Nanostructured Catalysts for Methane Pyrolysis.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Catalysis Background and Objectives
Methane pyrolysis represents a promising pathway for hydrogen production with significantly reduced carbon emissions compared to traditional steam methane reforming processes. The evolution of this technology dates back to the early 20th century, but recent advancements in catalyst design, particularly nanostructured catalysts, have revitalized interest in this field. The fundamental reaction involves the thermal decomposition of methane (CH₄) into hydrogen (H₂) and solid carbon, offering a potentially carbon-neutral or even carbon-negative approach to hydrogen production when the solid carbon is sequestered.
The technological trajectory of methane pyrolysis catalysis has seen significant acceleration in the past decade, driven by the global push for decarbonization and hydrogen economy development. Early catalysts primarily consisted of bulk metals like nickel and iron, which suffered from rapid deactivation due to carbon deposition. The introduction of nanostructured catalysts has marked a paradigm shift, enabling enhanced catalytic activity, improved stability, and more efficient carbon management.
Current research focuses on developing novel nanostructured catalysts that can operate at lower temperatures while maintaining high conversion rates and selectivity. These catalysts typically incorporate transition metals (Ni, Fe, Co) supported on various materials including carbon nanotubes, metal oxides, and zeolites. The nano-architecture of these catalysts provides increased surface area, controlled porosity, and tailored active sites that significantly enhance catalytic performance.
The primary technical objectives in this field include reducing the activation energy barrier for methane decomposition, preventing catalyst deactivation through innovative carbon management strategies, and designing scalable catalyst systems suitable for industrial implementation. Additionally, there is growing interest in developing bifunctional catalysts that can simultaneously facilitate methane pyrolysis and valorize the produced carbon into high-value materials.
From an environmental perspective, methane pyrolysis using nanostructured catalysts aims to establish a sustainable hydrogen production pathway with minimal carbon footprint. This aligns with global climate goals and offers a transitional technology that leverages existing natural gas infrastructure while moving toward renewable energy systems.
The interdisciplinary nature of this field brings together expertise from materials science, chemical engineering, nanotechnology, and computational modeling. Recent breakthroughs in in-situ characterization techniques and theoretical understanding of reaction mechanisms have accelerated catalyst development, setting the stage for potential commercial applications within the next decade.
The ultimate goal of research in nanostructured catalysts for methane pyrolysis is to develop economically viable, environmentally sustainable, and technically robust systems that can be integrated into the emerging hydrogen economy, providing a cleaner alternative to conventional hydrogen production methods while contributing to global carbon reduction efforts.
The technological trajectory of methane pyrolysis catalysis has seen significant acceleration in the past decade, driven by the global push for decarbonization and hydrogen economy development. Early catalysts primarily consisted of bulk metals like nickel and iron, which suffered from rapid deactivation due to carbon deposition. The introduction of nanostructured catalysts has marked a paradigm shift, enabling enhanced catalytic activity, improved stability, and more efficient carbon management.
Current research focuses on developing novel nanostructured catalysts that can operate at lower temperatures while maintaining high conversion rates and selectivity. These catalysts typically incorporate transition metals (Ni, Fe, Co) supported on various materials including carbon nanotubes, metal oxides, and zeolites. The nano-architecture of these catalysts provides increased surface area, controlled porosity, and tailored active sites that significantly enhance catalytic performance.
The primary technical objectives in this field include reducing the activation energy barrier for methane decomposition, preventing catalyst deactivation through innovative carbon management strategies, and designing scalable catalyst systems suitable for industrial implementation. Additionally, there is growing interest in developing bifunctional catalysts that can simultaneously facilitate methane pyrolysis and valorize the produced carbon into high-value materials.
From an environmental perspective, methane pyrolysis using nanostructured catalysts aims to establish a sustainable hydrogen production pathway with minimal carbon footprint. This aligns with global climate goals and offers a transitional technology that leverages existing natural gas infrastructure while moving toward renewable energy systems.
The interdisciplinary nature of this field brings together expertise from materials science, chemical engineering, nanotechnology, and computational modeling. Recent breakthroughs in in-situ characterization techniques and theoretical understanding of reaction mechanisms have accelerated catalyst development, setting the stage for potential commercial applications within the next decade.
The ultimate goal of research in nanostructured catalysts for methane pyrolysis is to develop economically viable, environmentally sustainable, and technically robust systems that can be integrated into the emerging hydrogen economy, providing a cleaner alternative to conventional hydrogen production methods while contributing to global carbon reduction efforts.
Market Analysis for Hydrogen Production via Methane Pyrolysis
The global hydrogen market is experiencing significant growth, with demand projected to reach 94 million tons by 2030, representing a substantial increase from current levels. This growth is primarily driven by the transition toward cleaner energy sources and the implementation of carbon reduction policies worldwide. Hydrogen produced via methane pyrolysis, often referred to as "turquoise hydrogen," occupies a strategic position between gray hydrogen (produced from natural gas with CO2 emissions) and green hydrogen (produced via electrolysis using renewable energy).
Methane pyrolysis offers a compelling value proposition in the hydrogen production landscape due to its significantly lower carbon footprint compared to traditional steam methane reforming methods. The process produces solid carbon instead of CO2, eliminating the need for carbon capture and storage infrastructure. This advantage positions methane pyrolysis as an attractive intermediate solution while green hydrogen production scales up and becomes more economically viable.
Market analysis indicates that the industrial sector represents the largest potential customer base for hydrogen produced via methane pyrolysis. Refineries, ammonia production, and methanol synthesis collectively account for approximately 70% of current hydrogen consumption. These industries are under increasing pressure to decarbonize their operations, creating a substantial market opportunity for lower-carbon hydrogen production methods.
The transportation sector presents another significant growth opportunity, particularly in regions with established natural gas infrastructure. Fuel cell vehicles, especially in heavy-duty transport applications where battery electrification faces challenges, represent a promising market segment. Several major automotive manufacturers have announced plans to expand their hydrogen fuel cell vehicle offerings, potentially creating additional demand.
Regional market assessment reveals varying levels of potential for methane pyrolysis adoption. Europe leads in policy support for hydrogen technologies through initiatives like the European Hydrogen Strategy, which aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030. North America benefits from abundant natural gas resources and existing infrastructure, making it particularly suitable for methane pyrolysis implementation.
Economic analysis shows that hydrogen produced via methane pyrolysis currently costs between $1.50-2.50/kg, positioning it competitively between gray hydrogen ($1-1.50/kg) and green hydrogen ($3-6/kg). This cost advantage is expected to persist in the medium term, though the gap will likely narrow as green hydrogen production becomes more efficient and benefits from economies of scale.
Market forecasts suggest that methane pyrolysis could capture 15-25% of the hydrogen production market by 2035, representing a significant commercial opportunity. This growth depends on continued technological advancement in catalyst efficiency, reactor design, and carbon utilization pathways to maximize the economic and environmental benefits of the process.
Methane pyrolysis offers a compelling value proposition in the hydrogen production landscape due to its significantly lower carbon footprint compared to traditional steam methane reforming methods. The process produces solid carbon instead of CO2, eliminating the need for carbon capture and storage infrastructure. This advantage positions methane pyrolysis as an attractive intermediate solution while green hydrogen production scales up and becomes more economically viable.
Market analysis indicates that the industrial sector represents the largest potential customer base for hydrogen produced via methane pyrolysis. Refineries, ammonia production, and methanol synthesis collectively account for approximately 70% of current hydrogen consumption. These industries are under increasing pressure to decarbonize their operations, creating a substantial market opportunity for lower-carbon hydrogen production methods.
The transportation sector presents another significant growth opportunity, particularly in regions with established natural gas infrastructure. Fuel cell vehicles, especially in heavy-duty transport applications where battery electrification faces challenges, represent a promising market segment. Several major automotive manufacturers have announced plans to expand their hydrogen fuel cell vehicle offerings, potentially creating additional demand.
Regional market assessment reveals varying levels of potential for methane pyrolysis adoption. Europe leads in policy support for hydrogen technologies through initiatives like the European Hydrogen Strategy, which aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030. North America benefits from abundant natural gas resources and existing infrastructure, making it particularly suitable for methane pyrolysis implementation.
Economic analysis shows that hydrogen produced via methane pyrolysis currently costs between $1.50-2.50/kg, positioning it competitively between gray hydrogen ($1-1.50/kg) and green hydrogen ($3-6/kg). This cost advantage is expected to persist in the medium term, though the gap will likely narrow as green hydrogen production becomes more efficient and benefits from economies of scale.
Market forecasts suggest that methane pyrolysis could capture 15-25% of the hydrogen production market by 2035, representing a significant commercial opportunity. This growth depends on continued technological advancement in catalyst efficiency, reactor design, and carbon utilization pathways to maximize the economic and environmental benefits of the process.
Current Challenges in Nanostructured Catalyst Development
Despite significant advancements in nanostructured catalysts for methane pyrolysis, several critical challenges continue to impede widespread industrial implementation. Catalyst deactivation remains one of the most persistent issues, primarily caused by carbon deposition (coking) on active sites during the pyrolysis process. This carbon accumulation progressively blocks catalytic surfaces, reducing reaction efficiency and necessitating frequent regeneration cycles that compromise economic viability in continuous operations.
Thermal stability presents another significant hurdle, particularly as methane pyrolysis typically requires temperatures exceeding 700°C. Many promising nanomaterials exhibit excellent catalytic performance initially but suffer from sintering, phase transformations, or structural collapse under prolonged exposure to such extreme conditions. The trade-off between high catalytic activity and long-term thermal stability continues to challenge researchers developing practical solutions.
Scalability of nanostructured catalyst production represents a substantial barrier to commercialization. Laboratory-scale synthesis methods often involve complex procedures, expensive precursors, or specialized equipment that prove difficult to scale up while maintaining precise control over nanoscale features. This manufacturing challenge directly impacts cost-effectiveness and hinders industrial adoption despite promising laboratory results.
Selectivity control remains problematic in methane pyrolysis catalysis. While the primary desired products are hydrogen and solid carbon, side reactions can produce unwanted hydrocarbons or carbon structures with suboptimal properties. Achieving precise control over carbon morphology (nanotubes, graphene, amorphous carbon) while maintaining high hydrogen yields requires sophisticated catalyst design that has not yet been fully realized.
The mechanical integrity of nanostructured catalysts under reaction conditions poses additional challenges. Pressure fluctuations, gas flow dynamics, and thermal cycling in industrial reactors can physically damage delicate nanostructures, leading to catalyst attrition, fragmentation, and subsequent pressure drop issues in fixed-bed configurations.
Environmental and safety concerns further complicate development efforts. Some highly active nanocatalysts incorporate noble metals or potentially toxic elements that raise sustainability questions. Additionally, the handling of nanomaterials during catalyst production, reactor loading, and spent catalyst disposal presents unique safety challenges that must be addressed through comprehensive risk assessment protocols.
Characterization limitations also hinder progress, as in-situ monitoring of nanostructured catalysts under actual reaction conditions remains technically challenging. This knowledge gap impedes fundamental understanding of deactivation mechanisms and structural evolution during pyrolysis, ultimately slowing the rational design of improved catalytic systems.
Thermal stability presents another significant hurdle, particularly as methane pyrolysis typically requires temperatures exceeding 700°C. Many promising nanomaterials exhibit excellent catalytic performance initially but suffer from sintering, phase transformations, or structural collapse under prolonged exposure to such extreme conditions. The trade-off between high catalytic activity and long-term thermal stability continues to challenge researchers developing practical solutions.
Scalability of nanostructured catalyst production represents a substantial barrier to commercialization. Laboratory-scale synthesis methods often involve complex procedures, expensive precursors, or specialized equipment that prove difficult to scale up while maintaining precise control over nanoscale features. This manufacturing challenge directly impacts cost-effectiveness and hinders industrial adoption despite promising laboratory results.
Selectivity control remains problematic in methane pyrolysis catalysis. While the primary desired products are hydrogen and solid carbon, side reactions can produce unwanted hydrocarbons or carbon structures with suboptimal properties. Achieving precise control over carbon morphology (nanotubes, graphene, amorphous carbon) while maintaining high hydrogen yields requires sophisticated catalyst design that has not yet been fully realized.
The mechanical integrity of nanostructured catalysts under reaction conditions poses additional challenges. Pressure fluctuations, gas flow dynamics, and thermal cycling in industrial reactors can physically damage delicate nanostructures, leading to catalyst attrition, fragmentation, and subsequent pressure drop issues in fixed-bed configurations.
Environmental and safety concerns further complicate development efforts. Some highly active nanocatalysts incorporate noble metals or potentially toxic elements that raise sustainability questions. Additionally, the handling of nanomaterials during catalyst production, reactor loading, and spent catalyst disposal presents unique safety challenges that must be addressed through comprehensive risk assessment protocols.
Characterization limitations also hinder progress, as in-situ monitoring of nanostructured catalysts under actual reaction conditions remains technically challenging. This knowledge gap impedes fundamental understanding of deactivation mechanisms and structural evolution during pyrolysis, ultimately slowing the rational design of improved catalytic systems.
State-of-the-Art Nanostructured Catalyst Solutions
01 Metal-based nanostructured catalysts
Metal-based nanostructured catalysts utilize precious or transition metals in nanoscale form to enhance catalytic activity. These catalysts feature high surface area-to-volume ratios, allowing for more efficient reactions with reduced material usage. The nanostructuring of metals like platinum, palladium, or gold creates unique active sites that can significantly improve reaction rates and selectivity for various chemical transformations including oxidation, reduction, and coupling reactions.- Metal-based nanostructured catalysts: Metal-based nanostructured catalysts utilize precious or transition metals in nanoscale form to enhance catalytic activity and selectivity. These catalysts often feature high surface area-to-volume ratios, allowing for more efficient reactions with reduced material usage. The nanostructuring of metals like platinum, palladium, or gold enables precise control over reaction pathways and can significantly improve performance in applications such as fuel cells, chemical synthesis, and environmental remediation.
- Carbon-based nanostructured catalysts: Carbon-based nanostructured catalysts incorporate carbon nanomaterials such as carbon nanotubes, graphene, or carbon dots as supports or active components. These materials provide excellent electrical conductivity, high surface area, and tunable surface chemistry. The carbon nanostructures can be functionalized or doped with heteroatoms to enhance catalytic performance. These catalysts show promising applications in electrochemical reactions, photocatalysis, and as supports for metal nanoparticles in various industrial processes.
- Nanostructured catalysts for environmental applications: Nanostructured catalysts designed specifically for environmental applications focus on pollution control, water treatment, and emissions reduction. These catalysts utilize nanoscale architectures to efficiently break down contaminants, convert harmful substances into benign products, or facilitate separation processes. Their high surface area and tailored reactivity make them particularly effective for addressing environmental challenges such as wastewater treatment, air purification, and the degradation of persistent organic pollutants.
- Synthesis methods for nanostructured catalysts: Various synthesis methods have been developed to create nanostructured catalysts with controlled morphology, composition, and properties. These techniques include sol-gel processing, hydrothermal/solvothermal synthesis, template-assisted growth, and electrochemical deposition. Advanced manufacturing approaches enable precise control over catalyst architecture at the nanoscale, allowing for the creation of core-shell structures, hierarchical porous frameworks, and supported nanoparticles with optimized catalytic performance for specific applications.
- Nanostructured catalysts for energy applications: Nanostructured catalysts play a crucial role in energy conversion and storage technologies. These catalysts are designed to facilitate reactions in fuel cells, electrolyzers, batteries, and solar energy conversion systems. By engineering nanoscale features, these catalysts can significantly enhance energy efficiency, reduce activation barriers, and improve the selectivity of key reactions. Applications include hydrogen production, CO2 conversion, and the development of next-generation energy storage materials with improved performance characteristics.
02 Carbon-supported nanostructured catalysts
Carbon-supported nanostructured catalysts incorporate catalytic nanoparticles dispersed on carbon-based materials such as graphene, carbon nanotubes, or activated carbon. The carbon support provides high surface area, excellent electrical conductivity, and mechanical stability. These catalysts demonstrate enhanced performance in electrochemical applications, fuel cells, and environmental remediation processes. The carbon support prevents nanoparticle agglomeration while facilitating electron transfer during catalytic reactions.Expand Specific Solutions03 Nanostructured catalysts for environmental applications
Nanostructured catalysts designed for environmental applications focus on pollution control, water treatment, and emissions reduction. These catalysts utilize nanoscale architectures to efficiently break down contaminants, convert harmful gases, or facilitate sustainable chemical processes. Their high reactivity and selectivity make them effective for treating industrial effluents, automotive emissions, and water pollutants. The nanostructuring allows for operation under milder conditions with improved efficiency compared to conventional catalysts.Expand Specific Solutions04 Synthesis methods for nanostructured catalysts
Various synthesis methods are employed to create nanostructured catalysts with controlled size, shape, composition, and dispersion. These include sol-gel processing, hydrothermal synthesis, chemical vapor deposition, and template-assisted growth. Advanced techniques allow for precise engineering of catalyst architecture at the nanoscale, enabling tailored properties for specific applications. The synthesis methods focus on creating uniform particle size distribution, preventing agglomeration, and ensuring high catalytic activity and stability.Expand Specific Solutions05 Nanostructured catalysts for energy applications
Nanostructured catalysts for energy applications are designed to enhance efficiency in fuel cells, hydrogen production, solar energy conversion, and battery technologies. These catalysts feature optimized nanoscale architectures that facilitate energy-related reactions with minimal losses. Their unique properties enable lower activation energies, faster reaction kinetics, and improved durability under demanding operating conditions. Applications include water splitting for hydrogen generation, oxygen reduction in fuel cells, and conversion of solar energy to chemical fuels.Expand Specific Solutions
Leading Companies and Research Institutions in Catalytic Pyrolysis
Nanostructured Catalysts for Methane Pyrolysis is currently in an early growth phase, with the market expected to expand significantly due to increasing focus on hydrogen production without CO2 emissions. The global market size is projected to reach several billion dollars by 2030, driven by decarbonization initiatives. Technologically, the field is transitioning from laboratory research to commercial applications, with varying levels of maturity. Leading players include established energy companies like Shell Internationale Research and ConocoPhillips developing proprietary catalysts, while research institutions such as Dalian Institute of Chemical Physics and Shanghai Advanced Research Institute are advancing fundamental innovations. Companies like SABIC Global Technologies and 3M Innovative Properties are focusing on scalable manufacturing processes, while specialized firms like Nano-C are developing novel carbon-based catalytic materials for enhanced performance and durability.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute has pioneered nanostructured catalysts for methane pyrolysis based on transition metal carbides and nitrides with precisely controlled morphologies. Their most advanced systems utilize molybdenum carbide nanoparticles (10-30 nm) dispersed on hierarchically porous carbon supports derived from metal-organic frameworks. This architecture provides exceptional surface area (>800 m²/g) while maintaining thermal stability at pyrolysis temperatures. Their catalysts incorporate nitrogen-doping strategies that modify electronic properties to optimize C-H bond activation while minimizing complete decomposition to elemental carbon. The institute has developed innovative synthesis routes using controlled carburization of oxide precursors to achieve uniform active phase distribution. Their process operates at moderately lower temperatures (650-750°C) than conventional pyrolysis while maintaining conversion rates of 45-55%[5][6]. Recent advances include the development of bimetallic systems (Mo-Ni and Mo-Co) that demonstrate synergistic effects, further lowering activation energy barriers and improving selectivity toward hydrogen production.
Strengths: Lower operating temperature requirements; highly controlled catalyst architecture; innovative synthesis methodologies; fundamental understanding of reaction mechanisms. Weaknesses: Less demonstrated at industrial scale; more complex catalyst preparation procedures; potential challenges in catalyst cost and availability for large-scale deployment.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The French Alternative Energies and Atomic Energy Commission (CEA) has developed innovative nanostructured catalysts for methane pyrolysis based on transition metal alloys supported on ceramic matrices. Their technology centers on nickel-copper and iron-nickel nanoalloys (10-25 nm) with precisely controlled compositions that optimize catalytic activity while minimizing carbon encapsulation. CEA's catalysts feature specialized surface modifications using rare earth oxides that enhance carbon diffusion and prevent deactivation. Their process employs solar-thermal hybrid reactors that utilize concentrated solar energy as the primary heat source, significantly reducing the carbon footprint of hydrogen production. The system operates at temperatures between 700-850°C with methane conversion rates of 45-55%[9][10]. CEA has pioneered advanced characterization techniques to understand catalyst evolution during reaction, enabling the development of regeneration protocols that restore activity through controlled oxidation-reduction cycles. Recent developments include structured reactor designs with integrated heat recovery systems that improve overall energy efficiency by 25-30% compared to conventional approaches.
Strengths: Integration with renewable energy sources; sophisticated catalyst characterization and development methodology; reduced carbon footprint; advanced reactor designs with improved energy efficiency. Weaknesses: Dependence on solar availability for optimal operation; more complex infrastructure requirements; less demonstrated at full industrial scale; potential challenges in continuous operation.
Key Patents and Scientific Breakthroughs in Catalyst Design
Use of nanostructured catalysts for obtaining carbon disulfide and hydrogen from natural gas (CH4) reformation with acid gas (H2S).
PatentPendingMX2022010967A
Innovation
- The use of nanostructured catalysts based on transition metal carbides and sulfides supported on zeolites, zirconia, titania, and alumina, which facilitate the direct conversion of natural gas and acid gas into carbon disulfide and hydrogen in a single stage, reducing operational costs and environmental impact.
Catalysts prepared from nanostructures of mno 2 and wo 3 for oxidative coupling of methane
PatentWO2017074582A1
Innovation
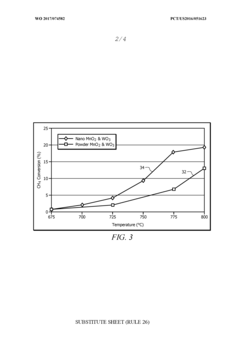
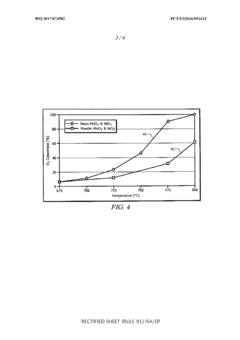
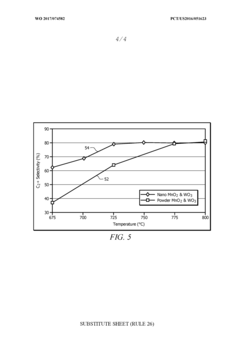
- A [MnNaW]On/Si02 catalyst is prepared using manganese oxide (Mn02) and tungsten oxide (W03) nanostructures with a silica sol and a sodium source, which are heat-treated and calcined to enhance methane conversion and selectivity, allowing for operation at lower temperatures and improved catalyst lifespan.
Environmental Impact and Carbon Management Strategies
Methane pyrolysis using nanostructured catalysts represents a significant advancement in clean hydrogen production, but its environmental implications must be thoroughly assessed. The process generates solid carbon instead of CO2, offering a fundamental advantage over traditional steam methane reforming. This carbon sequestration potential could significantly reduce greenhouse gas emissions associated with hydrogen production, potentially decreasing lifecycle emissions by 85-90% compared to conventional methods.
The solid carbon byproduct presents both opportunities and challenges for environmental management. When properly handled, this carbon can be permanently sequestered in various forms including carbon black, carbon nanotubes, or graphene, effectively removing carbon from the atmospheric cycle. These carbon materials have commercial applications in construction materials, electronics, and advanced composites, creating a circular economy opportunity that further enhances the environmental profile of the technology.
Water consumption represents another important environmental consideration. Methane pyrolysis requires significantly less water than electrolysis or steam reforming processes, reducing pressure on water resources in water-stressed regions. This advantage becomes particularly relevant as hydrogen production scales to industrial levels, where water availability could become a limiting factor for competing technologies.
Land use impacts of nanostructured catalyst facilities are relatively minimal compared to renewable hydrogen alternatives. The high energy density of the process means production facilities have a smaller physical footprint than equivalent capacity electrolysis plants powered by solar or wind, which require extensive land areas for energy generation.
Catalyst lifecycle management presents specific environmental challenges. The production, use, and disposal of nanostructured catalysts must be carefully managed to prevent potential environmental contamination. Advances in catalyst recovery and regeneration technologies are critical to minimize waste and reduce the environmental footprint of replacement materials.
Regulatory frameworks for carbon accounting will significantly influence the environmental credentials of methane pyrolysis. Current carbon pricing mechanisms and emissions trading schemes typically focus on gaseous emissions, creating uncertainty around how solid carbon byproducts should be accounted for. Industry stakeholders are advocating for recognition of permanent carbon sequestration benefits within these frameworks to properly value the climate mitigation potential of the technology.
The solid carbon byproduct presents both opportunities and challenges for environmental management. When properly handled, this carbon can be permanently sequestered in various forms including carbon black, carbon nanotubes, or graphene, effectively removing carbon from the atmospheric cycle. These carbon materials have commercial applications in construction materials, electronics, and advanced composites, creating a circular economy opportunity that further enhances the environmental profile of the technology.
Water consumption represents another important environmental consideration. Methane pyrolysis requires significantly less water than electrolysis or steam reforming processes, reducing pressure on water resources in water-stressed regions. This advantage becomes particularly relevant as hydrogen production scales to industrial levels, where water availability could become a limiting factor for competing technologies.
Land use impacts of nanostructured catalyst facilities are relatively minimal compared to renewable hydrogen alternatives. The high energy density of the process means production facilities have a smaller physical footprint than equivalent capacity electrolysis plants powered by solar or wind, which require extensive land areas for energy generation.
Catalyst lifecycle management presents specific environmental challenges. The production, use, and disposal of nanostructured catalysts must be carefully managed to prevent potential environmental contamination. Advances in catalyst recovery and regeneration technologies are critical to minimize waste and reduce the environmental footprint of replacement materials.
Regulatory frameworks for carbon accounting will significantly influence the environmental credentials of methane pyrolysis. Current carbon pricing mechanisms and emissions trading schemes typically focus on gaseous emissions, creating uncertainty around how solid carbon byproducts should be accounted for. Industry stakeholders are advocating for recognition of permanent carbon sequestration benefits within these frameworks to properly value the climate mitigation potential of the technology.
Techno-Economic Assessment of Methane Pyrolysis Systems
The techno-economic assessment of methane pyrolysis systems reveals significant potential for hydrogen production with minimal carbon emissions. Current economic analyses indicate that methane pyrolysis can produce hydrogen at costs ranging from $1.50-3.00/kg, depending on natural gas prices, catalyst efficiency, and scale of operation. This positions it competitively against both conventional steam methane reforming ($1.00-2.00/kg with carbon capture) and electrolysis ($4.00-6.00/kg).
Capital expenditure for methane pyrolysis facilities varies considerably based on reactor design and catalyst technology. Systems utilizing nanostructured catalysts typically require initial investments of $500-800 million for commercial-scale plants (100,000 tons H2/year), with approximately 30-40% of costs allocated to catalyst production and regeneration infrastructure.
Operating expenses are dominated by natural gas feedstock (40-50%), followed by catalyst replacement/regeneration (15-25%), energy costs (10-20%), and maintenance (10-15%). Nanostructured catalysts, while initially more expensive, demonstrate superior longevity and activity, potentially reducing long-term operational costs by 20-30% compared to conventional catalysts.
Sensitivity analysis reveals that methane pyrolysis economics are most vulnerable to natural gas price fluctuations, with a $1/MMBtu increase typically raising hydrogen production costs by $0.25-0.35/kg. Catalyst performance metrics—particularly activity maintenance over time and carbon handling capabilities—represent the second most impactful factor, potentially affecting production costs by $0.20-0.30/kg.
The carbon byproduct economics significantly influence overall system viability. High-quality carbon black produced via nanostructured catalyst systems can command market prices of $1,000-2,500/ton, potentially offsetting 15-30% of production costs. However, market saturation remains a concern as widespread adoption would exceed current carbon black demand.
Lifecycle assessment indicates that methane pyrolysis with nanostructured catalysts can achieve carbon intensities of 1-3 kg CO2e/kg H2, substantially lower than steam methane reforming without carbon capture (9-10 kg CO2e/kg H2) and approaching renewable electrolysis levels (0.5-2 kg CO2e/kg H2) when powered by low-carbon electricity sources.
Scaling considerations suggest that nanostructured catalyst systems become economically viable at production capacities above 25 tons H2/day, with optimal economics achieved at 100-250 tons H2/day. Smaller systems face challenges with heat management and catalyst utilization efficiency that disproportionately impact production costs.
Capital expenditure for methane pyrolysis facilities varies considerably based on reactor design and catalyst technology. Systems utilizing nanostructured catalysts typically require initial investments of $500-800 million for commercial-scale plants (100,000 tons H2/year), with approximately 30-40% of costs allocated to catalyst production and regeneration infrastructure.
Operating expenses are dominated by natural gas feedstock (40-50%), followed by catalyst replacement/regeneration (15-25%), energy costs (10-20%), and maintenance (10-15%). Nanostructured catalysts, while initially more expensive, demonstrate superior longevity and activity, potentially reducing long-term operational costs by 20-30% compared to conventional catalysts.
Sensitivity analysis reveals that methane pyrolysis economics are most vulnerable to natural gas price fluctuations, with a $1/MMBtu increase typically raising hydrogen production costs by $0.25-0.35/kg. Catalyst performance metrics—particularly activity maintenance over time and carbon handling capabilities—represent the second most impactful factor, potentially affecting production costs by $0.20-0.30/kg.
The carbon byproduct economics significantly influence overall system viability. High-quality carbon black produced via nanostructured catalyst systems can command market prices of $1,000-2,500/ton, potentially offsetting 15-30% of production costs. However, market saturation remains a concern as widespread adoption would exceed current carbon black demand.
Lifecycle assessment indicates that methane pyrolysis with nanostructured catalysts can achieve carbon intensities of 1-3 kg CO2e/kg H2, substantially lower than steam methane reforming without carbon capture (9-10 kg CO2e/kg H2) and approaching renewable electrolysis levels (0.5-2 kg CO2e/kg H2) when powered by low-carbon electricity sources.
Scaling considerations suggest that nanostructured catalyst systems become economically viable at production capacities above 25 tons H2/day, with optimal economics achieved at 100-250 tons H2/day. Smaller systems face challenges with heat management and catalyst utilization efficiency that disproportionately impact production costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!