Structural Materials Evaluation for Methane Pyrolysis.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Materials Background and Objectives
Methane pyrolysis represents a significant technological pathway in the evolution of hydrogen production methods, offering a potentially cleaner alternative to traditional steam methane reforming. The historical development of this technology dates back to the early 20th century, but recent environmental concerns and the push for decarbonization have revitalized interest in this process. The fundamental reaction involves the thermal decomposition of methane (CH₄) into hydrogen (H₂) and solid carbon, without direct CO₂ emissions, positioning it as a promising approach for low-carbon hydrogen production.
The technological evolution of methane pyrolysis has progressed through several distinct phases, from early laboratory demonstrations to more recent pilot-scale implementations. Initial research focused primarily on catalytic approaches, while recent innovations have expanded to include plasma, molten metal, and molten salt methods. Each evolutionary step has addressed specific challenges related to energy efficiency, carbon handling, and reactor design, gradually improving the commercial viability of the technology.
The primary objective of structural materials evaluation for methane pyrolysis is to identify and develop materials capable of withstanding the extreme conditions inherent to the process. These conditions include high temperatures (typically 700-1200°C), potential carbon deposition issues, and in some cases, exposure to corrosive molten media. Material selection represents a critical factor in determining process efficiency, operational lifespan, and ultimately economic feasibility of methane pyrolysis systems.
Current technical goals focus on developing reactor materials that demonstrate exceptional thermal stability, resistance to carbon fouling, and long-term structural integrity. Additionally, materials must maintain these properties while minimizing catalytic degradation and preventing unwanted side reactions. The ideal material solution would balance performance requirements with cost considerations to enable commercial-scale implementation.
The broader technological objective extends beyond material selection to encompass reactor design optimization, scaling methodologies, and integration strategies with existing energy infrastructure. Success in this domain could potentially position methane pyrolysis as a cornerstone technology in the transition toward hydrogen-based energy systems, particularly in regions with abundant natural gas resources and strong decarbonization imperatives.
Recent technological trends indicate growing interest in novel material compositions, including advanced ceramics, specialized metal alloys, and composite structures designed specifically for methane pyrolysis applications. These developments align with the overarching goal of establishing methane pyrolysis as a commercially viable, environmentally sustainable approach to hydrogen production within the evolving clean energy landscape.
The technological evolution of methane pyrolysis has progressed through several distinct phases, from early laboratory demonstrations to more recent pilot-scale implementations. Initial research focused primarily on catalytic approaches, while recent innovations have expanded to include plasma, molten metal, and molten salt methods. Each evolutionary step has addressed specific challenges related to energy efficiency, carbon handling, and reactor design, gradually improving the commercial viability of the technology.
The primary objective of structural materials evaluation for methane pyrolysis is to identify and develop materials capable of withstanding the extreme conditions inherent to the process. These conditions include high temperatures (typically 700-1200°C), potential carbon deposition issues, and in some cases, exposure to corrosive molten media. Material selection represents a critical factor in determining process efficiency, operational lifespan, and ultimately economic feasibility of methane pyrolysis systems.
Current technical goals focus on developing reactor materials that demonstrate exceptional thermal stability, resistance to carbon fouling, and long-term structural integrity. Additionally, materials must maintain these properties while minimizing catalytic degradation and preventing unwanted side reactions. The ideal material solution would balance performance requirements with cost considerations to enable commercial-scale implementation.
The broader technological objective extends beyond material selection to encompass reactor design optimization, scaling methodologies, and integration strategies with existing energy infrastructure. Success in this domain could potentially position methane pyrolysis as a cornerstone technology in the transition toward hydrogen-based energy systems, particularly in regions with abundant natural gas resources and strong decarbonization imperatives.
Recent technological trends indicate growing interest in novel material compositions, including advanced ceramics, specialized metal alloys, and composite structures designed specifically for methane pyrolysis applications. These developments align with the overarching goal of establishing methane pyrolysis as a commercially viable, environmentally sustainable approach to hydrogen production within the evolving clean energy landscape.
Market Analysis for Hydrogen Production Technologies
The global hydrogen production market is experiencing significant growth, driven by increasing demand for clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $130 billion, the market is projected to reach $220 billion by 2030, with a compound annual growth rate of 6.8%. This growth trajectory is supported by substantial government investments, with over $70 billion committed globally to hydrogen development initiatives.
Traditional hydrogen production methods dominate the current market landscape, with steam methane reforming (SMR) accounting for roughly 76% of global hydrogen production. This process, while cost-effective at $1-2 per kilogram of hydrogen, generates significant carbon emissions—approximately 9-12 kg CO2 per kg H2 produced. Electrolysis represents about 4% of production but is gaining momentum due to its zero-emission potential when powered by renewable energy sources.
Methane pyrolysis occupies a promising middle ground in the hydrogen production spectrum. This emerging technology produces "turquoise hydrogen" by decomposing methane into hydrogen and solid carbon, offering a significantly lower carbon footprint than SMR while maintaining competitive economics. The market potential for methane pyrolysis is substantial, with projections suggesting it could capture 15-20% of the hydrogen production market by 2035.
Regional market dynamics show varied adoption patterns. Europe leads in clean hydrogen initiatives with ambitious targets and regulatory frameworks supporting hydrogen infrastructure development. The European Hydrogen Strategy aims for 40 GW of electrolyzer capacity by 2030. North America focuses on both blue hydrogen (SMR with carbon capture) and innovative technologies like methane pyrolysis, supported by initiatives such as the U.S. Department of Energy's Hydrogen Shot program targeting $1/kg hydrogen production costs.
Asia-Pacific represents the fastest-growing market for hydrogen technologies, with China, Japan, and South Korea making significant investments. China alone plans to have 1 million hydrogen-powered vehicles on its roads by 2030, creating substantial demand for production technologies.
End-use market segmentation reveals diverse applications driving demand. Industrial processes currently consume approximately 70% of produced hydrogen, primarily in ammonia production and petroleum refining. Transportation applications are expected to grow at the highest rate, with a projected 25% CAGR through 2030, as fuel cell vehicles gain market acceptance. Energy storage applications are emerging as grid-scale solutions for managing intermittent renewable energy sources.
Traditional hydrogen production methods dominate the current market landscape, with steam methane reforming (SMR) accounting for roughly 76% of global hydrogen production. This process, while cost-effective at $1-2 per kilogram of hydrogen, generates significant carbon emissions—approximately 9-12 kg CO2 per kg H2 produced. Electrolysis represents about 4% of production but is gaining momentum due to its zero-emission potential when powered by renewable energy sources.
Methane pyrolysis occupies a promising middle ground in the hydrogen production spectrum. This emerging technology produces "turquoise hydrogen" by decomposing methane into hydrogen and solid carbon, offering a significantly lower carbon footprint than SMR while maintaining competitive economics. The market potential for methane pyrolysis is substantial, with projections suggesting it could capture 15-20% of the hydrogen production market by 2035.
Regional market dynamics show varied adoption patterns. Europe leads in clean hydrogen initiatives with ambitious targets and regulatory frameworks supporting hydrogen infrastructure development. The European Hydrogen Strategy aims for 40 GW of electrolyzer capacity by 2030. North America focuses on both blue hydrogen (SMR with carbon capture) and innovative technologies like methane pyrolysis, supported by initiatives such as the U.S. Department of Energy's Hydrogen Shot program targeting $1/kg hydrogen production costs.
Asia-Pacific represents the fastest-growing market for hydrogen technologies, with China, Japan, and South Korea making significant investments. China alone plans to have 1 million hydrogen-powered vehicles on its roads by 2030, creating substantial demand for production technologies.
End-use market segmentation reveals diverse applications driving demand. Industrial processes currently consume approximately 70% of produced hydrogen, primarily in ammonia production and petroleum refining. Transportation applications are expected to grow at the highest rate, with a projected 25% CAGR through 2030, as fuel cell vehicles gain market acceptance. Energy storage applications are emerging as grid-scale solutions for managing intermittent renewable energy sources.
Current Materials Challenges in Methane Pyrolysis
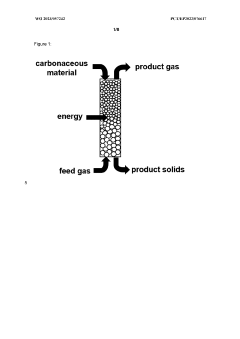
Methane pyrolysis represents a promising pathway for hydrogen production with significantly reduced carbon emissions compared to traditional steam methane reforming. However, the extreme operating conditions required for this process present substantial challenges for structural materials. Current reactor designs typically operate at temperatures between 700-1200°C, creating a highly demanding environment for materials integrity.
The primary challenge facing structural materials in methane pyrolysis is thermal stability. Materials must maintain mechanical strength and dimensional stability under prolonged exposure to high temperatures. Conventional steel alloys begin to lose their structural integrity above 600°C, making them unsuitable for many pyrolysis reactor designs. Nickel-based superalloys offer improved performance but still face limitations at the upper temperature ranges required for efficient pyrolysis.
Carbon deposition presents another significant materials challenge. The pyrolysis process inherently produces solid carbon, which tends to accumulate on reactor surfaces. This carbon deposition can lead to several detrimental effects: catalyst deactivation, flow restriction, and accelerated material degradation through carburization. Materials that resist carbon adhesion or facilitate easy carbon removal are urgently needed but remain underdeveloped.
Hydrogen embrittlement constitutes a third critical challenge. The high-temperature hydrogen-rich environment promotes hydrogen diffusion into metallic components, leading to reduced ductility and premature failure through crack formation and propagation. This phenomenon is particularly problematic for high-strength alloys commonly used in pressure-containing components.
Thermal cycling resistance represents another significant hurdle. Industrial-scale methane pyrolysis operations typically undergo multiple heating and cooling cycles during normal operation and maintenance. These thermal cycles induce mechanical stresses due to differential thermal expansion, leading to fatigue and potential failure. Current materials struggle to maintain performance under these cyclic conditions over extended operational periods.
Corrosion resistance in methane pyrolysis environments remains problematic. While the process environment is less oxidizing than combustion systems, trace impurities in the feedstock methane can lead to various corrosion mechanisms. Sulfur compounds, in particular, can cause accelerated degradation of many high-temperature alloys through sulfidation attacks at grain boundaries.
Cost-effectiveness presents a final but crucial challenge. Advanced ceramic materials and refractory metals offer superior high-temperature performance but at significantly higher costs than conventional alloys. This economic barrier has limited widespread adoption of potentially superior materials solutions, creating a persistent trade-off between performance and economic viability in reactor design.
The primary challenge facing structural materials in methane pyrolysis is thermal stability. Materials must maintain mechanical strength and dimensional stability under prolonged exposure to high temperatures. Conventional steel alloys begin to lose their structural integrity above 600°C, making them unsuitable for many pyrolysis reactor designs. Nickel-based superalloys offer improved performance but still face limitations at the upper temperature ranges required for efficient pyrolysis.
Carbon deposition presents another significant materials challenge. The pyrolysis process inherently produces solid carbon, which tends to accumulate on reactor surfaces. This carbon deposition can lead to several detrimental effects: catalyst deactivation, flow restriction, and accelerated material degradation through carburization. Materials that resist carbon adhesion or facilitate easy carbon removal are urgently needed but remain underdeveloped.
Hydrogen embrittlement constitutes a third critical challenge. The high-temperature hydrogen-rich environment promotes hydrogen diffusion into metallic components, leading to reduced ductility and premature failure through crack formation and propagation. This phenomenon is particularly problematic for high-strength alloys commonly used in pressure-containing components.
Thermal cycling resistance represents another significant hurdle. Industrial-scale methane pyrolysis operations typically undergo multiple heating and cooling cycles during normal operation and maintenance. These thermal cycles induce mechanical stresses due to differential thermal expansion, leading to fatigue and potential failure. Current materials struggle to maintain performance under these cyclic conditions over extended operational periods.
Corrosion resistance in methane pyrolysis environments remains problematic. While the process environment is less oxidizing than combustion systems, trace impurities in the feedstock methane can lead to various corrosion mechanisms. Sulfur compounds, in particular, can cause accelerated degradation of many high-temperature alloys through sulfidation attacks at grain boundaries.
Cost-effectiveness presents a final but crucial challenge. Advanced ceramic materials and refractory metals offer superior high-temperature performance but at significantly higher costs than conventional alloys. This economic barrier has limited widespread adoption of potentially superior materials solutions, creating a persistent trade-off between performance and economic viability in reactor design.
Existing Material Solutions for Methane Decomposition
01 Heat-resistant composite materials
Advanced composite materials designed specifically for high-temperature applications that maintain structural integrity under extreme heat conditions. These materials often combine ceramic matrices with reinforcing fibers or incorporate specialized polymers with enhanced thermal stability. The composites are engineered to resist thermal degradation, prevent deformation, and maintain mechanical properties at elevated temperatures, making them suitable for aerospace, industrial, and automotive applications where heat resistance is critical.- Heat-resistant composite materials: Advanced composite materials designed specifically for high-temperature applications, combining different substances to create structures with superior heat resistance. These composites often incorporate ceramic components, metal alloys, or specialized polymers that maintain structural integrity under extreme thermal conditions. The formulations are engineered to withstand thermal cycling, prevent degradation, and maintain mechanical properties at elevated temperatures.
- Thermal barrier coatings and surface treatments: Specialized coatings and surface treatments that enhance the heat resistance of structural materials. These treatments create protective layers that insulate the base material from thermal damage, prevent oxidation, and extend service life in high-temperature environments. The coatings may include ceramic layers, reflective materials, or multi-layer systems designed to manage heat transfer and protect the underlying structure.
- Fire-resistant construction materials: Materials specifically formulated for building applications that require enhanced fire resistance and structural integrity during exposure to flames or high temperatures. These materials incorporate flame retardants, intumescent compounds, or naturally fire-resistant substances that prevent or delay combustion while maintaining load-bearing capabilities. The formulations are designed to meet building safety codes and protect occupants by extending evacuation time during fire events.
- Durability enhancement through material reinforcement: Methods to improve the durability of structural materials through reinforcement techniques such as fiber inclusion, particle dispersion, or structural modifications. These approaches enhance resistance to mechanical stress, environmental factors, and repeated loading cycles. The reinforcements distribute forces throughout the material, prevent crack propagation, and maintain structural integrity over extended periods of use in challenging conditions.
- Temperature-adaptive structural systems: Innovative structural systems that can adapt to temperature changes while maintaining performance and safety. These include materials with shape memory properties, thermal expansion compensation mechanisms, or dynamic response capabilities. Such systems can adjust their properties or configuration in response to temperature fluctuations, preventing damage from thermal stress and ensuring continued functionality across varying environmental conditions.
02 Durability enhancement through surface treatments
Various surface treatment technologies that improve the durability of structural materials against environmental factors and mechanical stress. These treatments include specialized coatings, surface hardening processes, and chemical modifications that create protective barriers against corrosion, oxidation, and wear. The enhanced surface properties extend the service life of materials in harsh environments while maintaining their core structural characteristics and heat resistance properties.Expand Specific Solutions03 Thermal barrier systems for structural materials
Engineered systems designed to provide thermal insulation and protection to structural components exposed to high temperatures. These systems typically consist of multiple layers of materials with low thermal conductivity, reflective properties, or phase-change capabilities that absorb or redirect heat. The barrier systems prevent heat transfer to underlying structural elements, thereby preserving their mechanical properties and extending their operational lifespan in high-temperature environments.Expand Specific Solutions04 Novel alloy compositions for extreme environments
Innovative metal alloy formulations specifically developed to withstand extreme environmental conditions including high temperatures, corrosive atmospheres, and mechanical stress. These alloys incorporate precise combinations of elements that create microstructures resistant to creep, oxidation, and thermal fatigue. The compositions are tailored to maintain dimensional stability and mechanical strength at elevated temperatures while offering improved processability compared to traditional heat-resistant materials.Expand Specific Solutions05 Testing and evaluation methods for heat resistance
Specialized techniques and methodologies for assessing the heat resistance and durability of structural materials under simulated extreme conditions. These methods include accelerated aging tests, thermal cycling protocols, and advanced analytical techniques that predict long-term performance based on short-term testing data. The evaluation approaches help identify failure mechanisms, establish performance limits, and validate material selections for applications requiring exceptional thermal stability and structural integrity.Expand Specific Solutions
Leading Companies and Research Institutions in Pyrolysis Technology
Methane pyrolysis for hydrogen production is in an early growth phase, with a market expected to expand significantly as clean hydrogen demand rises. The technology is approaching commercial readiness, with varying maturity levels across key players. Shell, BASF, and Molten Industries are leading commercial development with pilot-scale demonstrations, while academic institutions like Zhejiang University and Dalian Institute of Chemical Physics are advancing fundamental research on catalyst materials and reactor designs. Energy companies including China Petroleum & Chemical Corp., Saudi Basic Industries, and ExxonMobil are investing in structural materials research to overcome high-temperature challenges. The competitive landscape features both established petrochemical giants and specialized startups, indicating growing industry recognition of methane pyrolysis as a promising decarbonization pathway.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive materials evaluation system for methane pyrolysis applications focusing on cost-effective solutions suitable for large-scale implementation. Their approach centers on modified stainless steel alloys with specialized surface treatments to enhance carbon resistance while maintaining mechanical properties at elevated temperatures. Sinopec's material development program has created a proprietary nickel-chromium-molybdenum alloy that demonstrates superior resistance to carburization and metal dusting, common failure mechanisms in methane pyrolysis environments. Their evaluation methodology incorporates both laboratory testing and pilot-scale validation, with materials subjected to actual process conditions for extended periods. Sinopec has also pioneered the use of advanced ceramic coatings applied through plasma spray techniques that can be retrofitted to existing equipment, enabling gradual technology adoption without complete system replacement. Their materials selection framework incorporates lifecycle cost analysis, balancing initial material costs against operational longevity and maintenance requirements.
Strengths: Cost-effective material solutions suitable for large-scale deployment; extensive testing facilities and capabilities; strong integration with actual operational environments. Weaknesses: Some materials show performance limitations at the highest operating temperatures (>1100°C); less experience with molten metal reactor environments; potential challenges with intellectual property protection for certain innovations.
Shell Oil Co.
Technical Solution: Shell has developed advanced reactor designs for methane pyrolysis utilizing specialized high-temperature resistant materials. Their technology employs molten metal reactors, particularly using nickel-based alloys and ceramic materials that can withstand temperatures exceeding 1000°C while maintaining structural integrity. Shell's approach incorporates a dual-layer protection system where the primary reactor vessel is constructed from high-chromium stainless steel with an inner lining of specialized ceramics that resist carbon deposition. Their materials evaluation program includes comprehensive testing under cyclic thermal conditions and exposure to carbon-rich environments to simulate long-term operational conditions. Shell has also pioneered the use of silicon carbide composites for critical components, demonstrating a 40% improvement in thermal shock resistance compared to conventional materials used in pyrolysis applications.
Strengths: Superior resistance to carbon fouling and thermal cycling; established global supply chain for specialized materials; extensive operational data from pilot plants. Weaknesses: Higher initial capital costs compared to conventional materials; requires specialized fabrication techniques; potential challenges in scaling up production of advanced ceramic components.
Critical Patents and Innovations in Pyrolysis Reactor Materials
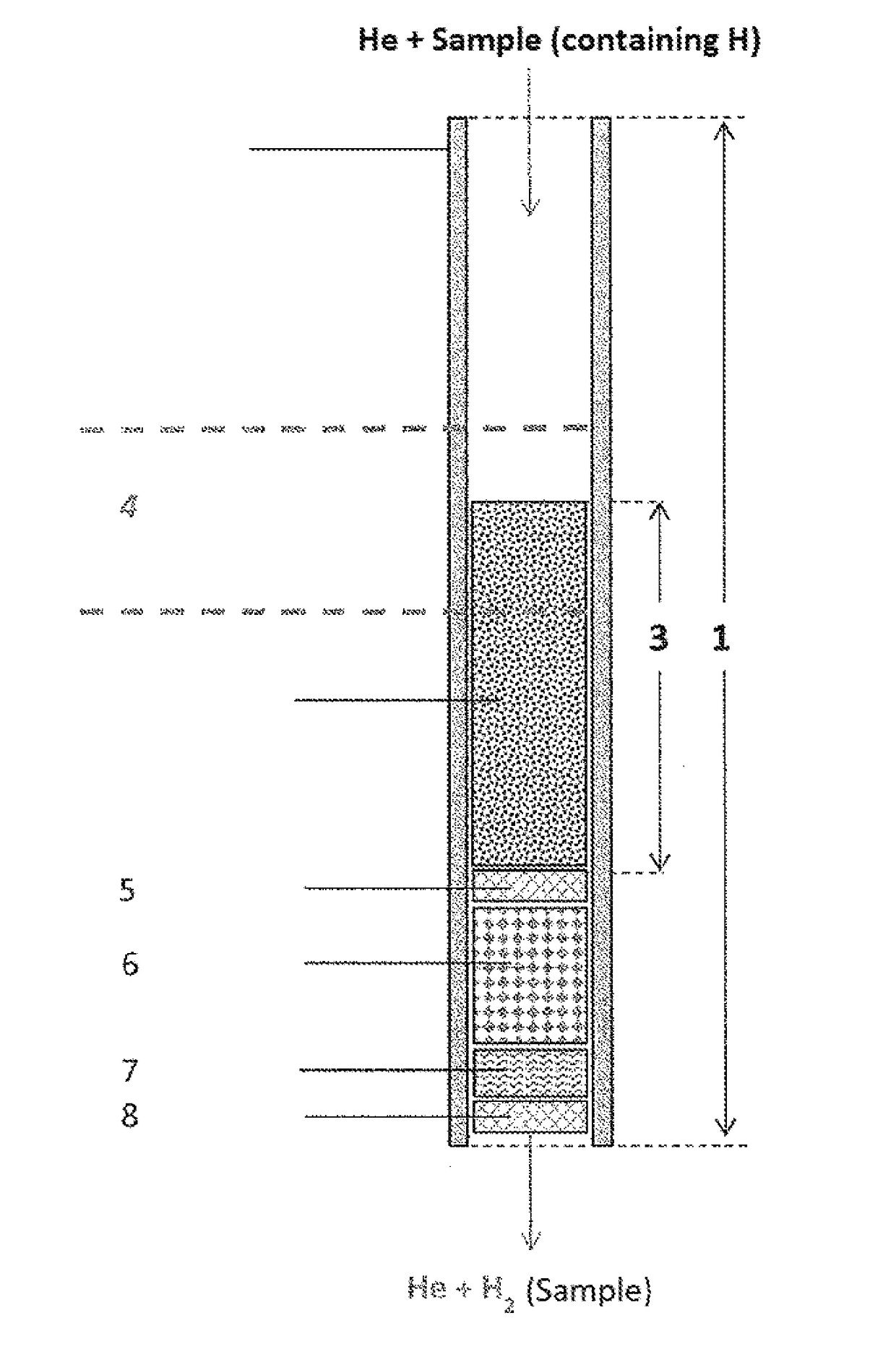
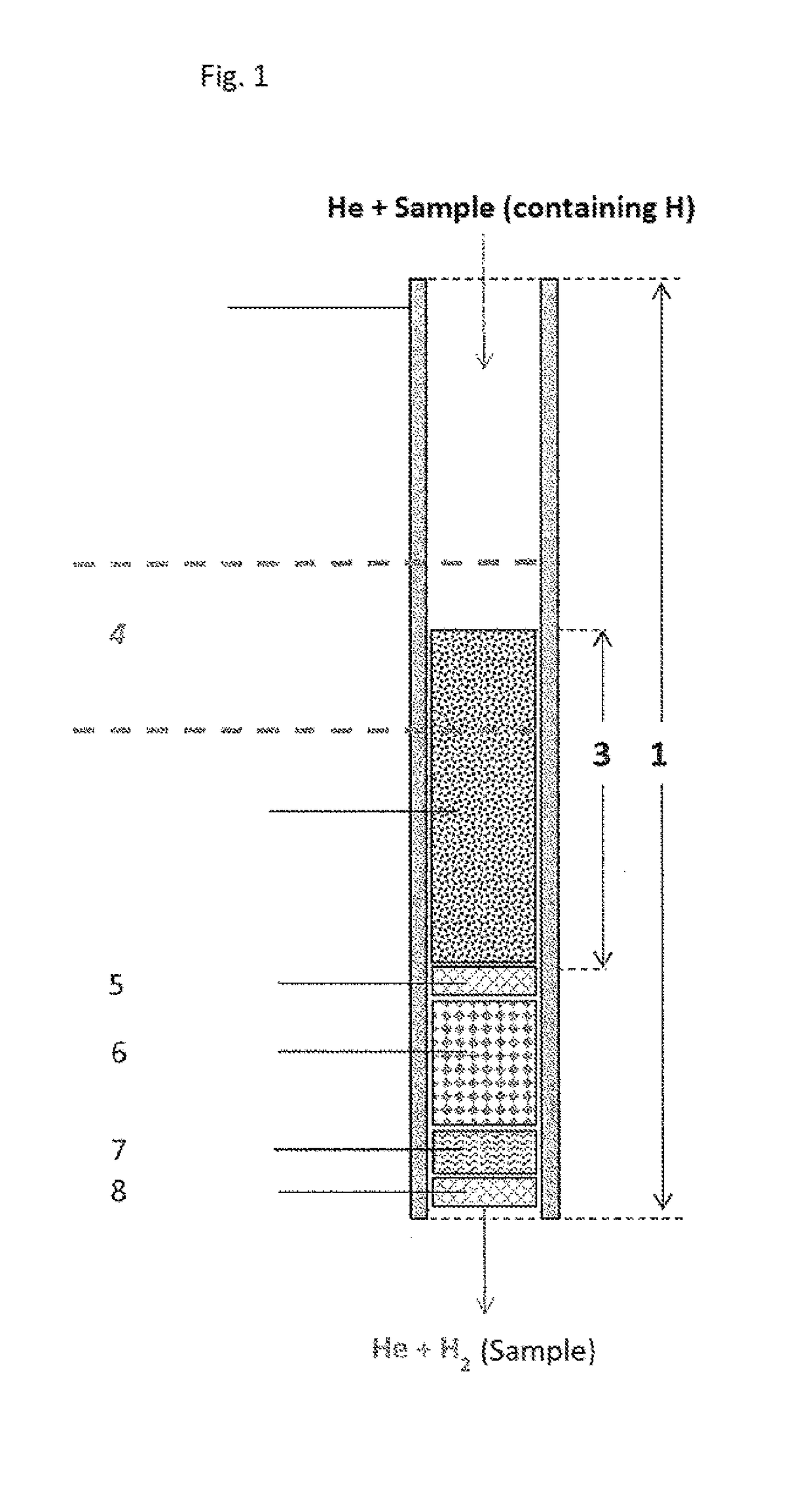
Use of a reactor, methods, and device for quantitatively obtaining molecular hydrogen from substances
PatentActiveUS20180021746A1
Innovation
- A reactor with a chromium-containing material is used for pyrolysis at temperatures above 1100°C, ensuring nearly 100% recovery of molecular hydrogen by irreversibly removing reactive elements, thereby preventing byproduct formation and isotope fractionation.
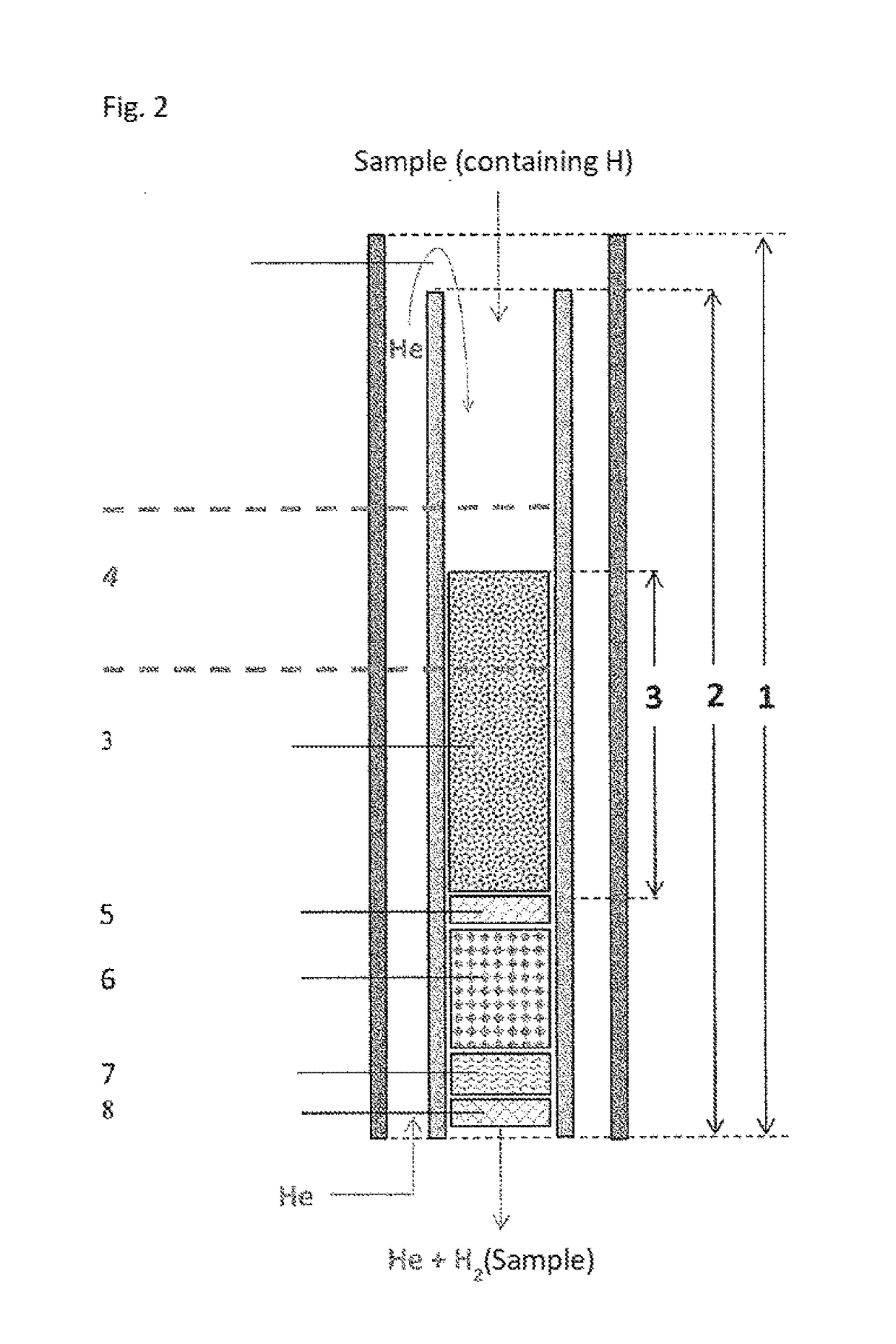
Use of carbonaceous carrier material in bed reactors
PatentWO2023057242A1
Innovation
- The use of macro-structured carbonaceous materials with specific porosity, carbon content, and minimal impurities as carrier materials in bed reactors, which prevents the accumulation of inorganic compounds and allows for uniform carbon deposition, enhancing mechanical stability and reducing soot formation.
Environmental Impact Assessment of Methane Pyrolysis Materials
The environmental impact assessment of methane pyrolysis materials reveals significant advantages over traditional methane utilization methods. Methane pyrolysis produces solid carbon instead of CO2, potentially reducing greenhouse gas emissions by up to 90% compared to steam methane reforming when using renewable energy sources. This process generates hydrogen with substantially lower carbon intensity, estimated at 0.91 kg CO2e/kg H2 versus 8-12 kg CO2e/kg H2 for conventional methods.
Material selection for pyrolysis reactors directly influences environmental performance. Molten metal reactors using nickel, iron, or tin demonstrate excellent methane conversion efficiency but require careful consideration of metal mining impacts and potential contamination risks. Studies indicate that nickel-based systems, while efficient, have higher upstream environmental footprints than iron-based alternatives due to more energy-intensive extraction processes.
Ceramic and carbon-based materials used in thermal decomposition reactors present different environmental considerations. Silicon carbide and alumina ceramics offer exceptional thermal stability with minimal degradation over thousands of operational hours, reducing replacement frequency and associated environmental impacts. However, their production involves high-temperature sintering processes that consume significant energy.
Life cycle assessments of various reactor materials indicate that carbon-based materials may offer the lowest cumulative environmental impact when considering manufacturing, operational lifespan, and end-of-life disposal. Carbon fiber reinforced composites, despite higher initial production emissions, demonstrate superior durability in methane pyrolysis conditions, potentially offsetting their environmental footprint through extended service life.
Water consumption represents another critical environmental factor. Molten metal systems typically require more sophisticated cooling systems than ceramic alternatives, potentially increasing water usage by 15-25%. This consideration becomes particularly relevant in water-stressed regions where industrial water consumption faces increasing scrutiny.
Material recyclability and circular economy potential vary significantly across different reactor designs. Metallic catalysts can be recovered and reprocessed with recovery rates exceeding 90% for precious metals, while ceramic materials present greater recycling challenges due to thermal degradation and carbon deposition. Recent innovations in material recovery techniques suggest potential improvements in closing material loops for all reactor types.
Land use impacts remain relatively minimal compared to other hydrogen production methods, with methane pyrolysis facilities requiring approximately 50-70% less land area than equivalent electrolysis installations when accounting for renewable energy generation requirements.
Material selection for pyrolysis reactors directly influences environmental performance. Molten metal reactors using nickel, iron, or tin demonstrate excellent methane conversion efficiency but require careful consideration of metal mining impacts and potential contamination risks. Studies indicate that nickel-based systems, while efficient, have higher upstream environmental footprints than iron-based alternatives due to more energy-intensive extraction processes.
Ceramic and carbon-based materials used in thermal decomposition reactors present different environmental considerations. Silicon carbide and alumina ceramics offer exceptional thermal stability with minimal degradation over thousands of operational hours, reducing replacement frequency and associated environmental impacts. However, their production involves high-temperature sintering processes that consume significant energy.
Life cycle assessments of various reactor materials indicate that carbon-based materials may offer the lowest cumulative environmental impact when considering manufacturing, operational lifespan, and end-of-life disposal. Carbon fiber reinforced composites, despite higher initial production emissions, demonstrate superior durability in methane pyrolysis conditions, potentially offsetting their environmental footprint through extended service life.
Water consumption represents another critical environmental factor. Molten metal systems typically require more sophisticated cooling systems than ceramic alternatives, potentially increasing water usage by 15-25%. This consideration becomes particularly relevant in water-stressed regions where industrial water consumption faces increasing scrutiny.
Material recyclability and circular economy potential vary significantly across different reactor designs. Metallic catalysts can be recovered and reprocessed with recovery rates exceeding 90% for precious metals, while ceramic materials present greater recycling challenges due to thermal degradation and carbon deposition. Recent innovations in material recovery techniques suggest potential improvements in closing material loops for all reactor types.
Land use impacts remain relatively minimal compared to other hydrogen production methods, with methane pyrolysis facilities requiring approximately 50-70% less land area than equivalent electrolysis installations when accounting for renewable energy generation requirements.
Techno-Economic Analysis of Material Selection
The techno-economic analysis of material selection for methane pyrolysis reveals significant cost implications across different structural materials. Initial capital expenditure varies substantially, with high-temperature alloys like Inconel and Hastelloy commanding premium prices of $50-100/kg compared to standard stainless steels at $10-20/kg. However, this analysis must extend beyond acquisition costs to consider total lifecycle economics.
Operational longevity presents a critical economic factor, as materials with superior thermal stability and carbon resistance demonstrate extended service life in pyrolysis environments. Nickel-based superalloys, despite higher upfront costs, may offer 3-5 times longer operational periods before replacement compared to conventional alternatives, substantially reducing long-term capital requirements and minimizing costly production interruptions.
Maintenance economics further differentiate material options. Ceramics and ceramic-metal composites typically require less frequent maintenance interventions, with estimated maintenance costs 30-40% lower than metallic alternatives over a 10-year operational period. This advantage stems from their superior resistance to carbon deposition and thermal cycling damage, two primary degradation mechanisms in methane pyrolysis systems.
Energy efficiency considerations reveal that material thermal conductivity properties directly impact operational economics. Materials with optimized thermal profiles can reduce energy consumption by 15-25%, representing significant operational savings in large-scale implementations. Thermal management characteristics must be evaluated against process temperature requirements, which typically range from 700-1200°C depending on the specific pyrolysis approach.
Scalability economics present another crucial dimension, as certain advanced materials face manufacturing constraints that increase costs exponentially at industrial scales. Silicon carbide components, for instance, demonstrate favorable performance characteristics but incur 200-300% cost premiums when scaled beyond laboratory dimensions, necessitating careful economic modeling for commercial deployment scenarios.
Risk assessment calculations further complicate material selection economics. Materials with limited operational history in methane pyrolysis environments introduce uncertainty premiums that must be quantified. Statistical modeling suggests contingency allocations of 15-25% for novel material implementations to account for potential unforeseen failure modes and associated production losses.
The comprehensive techno-economic analysis indicates that optimal material selection strategies often involve hybrid approaches, utilizing premium materials only in critical high-stress zones while employing more economical alternatives elsewhere, potentially reducing overall system costs by 30-40% compared to uniform material implementation strategies.
Operational longevity presents a critical economic factor, as materials with superior thermal stability and carbon resistance demonstrate extended service life in pyrolysis environments. Nickel-based superalloys, despite higher upfront costs, may offer 3-5 times longer operational periods before replacement compared to conventional alternatives, substantially reducing long-term capital requirements and minimizing costly production interruptions.
Maintenance economics further differentiate material options. Ceramics and ceramic-metal composites typically require less frequent maintenance interventions, with estimated maintenance costs 30-40% lower than metallic alternatives over a 10-year operational period. This advantage stems from their superior resistance to carbon deposition and thermal cycling damage, two primary degradation mechanisms in methane pyrolysis systems.
Energy efficiency considerations reveal that material thermal conductivity properties directly impact operational economics. Materials with optimized thermal profiles can reduce energy consumption by 15-25%, representing significant operational savings in large-scale implementations. Thermal management characteristics must be evaluated against process temperature requirements, which typically range from 700-1200°C depending on the specific pyrolysis approach.
Scalability economics present another crucial dimension, as certain advanced materials face manufacturing constraints that increase costs exponentially at industrial scales. Silicon carbide components, for instance, demonstrate favorable performance characteristics but incur 200-300% cost premiums when scaled beyond laboratory dimensions, necessitating careful economic modeling for commercial deployment scenarios.
Risk assessment calculations further complicate material selection economics. Materials with limited operational history in methane pyrolysis environments introduce uncertainty premiums that must be quantified. Statistical modeling suggests contingency allocations of 15-25% for novel material implementations to account for potential unforeseen failure modes and associated production losses.
The comprehensive techno-economic analysis indicates that optimal material selection strategies often involve hybrid approaches, utilizing premium materials only in critical high-stress zones while employing more economical alternatives elsewhere, potentially reducing overall system costs by 30-40% compared to uniform material implementation strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!