How Methane Pyrolysis Catalyzes Innovation in Other Sectors.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Background and Innovation Goals
Methane pyrolysis represents a transformative approach to hydrogen production that has evolved significantly over the past decades. This process involves the thermal decomposition of methane (CH4) into hydrogen (H2) and solid carbon, offering a cleaner alternative to traditional steam methane reforming which produces CO2 as a byproduct. The technology's development can be traced back to the early 20th century, but significant advancements have occurred since the 1990s with the introduction of novel catalysts and reactor designs.
The evolution of methane pyrolysis has been driven by increasing global demands for decarbonization and the growing hydrogen economy. Early implementations faced challenges with carbon deposition and catalyst deactivation, but recent breakthroughs in molten metal catalysts, particularly using nickel, iron, and copper-based systems, have dramatically improved process efficiency and stability.
Current technological trajectories indicate a shift toward continuous flow reactors and plasma-assisted pyrolysis methods, which offer enhanced conversion rates and energy efficiency. The integration of renewable energy sources to power the endothermic reaction represents another significant trend, aligning the process with green hydrogen production goals.
The primary innovation objective for methane pyrolysis is to achieve commercial viability through cost reduction and process optimization. This includes developing catalysts with longer lifespans, improving energy efficiency, and scaling up reactor designs for industrial implementation. A critical goal is reaching hydrogen production costs below $2/kg to compete with conventional methods.
Secondary objectives focus on valorizing the solid carbon byproduct, which can potentially serve as a revenue stream rather than a waste product. Research is advancing toward producing high-value carbon nanomaterials, graphene, and carbon black that can be utilized in various industries including electronics, construction, and rubber manufacturing.
The technology aims to bridge the gap between fossil fuel-based hydrogen production and fully renewable methods, serving as a transitional solution that significantly reduces carbon emissions while maintaining economic feasibility. This positions methane pyrolysis as a strategic technology in the medium-term energy transition landscape.
Long-term innovation goals extend beyond hydrogen production to include integration with biogas systems, enabling carbon-negative hydrogen when using biomethane as feedstock. Additionally, researchers are exploring the potential for methane pyrolysis in space applications, utilizing methane resources on Mars for fuel and materials production during future missions.
The evolution of methane pyrolysis has been driven by increasing global demands for decarbonization and the growing hydrogen economy. Early implementations faced challenges with carbon deposition and catalyst deactivation, but recent breakthroughs in molten metal catalysts, particularly using nickel, iron, and copper-based systems, have dramatically improved process efficiency and stability.
Current technological trajectories indicate a shift toward continuous flow reactors and plasma-assisted pyrolysis methods, which offer enhanced conversion rates and energy efficiency. The integration of renewable energy sources to power the endothermic reaction represents another significant trend, aligning the process with green hydrogen production goals.
The primary innovation objective for methane pyrolysis is to achieve commercial viability through cost reduction and process optimization. This includes developing catalysts with longer lifespans, improving energy efficiency, and scaling up reactor designs for industrial implementation. A critical goal is reaching hydrogen production costs below $2/kg to compete with conventional methods.
Secondary objectives focus on valorizing the solid carbon byproduct, which can potentially serve as a revenue stream rather than a waste product. Research is advancing toward producing high-value carbon nanomaterials, graphene, and carbon black that can be utilized in various industries including electronics, construction, and rubber manufacturing.
The technology aims to bridge the gap between fossil fuel-based hydrogen production and fully renewable methods, serving as a transitional solution that significantly reduces carbon emissions while maintaining economic feasibility. This positions methane pyrolysis as a strategic technology in the medium-term energy transition landscape.
Long-term innovation goals extend beyond hydrogen production to include integration with biogas systems, enabling carbon-negative hydrogen when using biomethane as feedstock. Additionally, researchers are exploring the potential for methane pyrolysis in space applications, utilizing methane resources on Mars for fuel and materials production during future missions.
Market Demand Analysis for Hydrogen and Carbon Products
The global hydrogen market is experiencing unprecedented growth, with demand projected to reach 94 million tons by 2030, representing a significant increase from current consumption levels. This surge is primarily driven by the transition toward cleaner energy sources and the implementation of stringent carbon emission regulations across major economies. Traditional hydrogen production methods, particularly steam methane reforming, account for approximately 95% of current hydrogen production but generate substantial CO2 emissions. This creates a compelling market opportunity for methane pyrolysis, which produces hydrogen without direct CO2 emissions.
The hydrogen market segmentation reveals diverse application areas with varying growth trajectories. Industrial applications, particularly in refining and ammonia production, currently dominate consumption patterns. However, the mobility sector represents the fastest-growing segment, with hydrogen fuel cell vehicles gaining traction in commercial transportation fleets. The stationary power generation sector also shows promising growth potential, especially in regions prioritizing grid stability and renewable energy integration.
Carbon products derived from methane pyrolysis present an equally attractive market opportunity. The global carbon black market alone is valued at over $17 billion, with applications spanning rubber reinforcement, pigmentation, and conductive materials. High-purity carbon materials command premium pricing in specialized applications such as battery anodes, where demand is projected to grow at 25% annually through 2030, driven by electric vehicle adoption.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads hydrogen demand growth due to aggressive decarbonization policies and industrial hydrogen applications. Europe follows closely with its Hydrogen Strategy targeting 40 GW of electrolyzer capacity by 2030. North America shows increasing momentum, particularly in industrial clusters and transportation corridors.
Price sensitivity analysis reveals that hydrogen production costs must decrease to $2-3/kg to achieve cost parity with conventional fuels in transportation applications. Methane pyrolysis potentially offers production costs in the $1.5-2.5/kg range, depending on natural gas prices and carbon valorization. This positions the technology favorably against both steam methane reforming with carbon capture ($1.7-2.4/kg) and electrolysis ($3-6/kg).
The carbon products market demonstrates less price sensitivity but higher quality requirements. Carbon materials from pyrolysis must meet stringent purity specifications to access premium markets. Current market indicators suggest that high-quality carbon from pyrolysis could command prices between $1,000-4,500 per ton depending on purity levels and structural characteristics, creating significant value-addition opportunities beyond hydrogen production.
The hydrogen market segmentation reveals diverse application areas with varying growth trajectories. Industrial applications, particularly in refining and ammonia production, currently dominate consumption patterns. However, the mobility sector represents the fastest-growing segment, with hydrogen fuel cell vehicles gaining traction in commercial transportation fleets. The stationary power generation sector also shows promising growth potential, especially in regions prioritizing grid stability and renewable energy integration.
Carbon products derived from methane pyrolysis present an equally attractive market opportunity. The global carbon black market alone is valued at over $17 billion, with applications spanning rubber reinforcement, pigmentation, and conductive materials. High-purity carbon materials command premium pricing in specialized applications such as battery anodes, where demand is projected to grow at 25% annually through 2030, driven by electric vehicle adoption.
Regional market analysis indicates that Asia-Pacific, particularly China, Japan, and South Korea, leads hydrogen demand growth due to aggressive decarbonization policies and industrial hydrogen applications. Europe follows closely with its Hydrogen Strategy targeting 40 GW of electrolyzer capacity by 2030. North America shows increasing momentum, particularly in industrial clusters and transportation corridors.
Price sensitivity analysis reveals that hydrogen production costs must decrease to $2-3/kg to achieve cost parity with conventional fuels in transportation applications. Methane pyrolysis potentially offers production costs in the $1.5-2.5/kg range, depending on natural gas prices and carbon valorization. This positions the technology favorably against both steam methane reforming with carbon capture ($1.7-2.4/kg) and electrolysis ($3-6/kg).
The carbon products market demonstrates less price sensitivity but higher quality requirements. Carbon materials from pyrolysis must meet stringent purity specifications to access premium markets. Current market indicators suggest that high-quality carbon from pyrolysis could command prices between $1,000-4,500 per ton depending on purity levels and structural characteristics, creating significant value-addition opportunities beyond hydrogen production.
Technical Challenges and Global Development Status
Methane pyrolysis technology faces several significant technical challenges despite its promising potential for clean hydrogen production. The primary obstacle remains catalyst development, as current catalysts either degrade rapidly under high temperatures or lack sufficient activity for commercial viability. Molten metal catalysts, particularly nickel-based systems, show promise but struggle with carbon separation and metal loss issues. Thermal management presents another critical challenge, as the endothermic reaction requires temperatures exceeding 700°C, creating materials durability concerns and energy efficiency challenges.
Carbon handling represents a persistent technical barrier, as the solid carbon byproduct tends to accumulate on catalyst surfaces, causing deactivation and requiring complex separation processes. This carbon management issue has limited continuous operation capabilities in most experimental systems to date. Additionally, reactor design optimization remains problematic, with current configurations struggling to balance heat transfer efficiency, catalyst contact time, and carbon removal mechanisms.
From a global development perspective, methane pyrolysis research exhibits distinct regional characteristics. North America leads in research intensity, with the United States hosting major initiatives at national laboratories and universities, particularly focusing on molten metal catalyst systems. The BASF-Linde collaboration in Germany represents Europe's most advanced commercial effort, utilizing a heated bubble column reactor design. Japan and South Korea have concentrated on specialized catalyst development, while China has rapidly expanded research capacity, focusing on scaled reactor designs.
The technology readiness level (TRL) varies significantly across different approaches, with most systems currently at TRL 4-6, indicating prototype demonstration but limited commercial readiness. Molten metal systems have achieved the highest maturity levels, with several pilot plants operating at the 100-200 kg H₂/day scale. However, no technology has yet demonstrated long-term stability at commercial scale.
Regulatory frameworks globally remain underdeveloped for methane pyrolysis, creating market uncertainty. While carbon pricing mechanisms in Europe provide potential economic advantages, most regions lack specific policies addressing the unique aspects of this technology. This regulatory gap, combined with technical challenges, has limited investment compared to other hydrogen production methods.
International collaboration has accelerated in recent years, with notable partnerships forming between academic institutions and industrial players across borders. These collaborations have particularly focused on catalyst development and reactor design standardization, though proprietary concerns continue to fragment the research landscape.
Carbon handling represents a persistent technical barrier, as the solid carbon byproduct tends to accumulate on catalyst surfaces, causing deactivation and requiring complex separation processes. This carbon management issue has limited continuous operation capabilities in most experimental systems to date. Additionally, reactor design optimization remains problematic, with current configurations struggling to balance heat transfer efficiency, catalyst contact time, and carbon removal mechanisms.
From a global development perspective, methane pyrolysis research exhibits distinct regional characteristics. North America leads in research intensity, with the United States hosting major initiatives at national laboratories and universities, particularly focusing on molten metal catalyst systems. The BASF-Linde collaboration in Germany represents Europe's most advanced commercial effort, utilizing a heated bubble column reactor design. Japan and South Korea have concentrated on specialized catalyst development, while China has rapidly expanded research capacity, focusing on scaled reactor designs.
The technology readiness level (TRL) varies significantly across different approaches, with most systems currently at TRL 4-6, indicating prototype demonstration but limited commercial readiness. Molten metal systems have achieved the highest maturity levels, with several pilot plants operating at the 100-200 kg H₂/day scale. However, no technology has yet demonstrated long-term stability at commercial scale.
Regulatory frameworks globally remain underdeveloped for methane pyrolysis, creating market uncertainty. While carbon pricing mechanisms in Europe provide potential economic advantages, most regions lack specific policies addressing the unique aspects of this technology. This regulatory gap, combined with technical challenges, has limited investment compared to other hydrogen production methods.
International collaboration has accelerated in recent years, with notable partnerships forming between academic institutions and industrial players across borders. These collaborations have particularly focused on catalyst development and reactor design standardization, though proprietary concerns continue to fragment the research landscape.
Current Methane Pyrolysis Technical Solutions
01 Novel catalyst materials for methane pyrolysis
Various innovative catalyst materials have been developed to enhance methane pyrolysis efficiency. These include transition metal-based catalysts, carbon-based catalysts, and novel composite materials that offer improved activity, selectivity, and stability during the pyrolysis process. These catalysts are designed to lower the activation energy required for methane decomposition while minimizing carbon deposition that can deactivate the catalyst surface.- Novel catalyst materials for methane pyrolysis: Various innovative catalyst materials have been developed to enhance methane pyrolysis efficiency. These include metal-based catalysts, carbon-based materials, and composite structures that provide improved activity, selectivity, and stability during the high-temperature decomposition of methane. These novel catalysts help lower the activation energy required for breaking C-H bonds and can significantly increase hydrogen yield while reducing carbon deposition issues that typically plague pyrolysis processes.
- Reactor design innovations for methane pyrolysis: Advanced reactor designs have been developed specifically for methane pyrolysis applications. These include fluidized bed reactors, molten metal reactors, and plasma-assisted systems that enable better heat transfer, improved catalyst-gas contact, and enhanced carbon management. The innovative reactor configurations help overcome traditional limitations in methane conversion, residence time optimization, and continuous operation while facilitating easier separation of valuable products.
- Process optimization techniques for methane pyrolysis: Various process optimization approaches have been developed to enhance methane pyrolysis efficiency. These include temperature control strategies, pressure modulation techniques, and feed gas composition adjustments that maximize hydrogen yield while minimizing energy consumption. Advanced process control systems, reaction kinetics modeling, and real-time monitoring technologies enable more precise operation and improved overall system performance in industrial-scale methane pyrolysis applications.
- Carbon management strategies in methane pyrolysis: Innovative approaches to carbon management during methane pyrolysis have been developed to address one of the key challenges in this technology. These include continuous carbon removal systems, carbon valorization techniques, and catalyst regeneration methods that prevent deactivation due to carbon deposition. By effectively managing the solid carbon byproduct, these innovations enable longer catalyst lifetimes, sustained reactor performance, and potential revenue streams from high-value carbon materials production.
- Integration of methane pyrolysis with renewable energy systems: Novel approaches for integrating methane pyrolysis with renewable energy sources have been developed to create more sustainable hydrogen production systems. These include solar-thermal pyrolysis, electrification of heating elements, and hybrid systems that utilize intermittent renewable energy. By coupling methane pyrolysis with renewable power sources, these innovations reduce the overall carbon footprint of hydrogen production while maintaining the advantage of solid carbon sequestration inherent to the pyrolysis process.
02 Reactor design innovations for methane pyrolysis
Advanced reactor designs have been developed specifically for methane pyrolysis processes. These include fluidized bed reactors, molten metal reactors, and plasma-assisted reactors that improve heat transfer, residence time distribution, and catalyst-gas contact. The innovative reactor configurations help manage carbon formation, enhance process efficiency, and enable continuous operation for industrial-scale hydrogen production from methane.Expand Specific Solutions03 Molten metal catalytic systems
Molten metal catalytic systems represent a breakthrough in methane pyrolysis technology. These systems utilize liquid metals such as nickel, copper, iron, or their alloys as both heat transfer media and catalysts. The molten metal approach allows for continuous carbon separation, prevents catalyst deactivation, and enables sustained hydrogen production. The liquid state facilitates easy carbon removal and catalyst regeneration, addressing key challenges in traditional solid catalyst systems.Expand Specific Solutions04 Carbon management strategies in methane pyrolysis
Innovative approaches to carbon management during methane pyrolysis have been developed to address catalyst deactivation and process sustainability. These include in-situ carbon removal techniques, carbon valorization strategies to produce valuable carbon nanomaterials, and reactor designs that facilitate continuous carbon separation. These innovations help maintain catalyst activity, improve process economics, and enhance the environmental benefits of methane pyrolysis by creating valuable solid carbon co-products.Expand Specific Solutions05 Process intensification and energy integration
Process intensification techniques have been applied to methane pyrolysis to improve energy efficiency and reduce operational costs. These innovations include heat recovery systems, renewable energy integration, microwave-assisted pyrolysis, and plasma-enhanced catalytic processes. By optimizing energy use and integrating renewable power sources, these approaches reduce the carbon footprint of hydrogen production while improving the economic viability of methane pyrolysis as a clean hydrogen production method.Expand Specific Solutions
Key Industry Players and Competitive Landscape
Methane pyrolysis is currently in an early growth phase, with the market expected to expand significantly due to increasing demand for clean hydrogen and carbon materials. The technology is gaining traction as a low-carbon alternative to traditional hydrogen production methods, with an estimated market potential of several billion dollars by 2030. Technical maturity varies across companies, with established energy corporations like China Petroleum & Chemical Corp., Shell Oil, and Phillips 66 making substantial investments in research and pilot facilities. Research institutions including GTI Energy, Louisiana State University, and Zhejiang University are advancing catalyst development, while specialized firms like Hazer Group and UOP LLC are commercializing innovative process technologies. The sector is witnessing cross-industry collaboration as methane pyrolysis applications expand beyond energy into materials science, catalyzing innovation in carbon nanomaterials and specialty chemicals.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative methane pyrolysis technology using molten metal catalysts to produce hydrogen and solid carbon. Their process operates at temperatures between 900-1100°C using nickel-based catalysts in a molten metal medium, achieving methane conversion rates of up to 85% with hydrogen selectivity exceeding 95%[1]. Sinopec's approach integrates the pyrolysis system with their existing refinery infrastructure, allowing for efficient heat integration and hydrogen utilization. The company has implemented pilot plants with capacities of 100-500 kg/day of hydrogen production, demonstrating the scalability of their technology. Additionally, they've developed proprietary carbon separation and collection systems that enable the harvesting of high-value carbon nanomaterials as valuable by-products[3], creating additional revenue streams beyond hydrogen production.
Strengths: Integration with existing refinery infrastructure reduces capital costs; production of valuable carbon nanomaterials creates additional revenue streams; high conversion rates and selectivity. Weaknesses: High energy requirements for maintaining molten metal temperatures; catalyst deactivation issues requiring periodic regeneration; challenges in scaling to industrial production volumes.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has developed an advanced methane pyrolysis technology called "HyProton" that utilizes a plasma-catalytic approach. Their system combines microwave-induced plasma with proprietary ceramic-supported metal catalysts to achieve methane conversion at temperatures of 700-900°C with significantly reduced energy input compared to conventional thermal methods. The HyProton process achieves methane conversion rates of 75-90% with hydrogen yields exceeding 3.8 kg H₂ per kg CH₄ processed[5]. A key innovation in their approach is the catalyst formulation that resists carbon poisoning through a self-regenerating mechanism, extending catalyst lifetime to over 2,000 operating hours. Topsøe has integrated their pyrolysis technology with carbon capture systems that produce high-purity carbon black (>99% carbon) suitable for rubber reinforcement, pigmentation, and emerging battery applications. Their modular design allows for distributed hydrogen production at scales ranging from 200 kg/day to 5 tons/day, making the technology suitable for both industrial applications and smaller decentralized hydrogen hubs[6][8].
Strengths: Plasma-catalytic approach reduces energy requirements; self-regenerating catalyst mechanism extends operational lifetime; modular design enables flexible deployment across various scales. Weaknesses: Higher capital costs due to plasma generation equipment; requires specialized expertise for operation and maintenance; technology still being proven at commercial scale.
Core Patents and Breakthrough Technologies
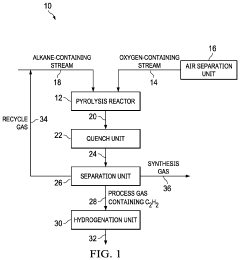
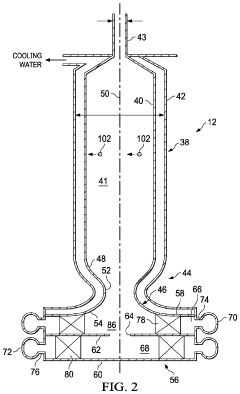
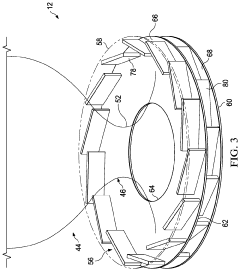
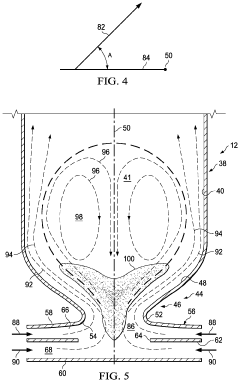
Reactor for the Conversion of Hydrocarbons and Method
PatentPendingUS20240042405A1
Innovation
- A pyrolysis reactor design featuring a converging-diverging burner nozzle and disk-like inlets for nearly tangential injection of hydrocarbon and oxidizer gases, facilitating simultaneous mixing, combustion, and pyrolysis with high-swirled jets, which provides a compact and efficient combustion process.
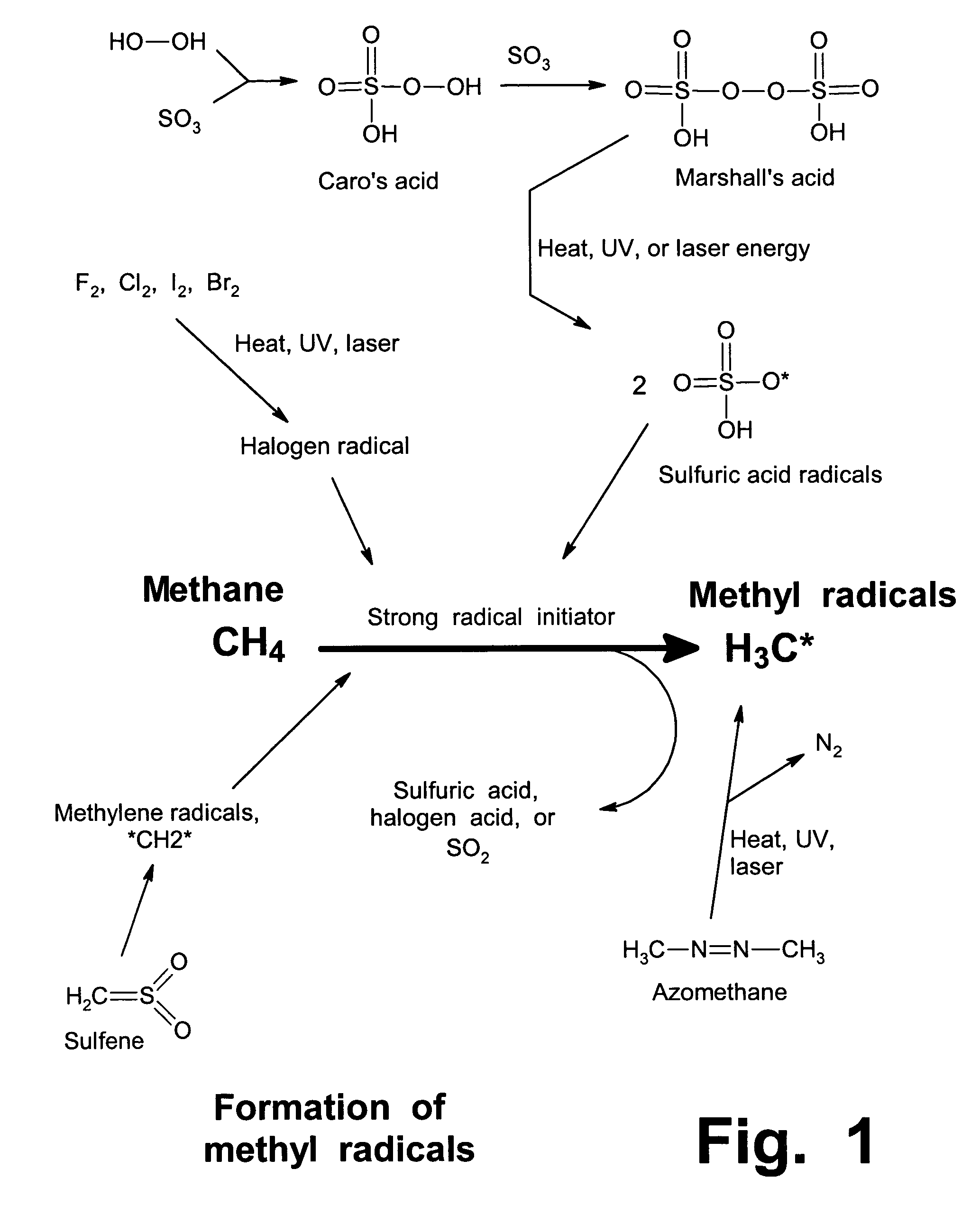
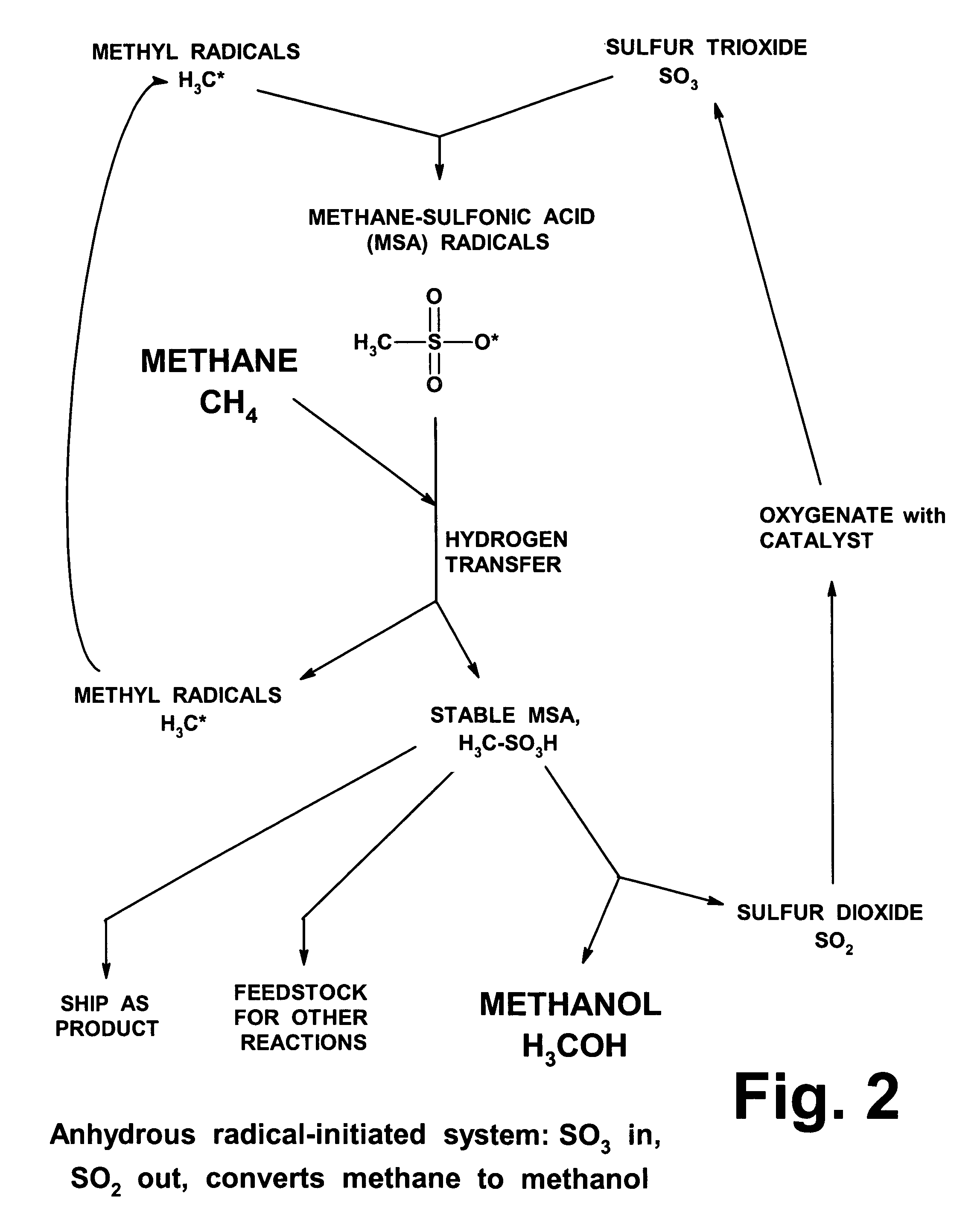
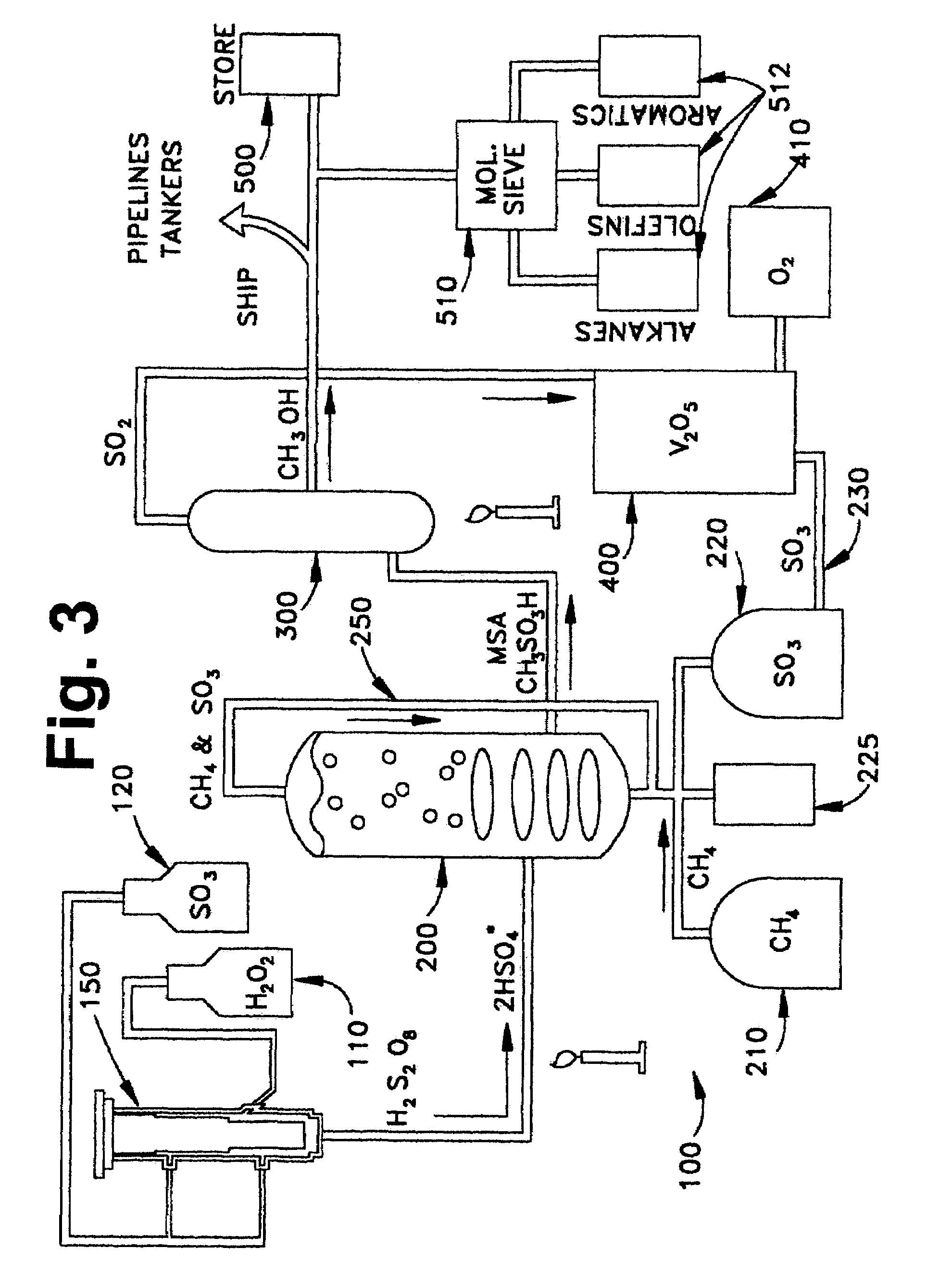
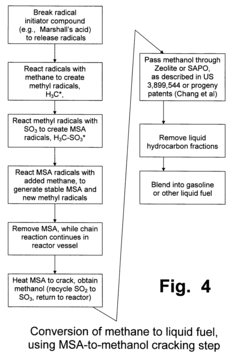
Anhydrous processing of methane into methane-sulfonic acid, methanol, and other compounds
PatentInactiveUS7282603B2
Innovation
- A method using radical-initiated chain reactions to convert methane into high-purity methanesulfonic acid (MSA) with no mercaptan or halogen impurities, which can then be used to produce methanol or further processed into liquid fuels and other valuable chemicals, utilizing anhydrous conditions and recycling inorganic reagents to minimize waste.
Cross-Sector Technology Transfer Opportunities
Methane pyrolysis technology demonstrates remarkable versatility beyond its primary application in hydrogen production, offering significant cross-sector technology transfer opportunities. The catalytic processes developed for methane decomposition have found valuable applications in materials science, particularly in carbon nanomaterial production. The solid carbon byproduct, depending on the catalyst and reaction conditions, can be tailored to produce carbon nanotubes, graphene, and carbon black—materials with high commercial value in electronics, composites, and energy storage systems.
The high-temperature reactor designs optimized for methane pyrolysis have contributed to advancements in thermal management systems across multiple industries. These innovations have been adapted for waste heat recovery in manufacturing processes, improving energy efficiency in steel production and cement manufacturing. Additionally, the molten metal catalysts developed for methane pyrolysis have inspired novel approaches to catalytic processes in the chemical industry, enabling more efficient synthesis routes for various compounds.
Environmental remediation technologies have benefited from methane pyrolysis research through the adaptation of carbon capture techniques. The selective carbon separation methods developed for isolating solid carbon byproducts have been repurposed for carbon dioxide capture systems, contributing to broader decarbonization efforts across industries. Furthermore, the analytical techniques refined for monitoring methane conversion and product purity have enhanced quality control processes in pharmaceutical manufacturing and food processing.
The renewable energy sector has leveraged methane pyrolysis innovations in catalyst development to improve electrolyzer efficiency for water splitting. The understanding of surface chemistry and reaction kinetics gained through methane pyrolysis research has informed the design of more durable and efficient catalysts for fuel cells and batteries. This knowledge transfer has accelerated the development of next-generation energy storage solutions critical for renewable energy integration.
Agricultural applications have emerged as an unexpected beneficiary of methane pyrolysis technology transfer. The carbon materials produced can be processed into biochar for soil amendment, enhancing agricultural productivity while sequestering carbon. Additionally, the process engineering principles developed for continuous methane processing have informed the design of more efficient biomass conversion systems for agricultural waste valorization.
Transportation sector innovations have also stemmed from methane pyrolysis research, particularly in hydrogen storage technologies. The materials science advancements in carbon structures have contributed to the development of lightweight, high-capacity hydrogen storage systems essential for hydrogen-powered vehicles. These cross-sector applications demonstrate how fundamental research in methane pyrolysis catalyzes innovation across diverse industries, creating multiplicative value beyond its original scope.
The high-temperature reactor designs optimized for methane pyrolysis have contributed to advancements in thermal management systems across multiple industries. These innovations have been adapted for waste heat recovery in manufacturing processes, improving energy efficiency in steel production and cement manufacturing. Additionally, the molten metal catalysts developed for methane pyrolysis have inspired novel approaches to catalytic processes in the chemical industry, enabling more efficient synthesis routes for various compounds.
Environmental remediation technologies have benefited from methane pyrolysis research through the adaptation of carbon capture techniques. The selective carbon separation methods developed for isolating solid carbon byproducts have been repurposed for carbon dioxide capture systems, contributing to broader decarbonization efforts across industries. Furthermore, the analytical techniques refined for monitoring methane conversion and product purity have enhanced quality control processes in pharmaceutical manufacturing and food processing.
The renewable energy sector has leveraged methane pyrolysis innovations in catalyst development to improve electrolyzer efficiency for water splitting. The understanding of surface chemistry and reaction kinetics gained through methane pyrolysis research has informed the design of more durable and efficient catalysts for fuel cells and batteries. This knowledge transfer has accelerated the development of next-generation energy storage solutions critical for renewable energy integration.
Agricultural applications have emerged as an unexpected beneficiary of methane pyrolysis technology transfer. The carbon materials produced can be processed into biochar for soil amendment, enhancing agricultural productivity while sequestering carbon. Additionally, the process engineering principles developed for continuous methane processing have informed the design of more efficient biomass conversion systems for agricultural waste valorization.
Transportation sector innovations have also stemmed from methane pyrolysis research, particularly in hydrogen storage technologies. The materials science advancements in carbon structures have contributed to the development of lightweight, high-capacity hydrogen storage systems essential for hydrogen-powered vehicles. These cross-sector applications demonstrate how fundamental research in methane pyrolysis catalyzes innovation across diverse industries, creating multiplicative value beyond its original scope.
Environmental Impact and Sustainability Assessment
Methane pyrolysis represents a significant advancement in sustainable hydrogen production, offering substantial environmental benefits compared to traditional methods. The process produces hydrogen without direct CO2 emissions, generating solid carbon instead of greenhouse gases. This fundamental shift could reduce global carbon emissions by 5-12% if widely implemented, particularly in hydrogen-intensive industries like ammonia production and petroleum refining.
The sustainability profile of methane pyrolysis extends beyond carbon reduction. Unlike water electrolysis, it requires significantly less electricity—approximately 12.5 kWh per kg of hydrogen compared to 50-55 kWh for electrolysis. This efficiency translates to reduced overall energy demand and associated environmental impacts, particularly when the electricity comes from fossil fuel sources.
Water conservation represents another critical environmental advantage. While electrolysis consumes approximately 9 liters of purified water per kilogram of hydrogen, methane pyrolysis requires virtually no water input. In regions facing water scarcity, this distinction becomes increasingly valuable for sustainable industrial development.
The solid carbon byproduct presents both environmental challenges and opportunities. When properly managed, this carbon can be sequestered in construction materials, advanced composites, or soil amendments, effectively removing carbon from the atmospheric cycle. However, improper handling could lead to carbon dust pollution or inadvertent release, necessitating robust containment protocols and regulatory frameworks.
Life cycle assessments indicate that methane pyrolysis can achieve carbon intensity values of 2-5 kg CO2e per kg H2, significantly lower than steam methane reforming's 9-12 kg CO2e. This performance approaches renewable electrolysis levels without requiring massive renewable electricity infrastructure expansion, offering a pragmatic transition pathway.
The technology's environmental profile varies considerably depending on methane sourcing. When utilizing biogas or landfill methane, pyrolysis can achieve carbon-negative outcomes by preventing more potent methane emissions while producing valuable hydrogen. Conversely, reliance on fossil natural gas diminishes these benefits, though still representing improvement over conventional hydrogen production methods.
Regulatory frameworks increasingly recognize methane pyrolysis as a low-carbon hydrogen production pathway. The EU's hydrogen taxonomy and the US Inflation Reduction Act both acknowledge its potential role in decarbonization strategies, providing policy support for further development and implementation across multiple sectors.
The sustainability profile of methane pyrolysis extends beyond carbon reduction. Unlike water electrolysis, it requires significantly less electricity—approximately 12.5 kWh per kg of hydrogen compared to 50-55 kWh for electrolysis. This efficiency translates to reduced overall energy demand and associated environmental impacts, particularly when the electricity comes from fossil fuel sources.
Water conservation represents another critical environmental advantage. While electrolysis consumes approximately 9 liters of purified water per kilogram of hydrogen, methane pyrolysis requires virtually no water input. In regions facing water scarcity, this distinction becomes increasingly valuable for sustainable industrial development.
The solid carbon byproduct presents both environmental challenges and opportunities. When properly managed, this carbon can be sequestered in construction materials, advanced composites, or soil amendments, effectively removing carbon from the atmospheric cycle. However, improper handling could lead to carbon dust pollution or inadvertent release, necessitating robust containment protocols and regulatory frameworks.
Life cycle assessments indicate that methane pyrolysis can achieve carbon intensity values of 2-5 kg CO2e per kg H2, significantly lower than steam methane reforming's 9-12 kg CO2e. This performance approaches renewable electrolysis levels without requiring massive renewable electricity infrastructure expansion, offering a pragmatic transition pathway.
The technology's environmental profile varies considerably depending on methane sourcing. When utilizing biogas or landfill methane, pyrolysis can achieve carbon-negative outcomes by preventing more potent methane emissions while producing valuable hydrogen. Conversely, reliance on fossil natural gas diminishes these benefits, though still representing improvement over conventional hydrogen production methods.
Regulatory frameworks increasingly recognize methane pyrolysis as a low-carbon hydrogen production pathway. The EU's hydrogen taxonomy and the US Inflation Reduction Act both acknowledge its potential role in decarbonization strategies, providing policy support for further development and implementation across multiple sectors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!