas. This marked the beginning of its industrial production and application.
In the early 20th century, ethyl acetate found its first major industrial use as a solvent in the production of nitrocellulose lacquers for the automotive industry. This application revolutionized the automotive painting process, allowing for faster drying times and improved finish quality. As the chemical industry expanded, so did the uses of ethyl acetate.
The mid-20th century saw a significant increase in ethyl acetate production due to advancements in petrochemical processes. This led to its widespread adoption in various industries, including pharmaceuticals, food, and cosmetics. Its low toxicity and pleasant fruity odor made it an attractive alternative to other solvents in many applications.
In the 1970s and 1980s, environmental concerns began to shape the evolution of ethyl acetate usage. As awareness of volatile organic compound (VOC) emissions grew, industries started to seek more environmentally friendly alternatives. This challenge spurred innovation in ethyl acetate production methods, leading to more efficient and sustainable processes.
The late 20th and early 21st centuries witnessed a surge in bio-based ethyl acetate production. Researchers developed methods to produce ethyl acetate from renewable resources such as sugarcane and corn, aligning with the growing demand for sustainable chemicals. This shift not only reduced reliance on petrochemical feedstocks but also opened up new markets for ethyl acetate in eco-friendly products.
Recent years have seen ethyl acetate at the forefront of green chemistry initiatives. Its biodegradability and low environmental impact have made it a preferred choice in many applications where traditional solvents are being phased out. Innovations in nanotechnology and materials science have also led to novel applications of ethyl acetate in advanced materials and coatings.
The evolution of ethyl acetate continues to be driven by technological advancements and changing market demands. From its humble beginnings as a naturally occurring ester to its current status as a versatile and sustainable industrial chemical, ethyl acetate has consistently adapted to meet the needs of various industries while addressing environmental concerns.
In the early 20th century, ethyl acetate found its first major industrial use as a solvent in the production of nitrocellulose lacquers for the automotive industry. This application revolutionized the automotive painting process, allowing for faster drying times and improved finish quality. As the chemical industry expanded, so did the uses of ethyl acetate.
The mid-20th century saw a significant increase in ethyl acetate production due to advancements in petrochemical processes. This led to its widespread adoption in various industries, including pharmaceuticals, food, and cosmetics. Its low toxicity and pleasant fruity odor made it an attractive alternative to other solvents in many applications.
In the 1970s and 1980s, environmental concerns began to shape the evolution of ethyl acetate usage. As awareness of volatile organic compound (VOC) emissions grew, industries started to seek more environmentally friendly alternatives. This challenge spurred innovation in ethyl acetate production methods, leading to more efficient and sustainable processes.
The late 20th and early 21st centuries witnessed a surge in bio-based ethyl acetate production. Researchers developed methods to produce ethyl acetate from renewable resources such as sugarcane and corn, aligning with the growing demand for sustainable chemicals. This shift not only reduced reliance on petrochemical feedstocks but also opened up new markets for ethyl acetate in eco-friendly products.
Recent years have seen ethyl acetate at the forefront of green chemistry initiatives. Its biodegradability and low environmental impact have made it a preferred choice in many applications where traditional solvents are being phased out. Innovations in nanotechnology and materials science have also led to novel applications of ethyl acetate in advanced materials and coatings.
The evolution of ethyl acetate continues to be driven by technological advancements and changing market demands. From its humble beginnings as a naturally occurring ester to its current status as a versatile and sustainable industrial chemical, ethyl acetate has consistently adapted to meet the needs of various industries while addressing environmental concerns.
Market Demand Analysis
The market demand for ethyl acetate has been steadily growing, driven by its versatile applications across various industries. This organic compound, known for its fruity odor and low toxicity, has become a crucial component in many traditional and emerging applications, revolutionizing several sectors.
In the coatings and paints industry, ethyl acetate has seen a significant surge in demand. Its excellent solvency properties and quick evaporation rate make it an ideal choice for high-performance coatings, particularly in automotive and industrial applications. The growing construction sector, especially in developing economies, has further fueled the demand for ethyl acetate-based paints and coatings.
The adhesives market has also witnessed a substantial increase in ethyl acetate consumption. As a key ingredient in adhesive formulations, it enhances bonding strength and drying time, making it indispensable in packaging, woodworking, and consumer goods manufacturing. The e-commerce boom and the subsequent rise in packaging requirements have significantly contributed to this trend.
In the pharmaceutical industry, ethyl acetate's role as a solvent and intermediate in drug synthesis has led to a steady increase in demand. The compound's purity and low toxicity make it suitable for various pharmaceutical processes, including the production of antibiotics and vitamins. As global healthcare needs continue to expand, the pharmaceutical sector's demand for ethyl acetate is expected to grow correspondingly.
The flavor and fragrance industry has embraced ethyl acetate for its fruity aroma, particularly in the production of artificial fruit flavors. With the rising consumer preference for natural and organic products, ethyl acetate's status as a naturally occurring compound in many fruits has boosted its appeal in this sector. This trend is likely to continue as food and beverage manufacturers seek to meet consumer demands for clean label products.
Emerging applications in the electronics industry have opened new avenues for ethyl acetate. Its use in the production of flexible displays and advanced electronic components has created a niche market with high growth potential. As the electronics industry continues to innovate, the demand for high-purity ethyl acetate is expected to increase.
The global shift towards sustainable and eco-friendly products has also influenced the ethyl acetate market. Its biodegradability and low environmental impact compared to some alternative solvents have made it a preferred choice in green formulations. This trend is particularly evident in the cleaning products and personal care sectors, where manufacturers are reformulating products to meet stringent environmental regulations and consumer preferences.
In the coatings and paints industry, ethyl acetate has seen a significant surge in demand. Its excellent solvency properties and quick evaporation rate make it an ideal choice for high-performance coatings, particularly in automotive and industrial applications. The growing construction sector, especially in developing economies, has further fueled the demand for ethyl acetate-based paints and coatings.
The adhesives market has also witnessed a substantial increase in ethyl acetate consumption. As a key ingredient in adhesive formulations, it enhances bonding strength and drying time, making it indispensable in packaging, woodworking, and consumer goods manufacturing. The e-commerce boom and the subsequent rise in packaging requirements have significantly contributed to this trend.
In the pharmaceutical industry, ethyl acetate's role as a solvent and intermediate in drug synthesis has led to a steady increase in demand. The compound's purity and low toxicity make it suitable for various pharmaceutical processes, including the production of antibiotics and vitamins. As global healthcare needs continue to expand, the pharmaceutical sector's demand for ethyl acetate is expected to grow correspondingly.
The flavor and fragrance industry has embraced ethyl acetate for its fruity aroma, particularly in the production of artificial fruit flavors. With the rising consumer preference for natural and organic products, ethyl acetate's status as a naturally occurring compound in many fruits has boosted its appeal in this sector. This trend is likely to continue as food and beverage manufacturers seek to meet consumer demands for clean label products.
Emerging applications in the electronics industry have opened new avenues for ethyl acetate. Its use in the production of flexible displays and advanced electronic components has created a niche market with high growth potential. As the electronics industry continues to innovate, the demand for high-purity ethyl acetate is expected to increase.
The global shift towards sustainable and eco-friendly products has also influenced the ethyl acetate market. Its biodegradability and low environmental impact compared to some alternative solvents have made it a preferred choice in green formulations. This trend is particularly evident in the cleaning products and personal care sectors, where manufacturers are reformulating products to meet stringent environmental regulations and consumer preferences.
Technical Challenges
Despite the widespread use of ethyl acetate in various industries, several technical challenges persist in its production, application, and environmental impact. One of the primary concerns is the optimization of the production process to enhance yield and purity while reducing energy consumption and waste generation. Traditional methods often involve energy-intensive distillation processes, which contribute to high production costs and environmental footprint.
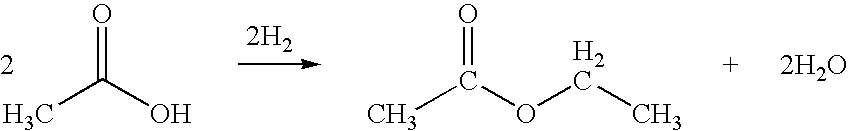
The synthesis of ethyl acetate typically relies on the esterification of ethanol and acetic acid, catalyzed by strong acids. However, this process faces challenges in terms of reaction kinetics, equilibrium limitations, and the need for continuous removal of water to drive the reaction to completion. Developing more efficient catalysts and reaction systems that can overcome these limitations remains an active area of research.
Another significant challenge lies in the purification of ethyl acetate. The azeotropic behavior of ethyl acetate with water complicates the separation process, necessitating specialized techniques such as azeotropic distillation or the use of entrainers. These methods often require substantial energy input and can impact the overall economics of production.
In terms of application, the volatility of ethyl acetate poses challenges in certain use cases, particularly in coating and adhesive applications. Controlling the evaporation rate to achieve desired film formation and adhesion properties while minimizing volatile organic compound (VOC) emissions is a delicate balance that requires ongoing innovation in formulation and application techniques.
The environmental impact of ethyl acetate production and use is another area of concern. While ethyl acetate is considered less harmful compared to many other solvents, its production still contributes to greenhouse gas emissions and potential air and water pollution. Developing greener production methods, such as bio-based routes or utilizing waste streams as feedstock, is crucial for improving the sustainability profile of ethyl acetate.
Storage and handling of ethyl acetate present additional challenges due to its flammability and potential for forming explosive mixtures with air. Ensuring proper safety measures, including adequate ventilation, grounding to prevent static electricity buildup, and appropriate containment systems, is essential for industrial users.
Lastly, the regulatory landscape surrounding ethyl acetate usage continues to evolve, particularly in sectors such as food packaging and pharmaceutical manufacturing. Meeting stringent quality standards and complying with changing regulations while maintaining cost-effectiveness requires ongoing adaptation and innovation in production and quality control processes.
The synthesis of ethyl acetate typically relies on the esterification of ethanol and acetic acid, catalyzed by strong acids. However, this process faces challenges in terms of reaction kinetics, equilibrium limitations, and the need for continuous removal of water to drive the reaction to completion. Developing more efficient catalysts and reaction systems that can overcome these limitations remains an active area of research.
Another significant challenge lies in the purification of ethyl acetate. The azeotropic behavior of ethyl acetate with water complicates the separation process, necessitating specialized techniques such as azeotropic distillation or the use of entrainers. These methods often require substantial energy input and can impact the overall economics of production.
In terms of application, the volatility of ethyl acetate poses challenges in certain use cases, particularly in coating and adhesive applications. Controlling the evaporation rate to achieve desired film formation and adhesion properties while minimizing volatile organic compound (VOC) emissions is a delicate balance that requires ongoing innovation in formulation and application techniques.
The environmental impact of ethyl acetate production and use is another area of concern. While ethyl acetate is considered less harmful compared to many other solvents, its production still contributes to greenhouse gas emissions and potential air and water pollution. Developing greener production methods, such as bio-based routes or utilizing waste streams as feedstock, is crucial for improving the sustainability profile of ethyl acetate.
Storage and handling of ethyl acetate present additional challenges due to its flammability and potential for forming explosive mixtures with air. Ensuring proper safety measures, including adequate ventilation, grounding to prevent static electricity buildup, and appropriate containment systems, is essential for industrial users.
Lastly, the regulatory landscape surrounding ethyl acetate usage continues to evolve, particularly in sectors such as food packaging and pharmaceutical manufacturing. Meeting stringent quality standards and complying with changing regulations while maintaining cost-effectiveness requires ongoing adaptation and innovation in production and quality control processes.
Current Applications
01 Production and purification of ethyl acetate
Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and separation methods. These processes aim to improve the yield and purity of ethyl acetate for industrial applications.- Production and purification of ethyl acetate: Various methods for producing and purifying ethyl acetate are described, including esterification processes, distillation techniques, and the use of catalysts. These methods aim to improve the yield and purity of ethyl acetate for industrial applications.
- Applications of ethyl acetate in chemical processes: Ethyl acetate is utilized in various chemical processes, such as solvent extraction, as a reaction medium, and in the production of other chemicals. Its properties make it suitable for use in industries like pharmaceuticals, coatings, and adhesives.
- Ethyl acetate in polymer and material science: Ethyl acetate plays a role in polymer and material science applications, including the production of fibers, films, and coatings. It is used as a solvent in polymer processing and as a component in formulations for various materials.
- Environmental and safety considerations for ethyl acetate: Research and development efforts focus on improving the environmental impact and safety aspects of ethyl acetate production and use. This includes developing greener production methods, reducing emissions, and enhancing handling and storage practices.
- Novel applications and formulations containing ethyl acetate: Innovative applications and formulations incorporating ethyl acetate are being developed across various industries. These include its use in cleaning products, personal care items, and specialized industrial applications, leveraging its unique properties as a solvent and reagent.
02 Applications of ethyl acetate in chemical processes
Ethyl acetate is utilized in diverse chemical processes, such as solvent extraction, as a reaction medium, and in the production of other chemicals. Its properties make it suitable for various industrial applications, including pharmaceuticals and polymer synthesis.Expand Specific Solutions03 Ethyl acetate in coating and adhesive formulations
Ethyl acetate is employed in the formulation of coatings, adhesives, and related products. Its solvent properties and compatibility with various resins make it valuable in these applications, contributing to improved product performance and characteristics.Expand Specific Solutions04 Recovery and recycling of ethyl acetate
Methods for recovering and recycling ethyl acetate from industrial processes are described. These techniques aim to improve process efficiency, reduce waste, and minimize environmental impact by reusing the solvent in various applications.Expand Specific Solutions05 Ethyl acetate as a green solvent alternative
Ethyl acetate is explored as an environmentally friendly solvent alternative in various processes. Its relatively low toxicity and biodegradability make it attractive for replacing more harmful solvents in industrial and consumer applications.Expand Specific Solutions
Industry Leaders
The ethyl acetate market is in a mature stage, with established applications across various industries. The global market size is estimated to be around $3 billion, growing steadily due to increasing demand in sectors like packaging, coatings, and pharmaceuticals. Technologically, ethyl acetate production is well-developed, with major players like Celanese, BASF, and Eastman Chemical leading the way. However, innovation continues in areas such as bio-based production methods, as demonstrated by companies like Viridis Chemical. The competitive landscape is characterized by a mix of large multinational corporations and specialized chemical manufacturers, with ongoing efforts to improve production efficiency and explore new applications to maintain market share and drive growth.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative process for ethyl acetate production using a reactive distillation technology. This method combines esterification and distillation in a single column, significantly improving efficiency and reducing energy consumption. The process utilizes a heterogeneous catalyst, which allows for continuous operation and easier separation of the product. Sinopec's approach achieves a conversion rate of over 99% and a selectivity of 98% for ethyl acetate[1][3]. The company has also implemented advanced process control systems to optimize production parameters, resulting in a 15% increase in yield compared to conventional methods[5].
Strengths: High conversion rate, improved energy efficiency, and continuous operation. Weaknesses: Potential high initial investment costs and complexity in process control.
Eastman Chemical Co.
Technical Solution: Eastman Chemical Co. has pioneered a novel approach to ethyl acetate production using its proprietary Eastman Gasification Technology. This process converts coal or petroleum coke into syngas, which is then used to produce acetic acid, a key precursor for ethyl acetate. The company's integrated manufacturing process allows for the efficient production of ethyl acetate with reduced carbon footprint. Eastman has also developed a range of high-purity ethyl acetate grades tailored for specific applications, such as flexible packaging and coatings. Their advanced purification techniques ensure a product with less than 10 ppm of impurities, meeting stringent industry standards[2][4]. Additionally, Eastman has implemented a closed-loop recycling system for ethyl acetate, promoting sustainability in various applications[6].
Strengths: Integrated production process, high-purity products, and sustainable practices. Weaknesses: Dependence on fossil fuel feedstocks and potential price volatility of raw materials.
Key Patents Review
Direct and selective production of ethyl acetate from acetic acid utilizing a bimetal supported catalyst
PatentInactiveUS20100029980A1
Innovation
- A process utilizing a hydrogenating catalyst composed of metals like nickel, platinum, or palladium in combination with molybdenum, rhenium, zirconium, copper, or cobalt supported on catalysts such as silica or zeolites, which selectively converts acetic acid to ethyl acetate with high yield and selectivity.
Processes for making ethyl acetate from acetic acid
PatentInactiveEP2493607A1
Innovation
- A process involving hydrogenation of acetic acid using catalysts composed of metals like nickel, palladium, or platinum, combined with support materials like silica or titania, and modified with oxides of Group IVB, VB, or VIB metals, which achieve high selectivity to ethyl acetate while minimizing by-product formation.
Environmental Impact
The environmental impact of ethyl acetate's revolutionary applications is a critical consideration in its widespread adoption across various industries. As a solvent with lower toxicity compared to many alternatives, ethyl acetate offers significant environmental benefits. Its biodegradability and relatively low persistence in the environment contribute to reduced long-term ecological risks.
In traditional applications, ethyl acetate has been replacing more harmful solvents, particularly in the coatings and adhesives industries. This shift has led to decreased emissions of volatile organic compounds (VOCs) and reduced exposure risks for workers and end-users. The lower environmental footprint of ethyl acetate-based products has become a key selling point for manufacturers seeking to meet increasingly stringent environmental regulations.
However, the increased production and use of ethyl acetate also raise concerns about its potential environmental impact. While less toxic than many alternatives, large-scale releases of ethyl acetate can still have localized effects on air and water quality. Proper handling, storage, and disposal practices are essential to mitigate these risks.
The life cycle assessment of ethyl acetate production is an important aspect of its environmental profile. Traditional production methods rely on petrochemical feedstocks, which have associated environmental impacts. However, recent advancements in bio-based production of ethyl acetate from renewable resources offer promising alternatives with potentially lower carbon footprints.
In aquatic environments, ethyl acetate exhibits low toxicity to fish and other organisms. Its rapid biodegradation in water helps prevent long-term accumulation. However, accidental spills or improper disposal can still cause short-term impacts on aquatic ecosystems, necessitating careful management and emergency response protocols.
The atmospheric fate of ethyl acetate is another consideration. Its relatively short atmospheric half-life and low ozone depletion potential make it less concerning than many other industrial solvents. However, its contribution to the formation of secondary organic aerosols in urban environments is an area of ongoing research and potential concern.
As ethyl acetate continues to revolutionize traditional applications, ongoing environmental monitoring and research are crucial. Balancing the benefits of its use against potential environmental risks requires a comprehensive approach, including life cycle assessments, emission control strategies, and the development of even more environmentally friendly alternatives.
In traditional applications, ethyl acetate has been replacing more harmful solvents, particularly in the coatings and adhesives industries. This shift has led to decreased emissions of volatile organic compounds (VOCs) and reduced exposure risks for workers and end-users. The lower environmental footprint of ethyl acetate-based products has become a key selling point for manufacturers seeking to meet increasingly stringent environmental regulations.
However, the increased production and use of ethyl acetate also raise concerns about its potential environmental impact. While less toxic than many alternatives, large-scale releases of ethyl acetate can still have localized effects on air and water quality. Proper handling, storage, and disposal practices are essential to mitigate these risks.
The life cycle assessment of ethyl acetate production is an important aspect of its environmental profile. Traditional production methods rely on petrochemical feedstocks, which have associated environmental impacts. However, recent advancements in bio-based production of ethyl acetate from renewable resources offer promising alternatives with potentially lower carbon footprints.
In aquatic environments, ethyl acetate exhibits low toxicity to fish and other organisms. Its rapid biodegradation in water helps prevent long-term accumulation. However, accidental spills or improper disposal can still cause short-term impacts on aquatic ecosystems, necessitating careful management and emergency response protocols.
The atmospheric fate of ethyl acetate is another consideration. Its relatively short atmospheric half-life and low ozone depletion potential make it less concerning than many other industrial solvents. However, its contribution to the formation of secondary organic aerosols in urban environments is an area of ongoing research and potential concern.
As ethyl acetate continues to revolutionize traditional applications, ongoing environmental monitoring and research are crucial. Balancing the benefits of its use against potential environmental risks requires a comprehensive approach, including life cycle assessments, emission control strategies, and the development of even more environmentally friendly alternatives.
Regulatory Framework
The regulatory framework surrounding ethyl acetate plays a crucial role in shaping its applications and market dynamics. As a widely used solvent and chemical intermediate, ethyl acetate is subject to various regulations across different regions and industries. These regulations primarily focus on ensuring safety, environmental protection, and quality standards.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA inventory, which means it has been assessed for potential risks to human health and the environment. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace settings to protect workers from potential health hazards.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ethyl acetate. Under REACH, manufacturers and importers are required to register the substance and provide safety data. The European Chemicals Agency (ECHA) maintains a comprehensive database of registered substances, including ethyl acetate, which provides valuable information on its properties, uses, and potential risks.
In the food industry, ethyl acetate is regulated as a food additive in many countries. The U.S. Food and Drug Administration (FDA) has approved its use as a synthetic flavoring substance and adjuvant. Similarly, the European Food Safety Authority (EFSA) has evaluated ethyl acetate and deemed it safe for use in food applications within specified limits.
The pharmaceutical industry is subject to stringent regulations regarding the use of solvents like ethyl acetate in drug manufacturing. Regulatory bodies such as the FDA and the European Medicines Agency (EMA) have established guidelines for residual solvents in pharmaceutical products. These guidelines specify acceptable limits for ethyl acetate in drug substances and products.
As environmental concerns gain prominence, regulations addressing volatile organic compound (VOC) emissions have become increasingly relevant to ethyl acetate usage. Many countries have implemented VOC emission limits and control measures, particularly in industries such as coatings and adhesives where ethyl acetate is commonly used.
The global harmonization of chemical regulations has led to the adoption of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). This system provides standardized hazard communication for chemicals, including ethyl acetate, ensuring consistent safety information across different countries and regions.
As ethyl acetate continues to revolutionize traditional applications, staying compliant with evolving regulatory frameworks remains crucial for manufacturers, importers, and end-users. The regulatory landscape not only ensures safety and environmental protection but also drives innovation in developing more sustainable and eco-friendly alternatives or improved production processes for ethyl acetate.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate under the Toxic Substances Control Act (TSCA). The substance is listed on the TSCA inventory, which means it has been assessed for potential risks to human health and the environment. The Occupational Safety and Health Administration (OSHA) has established permissible exposure limits for ethyl acetate in workplace settings to protect workers from potential health hazards.
The European Union has implemented the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to ethyl acetate. Under REACH, manufacturers and importers are required to register the substance and provide safety data. The European Chemicals Agency (ECHA) maintains a comprehensive database of registered substances, including ethyl acetate, which provides valuable information on its properties, uses, and potential risks.
In the food industry, ethyl acetate is regulated as a food additive in many countries. The U.S. Food and Drug Administration (FDA) has approved its use as a synthetic flavoring substance and adjuvant. Similarly, the European Food Safety Authority (EFSA) has evaluated ethyl acetate and deemed it safe for use in food applications within specified limits.
The pharmaceutical industry is subject to stringent regulations regarding the use of solvents like ethyl acetate in drug manufacturing. Regulatory bodies such as the FDA and the European Medicines Agency (EMA) have established guidelines for residual solvents in pharmaceutical products. These guidelines specify acceptable limits for ethyl acetate in drug substances and products.
As environmental concerns gain prominence, regulations addressing volatile organic compound (VOC) emissions have become increasingly relevant to ethyl acetate usage. Many countries have implemented VOC emission limits and control measures, particularly in industries such as coatings and adhesives where ethyl acetate is commonly used.
The global harmonization of chemical regulations has led to the adoption of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). This system provides standardized hazard communication for chemicals, including ethyl acetate, ensuring consistent safety information across different countries and regions.
As ethyl acetate continues to revolutionize traditional applications, staying compliant with evolving regulatory frameworks remains crucial for manufacturers, importers, and end-users. The regulatory landscape not only ensures safety and environmental protection but also drives innovation in developing more sustainable and eco-friendly alternatives or improved production processes for ethyl acetate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!