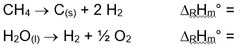Methane Pyrolysis and the Hydrogen Economy.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Evolution and Hydrogen Production Goals
Methane pyrolysis represents a significant technological pathway in the evolution of hydrogen production methods, offering a potentially cleaner alternative to traditional steam methane reforming (SMR). The concept of methane decomposition dates back to the early 20th century, but has gained renewed attention in recent decades as the world seeks decarbonization solutions. The fundamental reaction (CH₄ → C + 2H₂) produces hydrogen without direct CO₂ emissions, positioning it as a promising bridge technology in the transition toward a hydrogen economy.
The technological evolution of methane pyrolysis has progressed through several distinct phases. Initial research in the 1960s-1980s focused on thermal decomposition at high temperatures (>1000°C), which proved energy-intensive and economically challenging. The 1990s saw the emergence of catalytic approaches using nickel, iron, and carbon-based catalysts to reduce reaction temperatures to 500-900°C, improving energy efficiency. The 2000s brought plasma-assisted methods and molten metal technologies, with the latter gaining significant momentum in the 2010s through innovations like the molten nickel process developed by BASF and the molten salt approach pioneered by researchers at the Karlsruhe Institute of Technology.
Current technological goals for methane pyrolysis center on achieving economic viability at industrial scale while maintaining environmental benefits. Key objectives include reducing energy requirements to below 37 kWh/kg H₂, developing catalysts with extended lifespans exceeding 1,000 hours, and designing reactor systems capable of continuous carbon removal without process interruption. Additionally, researchers aim to achieve hydrogen production costs under $2/kg to compete with conventional methods.
The hydrogen production landscape is evolving toward more ambitious targets aligned with global climate objectives. By 2030, the industry aims to scale methane pyrolysis to gigawatt-level production capacity, with carbon utilization pathways established for the solid carbon byproduct. The 2040 vision includes fully integrated pyrolysis systems powered by renewable electricity, potentially achieving carbon-negative hydrogen when combined with carbon sequestration or utilization.
The ultimate technological goal extends beyond mere production efficiency to creating a circular carbon economy where methane pyrolysis serves as a cornerstone technology. This involves developing versatile systems capable of processing various carbon feedstocks, including biogas and recycled methane, while producing hydrogen at costs competitive with fossil-based alternatives but without the associated emissions burden.
The technological evolution of methane pyrolysis has progressed through several distinct phases. Initial research in the 1960s-1980s focused on thermal decomposition at high temperatures (>1000°C), which proved energy-intensive and economically challenging. The 1990s saw the emergence of catalytic approaches using nickel, iron, and carbon-based catalysts to reduce reaction temperatures to 500-900°C, improving energy efficiency. The 2000s brought plasma-assisted methods and molten metal technologies, with the latter gaining significant momentum in the 2010s through innovations like the molten nickel process developed by BASF and the molten salt approach pioneered by researchers at the Karlsruhe Institute of Technology.
Current technological goals for methane pyrolysis center on achieving economic viability at industrial scale while maintaining environmental benefits. Key objectives include reducing energy requirements to below 37 kWh/kg H₂, developing catalysts with extended lifespans exceeding 1,000 hours, and designing reactor systems capable of continuous carbon removal without process interruption. Additionally, researchers aim to achieve hydrogen production costs under $2/kg to compete with conventional methods.
The hydrogen production landscape is evolving toward more ambitious targets aligned with global climate objectives. By 2030, the industry aims to scale methane pyrolysis to gigawatt-level production capacity, with carbon utilization pathways established for the solid carbon byproduct. The 2040 vision includes fully integrated pyrolysis systems powered by renewable electricity, potentially achieving carbon-negative hydrogen when combined with carbon sequestration or utilization.
The ultimate technological goal extends beyond mere production efficiency to creating a circular carbon economy where methane pyrolysis serves as a cornerstone technology. This involves developing versatile systems capable of processing various carbon feedstocks, including biogas and recycled methane, while producing hydrogen at costs competitive with fossil-based alternatives but without the associated emissions burden.
Market Analysis for Clean Hydrogen Demand
The global hydrogen market is experiencing significant growth, driven by the increasing focus on decarbonization and sustainable energy solutions. Current estimates place the global hydrogen market at approximately $130 billion, with projections suggesting expansion to $500 billion by 2030. Clean hydrogen, particularly from methane pyrolysis, represents a crucial segment within this growing market.
Industrial applications currently dominate hydrogen demand, with ammonia production accounting for roughly 55% of global hydrogen consumption. Petroleum refining follows at 25%, while methanol production utilizes about 10%. These established sectors provide a stable baseline demand for hydrogen producers.
Transportation emerges as a rapidly expanding market for clean hydrogen. The fuel cell electric vehicle (FCEV) sector is growing at a compound annual growth rate of 31.4% through 2028. Major automotive manufacturers including Toyota, Hyundai, and Honda have commercially available hydrogen vehicles, while heavy transport applications in trucking, shipping, and aviation show increasing adoption potential due to hydrogen's energy density advantages over battery technologies.
Power generation represents another significant growth area, with hydrogen increasingly viewed as a solution for grid-scale energy storage and baseload power generation. The market for hydrogen power systems is projected to grow at 14.3% annually through 2027, with particular strength in regions pursuing aggressive renewable energy integration.
Regional analysis reveals distinct market characteristics. Asia-Pacific leads global hydrogen demand, with China and Japan implementing ambitious hydrogen strategies. Europe follows closely, driven by the European Green Deal and national hydrogen roadmaps from Germany, France, and the Netherlands. North America shows growing momentum, particularly in California and Canada's hydrogen corridors.
Clean hydrogen produced via methane pyrolysis holds particular appeal across these markets due to its carbon-neutral profile and cost advantages over electrolysis. Industries with hard-to-abate emissions, including steel production, cement manufacturing, and chemical processing, represent prime targets for pyrolytic hydrogen adoption.
Market barriers include infrastructure limitations, with hydrogen distribution networks requiring significant investment. Regulatory frameworks remain inconsistent across regions, though carbon pricing mechanisms increasingly favor clean hydrogen solutions. Price sensitivity varies by sector, with industrial users demonstrating greater price elasticity than emerging applications in transportation and power generation.
The competitive landscape features traditional industrial gas companies expanding clean hydrogen capabilities alongside energy majors pivoting toward hydrogen as part of decarbonization strategies. Specialized technology providers focused on methane pyrolysis are attracting significant venture capital, indicating strong market confidence in this production pathway.
Industrial applications currently dominate hydrogen demand, with ammonia production accounting for roughly 55% of global hydrogen consumption. Petroleum refining follows at 25%, while methanol production utilizes about 10%. These established sectors provide a stable baseline demand for hydrogen producers.
Transportation emerges as a rapidly expanding market for clean hydrogen. The fuel cell electric vehicle (FCEV) sector is growing at a compound annual growth rate of 31.4% through 2028. Major automotive manufacturers including Toyota, Hyundai, and Honda have commercially available hydrogen vehicles, while heavy transport applications in trucking, shipping, and aviation show increasing adoption potential due to hydrogen's energy density advantages over battery technologies.
Power generation represents another significant growth area, with hydrogen increasingly viewed as a solution for grid-scale energy storage and baseload power generation. The market for hydrogen power systems is projected to grow at 14.3% annually through 2027, with particular strength in regions pursuing aggressive renewable energy integration.
Regional analysis reveals distinct market characteristics. Asia-Pacific leads global hydrogen demand, with China and Japan implementing ambitious hydrogen strategies. Europe follows closely, driven by the European Green Deal and national hydrogen roadmaps from Germany, France, and the Netherlands. North America shows growing momentum, particularly in California and Canada's hydrogen corridors.
Clean hydrogen produced via methane pyrolysis holds particular appeal across these markets due to its carbon-neutral profile and cost advantages over electrolysis. Industries with hard-to-abate emissions, including steel production, cement manufacturing, and chemical processing, represent prime targets for pyrolytic hydrogen adoption.
Market barriers include infrastructure limitations, with hydrogen distribution networks requiring significant investment. Regulatory frameworks remain inconsistent across regions, though carbon pricing mechanisms increasingly favor clean hydrogen solutions. Price sensitivity varies by sector, with industrial users demonstrating greater price elasticity than emerging applications in transportation and power generation.
The competitive landscape features traditional industrial gas companies expanding clean hydrogen capabilities alongside energy majors pivoting toward hydrogen as part of decarbonization strategies. Specialized technology providers focused on methane pyrolysis are attracting significant venture capital, indicating strong market confidence in this production pathway.
Global Methane Pyrolysis Technology Status and Barriers
Methane pyrolysis technology has emerged as a promising pathway for hydrogen production with significantly reduced carbon emissions compared to conventional methods. Currently, the global landscape of methane pyrolysis is characterized by varied technological approaches at different stages of development, from laboratory-scale experiments to early commercial demonstrations.
The most advanced methane pyrolysis technologies can be categorized into three main types: thermal decomposition using various heat sources, catalytic methods employing metal catalysts, and plasma-based approaches. Each presents distinct advantages and challenges in terms of energy efficiency, carbon quality, and scalability potential.
Thermal decomposition methods, particularly those utilizing molten metals as heat transfer media, have gained significant traction. Companies like Monolith Materials in the United States and BASF in Germany have made notable progress in this area. Monolith's technology has reached commercial scale, producing hydrogen and carbon black from natural gas with substantially lower emissions than traditional methods.
Catalytic approaches, while promising for their lower energy requirements, face challenges related to catalyst deactivation due to carbon deposition. Research groups in Japan, Germany, and the United States are actively working to develop more resilient catalysts and innovative reactor designs to overcome these limitations.
Despite these advancements, several significant barriers impede widespread adoption of methane pyrolysis. The high energy input requirement remains a primary challenge, with most processes requiring temperatures exceeding 1000°C to achieve acceptable methane conversion rates. This translates to substantial energy costs that can undermine economic viability without access to low-cost renewable electricity or efficient heat recovery systems.
Material limitations present another critical barrier. Reactor materials must withstand extreme temperatures and resist carbon fouling while maintaining structural integrity over extended operational periods. Current materials often fail to meet these demanding requirements, necessitating frequent maintenance and replacement.
Scale-up challenges also persist across all technology variants. Laboratory successes have proven difficult to translate to industrial scales while maintaining performance metrics. The handling and valorization of solid carbon byproducts represent both a technical challenge and a potential economic opportunity, but markets for high-volume carbon materials remain underdeveloped.
Regulatory frameworks and infrastructure requirements further complicate deployment. The nascent nature of the technology means that specific regulations governing its operation and carbon handling are still evolving in most jurisdictions. Additionally, integration with existing natural gas and hydrogen infrastructure requires significant investment and planning.
The most advanced methane pyrolysis technologies can be categorized into three main types: thermal decomposition using various heat sources, catalytic methods employing metal catalysts, and plasma-based approaches. Each presents distinct advantages and challenges in terms of energy efficiency, carbon quality, and scalability potential.
Thermal decomposition methods, particularly those utilizing molten metals as heat transfer media, have gained significant traction. Companies like Monolith Materials in the United States and BASF in Germany have made notable progress in this area. Monolith's technology has reached commercial scale, producing hydrogen and carbon black from natural gas with substantially lower emissions than traditional methods.
Catalytic approaches, while promising for their lower energy requirements, face challenges related to catalyst deactivation due to carbon deposition. Research groups in Japan, Germany, and the United States are actively working to develop more resilient catalysts and innovative reactor designs to overcome these limitations.
Despite these advancements, several significant barriers impede widespread adoption of methane pyrolysis. The high energy input requirement remains a primary challenge, with most processes requiring temperatures exceeding 1000°C to achieve acceptable methane conversion rates. This translates to substantial energy costs that can undermine economic viability without access to low-cost renewable electricity or efficient heat recovery systems.
Material limitations present another critical barrier. Reactor materials must withstand extreme temperatures and resist carbon fouling while maintaining structural integrity over extended operational periods. Current materials often fail to meet these demanding requirements, necessitating frequent maintenance and replacement.
Scale-up challenges also persist across all technology variants. Laboratory successes have proven difficult to translate to industrial scales while maintaining performance metrics. The handling and valorization of solid carbon byproducts represent both a technical challenge and a potential economic opportunity, but markets for high-volume carbon materials remain underdeveloped.
Regulatory frameworks and infrastructure requirements further complicate deployment. The nascent nature of the technology means that specific regulations governing its operation and carbon handling are still evolving in most jurisdictions. Additionally, integration with existing natural gas and hydrogen infrastructure requires significant investment and planning.
Current Methane Pyrolysis Reactor Designs and Processes
01 Catalytic methane pyrolysis processes
Catalytic processes enhance methane pyrolysis by lowering activation energy and improving conversion efficiency. Various catalysts including transition metals, metal oxides, and carbon-based materials can be used to facilitate the decomposition of methane into hydrogen and solid carbon. These catalytic systems operate at lower temperatures compared to thermal pyrolysis, making the process more energy-efficient while producing high-purity hydrogen and valuable carbon materials.- Catalytic methane pyrolysis processes: Catalytic processes for methane pyrolysis involve the use of specific catalysts to enhance the decomposition of methane into hydrogen and solid carbon. These catalysts typically include transition metals, metal oxides, or supported metal systems that lower the activation energy required for breaking carbon-hydrogen bonds. The catalytic approach allows for operation at lower temperatures compared to thermal pyrolysis, improving energy efficiency and reducing operational costs. The process design often includes specialized reactor configurations to maintain catalyst activity and manage carbon deposition.
- Thermal methane pyrolysis systems: Thermal methane pyrolysis systems utilize high temperatures (typically 800-1200°C) to break down methane molecules without catalysts. These systems often employ specialized reactor designs such as plasma reactors, molten metal reactors, or fluidized bed systems to achieve efficient heat transfer and reaction kinetics. The process produces hydrogen gas and solid carbon as the main products. Thermal approaches can handle impure methane streams but require significant energy input to maintain the high temperatures necessary for the pyrolysis reaction.
- Carbon management in methane pyrolysis: Managing the solid carbon byproduct is a critical aspect of methane pyrolysis technology. Various approaches have been developed to handle carbon deposition, including continuous carbon removal systems, reactor designs that facilitate carbon separation, and methods to produce valuable carbon allotropes such as carbon nanotubes or graphene. Some systems incorporate molten metal or salt media that allow carbon to float to the surface for easy collection. The quality and form of the carbon product can be controlled through process parameters, potentially creating additional value streams beyond hydrogen production.
- Reactor designs for methane pyrolysis: Specialized reactor designs for methane pyrolysis include molten metal reactors, plasma-assisted reactors, moving bed reactors, and fluidized bed systems. These designs address challenges such as heat transfer efficiency, carbon management, and continuous operation. Molten metal reactors use liquid metals like tin or nickel as heat transfer media and sometimes as catalysts. Plasma reactors use electrical energy to create high-temperature plasma for methane decomposition. Moving bed and fluidized bed designs help manage solid carbon formation while maintaining reaction efficiency. Reactor materials must withstand high temperatures and resist carbon fouling.
- Integration of methane pyrolysis with hydrogen production systems: Methane pyrolysis can be integrated into broader hydrogen production and energy systems. These integrated approaches include combining pyrolysis with hydrogen purification technologies, incorporating renewable energy sources for process heat, and developing closed-loop systems that utilize byproducts. Some designs integrate methane pyrolysis with existing natural gas infrastructure or industrial processes to improve overall efficiency. The integration can include heat recovery systems, hydrogen storage solutions, and carbon utilization pathways. These integrated systems aim to optimize energy efficiency, reduce costs, and minimize environmental impact in hydrogen production.
02 Reactor designs for methane pyrolysis
Specialized reactor designs are crucial for efficient methane pyrolysis. These include molten metal reactors, fluidized bed reactors, and plasma reactors that provide optimal conditions for the thermal decomposition of methane. Advanced reactor configurations incorporate features for continuous carbon removal, heat management, and gas separation to maintain process efficiency and prevent catalyst deactivation. These designs aim to maximize hydrogen yield while ensuring operational stability for industrial-scale applications.Expand Specific Solutions03 Carbon management and utilization from methane pyrolysis
Methane pyrolysis produces solid carbon as a co-product, which requires effective management and offers potential for value-added applications. The carbon can be processed into various forms including carbon black, carbon nanotubes, and graphene depending on process conditions. Systems for continuous carbon removal, collection, and processing are essential for maintaining process efficiency. The recovered carbon materials can be utilized in manufacturing, construction, agriculture, and other industries, creating additional revenue streams.Expand Specific Solutions04 Integration of methane pyrolysis with renewable energy systems
Methane pyrolysis can be integrated with renewable energy sources to create sustainable hydrogen production systems. By utilizing solar, wind, or geothermal energy to provide the heat required for pyrolysis, the process can achieve near-zero carbon emissions. These integrated systems may incorporate thermal energy storage, electrification of heating elements, and smart control systems to manage intermittent renewable energy inputs. This approach enables the production of clean hydrogen while minimizing environmental impact.Expand Specific Solutions05 Process optimization and scale-up technologies
Optimizing methane pyrolysis for industrial scale requires advanced technologies for process control, heat management, and system integration. Techniques include precise temperature control, pressure regulation, residence time optimization, and feed gas purification. Computational modeling and simulation tools help predict process behavior and optimize operating parameters. Scale-up strategies address challenges related to heat transfer, carbon handling, and catalyst regeneration to maintain efficiency at commercial scales while ensuring economic viability and operational reliability.Expand Specific Solutions
Leading Companies and Research Institutions in Methane Pyrolysis
The methane pyrolysis hydrogen market is in its early growth phase, characterized by increasing R&D investments and pilot projects. The global hydrogen economy is projected to reach $500-700 billion by 2050, with methane pyrolysis emerging as a promising low-carbon hydrogen production method. Technologically, the field shows varying maturity levels across different approaches. Leading players include established energy corporations (ExxonMobil, Air Products, BASF, Shell, Saudi Aramco) investing in commercial-scale development, alongside innovative startups like Molten Industries developing novel reactor designs. Academic institutions (University of California, China University of Petroleum) are advancing fundamental research, while research organizations (TNO) are bridging the gap between laboratory discoveries and industrial applications, creating a dynamic competitive landscape poised for significant growth.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has developed an advanced methane pyrolysis technology that uses a proprietary molten metal catalyst system to decompose natural gas into hydrogen and solid carbon. Their process operates at temperatures around 1000°C in a bubble column reactor where methane passes through molten metal (typically nickel-bismuth alloy), enabling efficient thermal decomposition. The technology incorporates a continuous carbon removal system that prevents catalyst deactivation and ensures sustained hydrogen production. ExxonMobil's approach integrates with existing natural gas infrastructure, allowing for scalable implementation at industrial sites. Their system achieves approximately 80% conversion efficiency while producing hydrogen with over 99% purity without direct CO2 emissions. The company has demonstrated this technology at pilot scale facilities and is working toward commercial-scale deployment as part of their broader low-carbon hydrogen strategy.
Strengths: Produces hydrogen without direct CO2 emissions; generates valuable solid carbon byproduct; leverages existing natural gas infrastructure; achieves high hydrogen purity. Weaknesses: High energy requirements for maintaining molten metal temperatures; challenges in scaling up the continuous carbon removal system; potential metal catalyst contamination issues over extended operation periods.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has developed an innovative methane pyrolysis technology called HydrogenPro™ that utilizes a fluidized bed reactor system with specialized carbon-resistant catalysts. Their approach operates at moderate temperatures (700-900°C) and employs proprietary metal alloy catalysts supported on ceramic materials that resist carbon deposition and deactivation. The process features a continuous catalyst regeneration system that maintains activity over thousands of operating hours. Topsøe's technology achieves methane conversion rates of approximately 75-85% with hydrogen selectivity exceeding 95%. A key innovation is their integrated heat management system that recovers thermal energy from the process to minimize external energy requirements. The company has successfully demonstrated this technology at pilot scale, processing up to 200 kg of methane daily with stable performance. Their system produces solid carbon in a granular form that can be easily handled and potentially valorized in materials applications. Topsøe has integrated this technology into their broader hydrogen production portfolio as a complementary approach to their established steam reforming technologies.
Strengths: Lower operating temperatures than molten metal approaches; robust catalyst system with extended lifetime; efficient heat integration; produces high-purity hydrogen suitable for fuel cells. Weaknesses: Lower single-pass conversion than some competing technologies; requires periodic catalyst replacement; carbon product quality may vary depending on operating conditions; scaling challenges for the fluidized bed system at very large capacities.
Critical Patents and Catalytic Innovations in Methane Decomposition
Exploitation of natural hydrogen deposits using methane pyrolysis
PatentWO2025073666A1
Innovation
- A process involving methane pyrolysis, where natural hydrogen-hydrocarbon mixtures are fed into a hydrocarbon pyrolysis unit, causing the methane to decompose into hydrogen and solid carbon, with the solid carbon being separated from the hydrogen product stream.
Process of selectively hydrogenating gas mixture having high acetylene content
PatentActiveUS11858886B2
Innovation
- A selective hydrogenation process using a bimetallic catalyst with Pd and Cu supported on a porous substrate, where the catalysts are prepared by spray impregnation and reduction, allowing for efficient conversion of high-concentration acetylene in methane pyrolysis products to ethylene without external hydrogen supply, optimizing the weight ratio of metals to enhance acetylene conversion and ethylene selectivity.
Carbon Management Strategies and Solid Carbon Valorization
Carbon management is a critical component of methane pyrolysis implementation within the hydrogen economy framework. As methane pyrolysis produces solid carbon as a byproduct, effective strategies for handling this carbon output are essential for both environmental sustainability and economic viability of the process. Current carbon management approaches include sequestration, utilization in industrial applications, and development of high-value carbon products.
Carbon sequestration options for solid carbon from methane pyrolysis include underground storage in depleted oil and gas reservoirs or saline aquifers. This approach offers permanent carbon removal but faces challenges related to transportation logistics and long-term monitoring requirements. The stability of pyrolytic carbon makes it potentially suitable for long-term sequestration with minimal risk of re-release into the atmosphere.
Carbon utilization pathways represent a more economically attractive option. The solid carbon produced through methane pyrolysis can be processed into various forms including carbon black, graphite, carbon nanotubes, and graphene. These materials have established markets in industries ranging from rubber manufacturing to electronics and construction. Carbon black, for instance, has a global market exceeding $17 billion annually, primarily driven by tire manufacturing.
Advanced valorization strategies focus on converting pyrolytic carbon into higher-value products. Research indicates that controlling pyrolysis conditions can influence carbon morphology and properties, potentially enabling targeted production of specific carbon allotropes. Catalytic post-processing can further enhance carbon quality for specialized applications. Recent innovations include carbon-reinforced concrete additives that improve strength while permanently sequestering carbon, and advanced carbon materials for energy storage applications.
The economic implications of carbon valorization are substantial. Analysis suggests that revenue from carbon byproducts could offset 20-40% of hydrogen production costs in methane pyrolysis systems, significantly improving the economic competitiveness against other hydrogen production methods. This "carbon credit" effectively reduces the breakeven price for turquoise hydrogen, potentially accelerating market adoption.
Environmental life cycle assessments indicate that carbon utilization pathways generally offer superior sustainability outcomes compared to sequestration alone. By displacing carbon-intensive conventional production methods for materials like carbon black, the net environmental benefit extends beyond the hydrogen production process itself. However, market absorption capacity remains a constraint, as large-scale methane pyrolysis deployment would produce carbon volumes that could potentially saturate existing markets.
Carbon sequestration options for solid carbon from methane pyrolysis include underground storage in depleted oil and gas reservoirs or saline aquifers. This approach offers permanent carbon removal but faces challenges related to transportation logistics and long-term monitoring requirements. The stability of pyrolytic carbon makes it potentially suitable for long-term sequestration with minimal risk of re-release into the atmosphere.
Carbon utilization pathways represent a more economically attractive option. The solid carbon produced through methane pyrolysis can be processed into various forms including carbon black, graphite, carbon nanotubes, and graphene. These materials have established markets in industries ranging from rubber manufacturing to electronics and construction. Carbon black, for instance, has a global market exceeding $17 billion annually, primarily driven by tire manufacturing.
Advanced valorization strategies focus on converting pyrolytic carbon into higher-value products. Research indicates that controlling pyrolysis conditions can influence carbon morphology and properties, potentially enabling targeted production of specific carbon allotropes. Catalytic post-processing can further enhance carbon quality for specialized applications. Recent innovations include carbon-reinforced concrete additives that improve strength while permanently sequestering carbon, and advanced carbon materials for energy storage applications.
The economic implications of carbon valorization are substantial. Analysis suggests that revenue from carbon byproducts could offset 20-40% of hydrogen production costs in methane pyrolysis systems, significantly improving the economic competitiveness against other hydrogen production methods. This "carbon credit" effectively reduces the breakeven price for turquoise hydrogen, potentially accelerating market adoption.
Environmental life cycle assessments indicate that carbon utilization pathways generally offer superior sustainability outcomes compared to sequestration alone. By displacing carbon-intensive conventional production methods for materials like carbon black, the net environmental benefit extends beyond the hydrogen production process itself. However, market absorption capacity remains a constraint, as large-scale methane pyrolysis deployment would produce carbon volumes that could potentially saturate existing markets.
Economic Viability and Energy Transition Integration
The economic viability of methane pyrolysis as a hydrogen production method represents a critical factor in determining its role in the emerging hydrogen economy. Current cost analyses indicate that methane pyrolysis can produce hydrogen at approximately $1.50-2.00 per kilogram, positioning it competitively between conventional steam methane reforming ($1.00-1.50/kg) and green hydrogen from electrolysis ($3.00-6.00/kg). This intermediate cost profile creates a strategic advantage during the transition period when renewable electricity remains relatively expensive.
Capital expenditure requirements for methane pyrolysis facilities are estimated at $600-900 per kilowatt of hydrogen production capacity, significantly lower than electrolysis systems which typically range from $1,000-1,500 per kilowatt. This reduced initial investment threshold lowers barriers to entry and accelerates deployment potential across diverse markets.
The solid carbon co-product generated through methane pyrolysis introduces a valuable revenue stream that substantially improves the economic equation. High-quality carbon black commands market prices between $1,000-2,500 per ton, potentially offsetting 20-40% of production costs depending on purity levels and market applications. This dual-product model creates economic resilience against hydrogen price volatility.
Integration of methane pyrolysis into existing natural gas infrastructure presents significant transition advantages. The process can leverage established gas distribution networks, reducing the need for new transportation infrastructure investments. This compatibility enables a phased approach to hydrogen economy development, allowing gradual scaling while maintaining energy security through existing systems.
From an energy transition perspective, methane pyrolysis occupies a strategic middle ground between fossil fuel-dependent and fully renewable hydrogen pathways. Its carbon intensity (2-5 kg CO₂e/kg H₂) represents an 85-95% reduction compared to conventional hydrogen production methods, enabling immediate emissions reductions while renewable capacity expands. This characteristic positions pyrolysis as an effective "bridge technology" in decarbonization roadmaps.
Policy frameworks increasingly recognize this transitional value, with several jurisdictions developing tiered incentive structures that acknowledge the emissions reduction benefits of pyrolysis-derived hydrogen. The EU's hydrogen taxonomy and the US Inflation Reduction Act both incorporate provisions that potentially benefit turquoise hydrogen producers, enhancing economic viability through regulatory support mechanisms.
Capital expenditure requirements for methane pyrolysis facilities are estimated at $600-900 per kilowatt of hydrogen production capacity, significantly lower than electrolysis systems which typically range from $1,000-1,500 per kilowatt. This reduced initial investment threshold lowers barriers to entry and accelerates deployment potential across diverse markets.
The solid carbon co-product generated through methane pyrolysis introduces a valuable revenue stream that substantially improves the economic equation. High-quality carbon black commands market prices between $1,000-2,500 per ton, potentially offsetting 20-40% of production costs depending on purity levels and market applications. This dual-product model creates economic resilience against hydrogen price volatility.
Integration of methane pyrolysis into existing natural gas infrastructure presents significant transition advantages. The process can leverage established gas distribution networks, reducing the need for new transportation infrastructure investments. This compatibility enables a phased approach to hydrogen economy development, allowing gradual scaling while maintaining energy security through existing systems.
From an energy transition perspective, methane pyrolysis occupies a strategic middle ground between fossil fuel-dependent and fully renewable hydrogen pathways. Its carbon intensity (2-5 kg CO₂e/kg H₂) represents an 85-95% reduction compared to conventional hydrogen production methods, enabling immediate emissions reductions while renewable capacity expands. This characteristic positions pyrolysis as an effective "bridge technology" in decarbonization roadmaps.
Policy frameworks increasingly recognize this transitional value, with several jurisdictions developing tiered incentive structures that acknowledge the emissions reduction benefits of pyrolysis-derived hydrogen. The EU's hydrogen taxonomy and the US Inflation Reduction Act both incorporate provisions that potentially benefit turquoise hydrogen producers, enhancing economic viability through regulatory support mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!