Innovations in Reactor Design for Methane Pyrolysis.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Reactor Evolution and Objectives
Methane pyrolysis, the process of decomposing methane into hydrogen and solid carbon, has evolved significantly over the past decades as researchers and industry professionals seek cleaner methods for hydrogen production. The evolution of reactor designs for methane pyrolysis reflects the growing understanding of reaction kinetics, heat transfer mechanisms, and material science advancements in high-temperature applications.
In the 1960s, early methane pyrolysis reactors were primarily focused on carbon black production rather than hydrogen generation. These initial designs utilized thermal decomposition in furnaces with limited control over reaction parameters and product characteristics. The 1980s marked a shift toward more sophisticated reactor concepts, including fluidized bed reactors that improved heat transfer efficiency and reaction rates.
The 1990s witnessed the emergence of plasma-based reactors, which enabled methane decomposition at lower temperatures through the use of non-thermal plasma. This period also saw increased interest in catalytic pyrolysis approaches, with various transition metals being explored as potential catalysts to reduce the energy requirements of the process.
The early 2000s brought significant advancements in molten metal reactor designs, particularly those utilizing liquid metals like tin, nickel, and various alloys. These reactors offered superior heat transfer characteristics and continuous carbon separation capabilities, addressing key challenges in scaling methane pyrolysis technology.
Recent innovations have focused on hybrid reactor designs that combine multiple approaches, such as plasma-catalytic systems and concentrated solar-thermal reactors. These hybrid systems aim to optimize energy efficiency while maximizing hydrogen yield and facilitating continuous carbon removal.
The primary objectives driving methane pyrolysis reactor development include reducing the energy intensity of the process, enhancing reaction selectivity toward hydrogen production, enabling continuous operation without catalyst deactivation or carbon buildup, and developing cost-effective scaling strategies for industrial implementation.
Current research goals focus on achieving thermal efficiency exceeding 70%, hydrogen production costs below $2/kg, reactor lifespans of 10+ years under continuous operation, and carbon products with market value to offset production costs. Additionally, there is growing emphasis on reactor designs compatible with renewable energy sources to further reduce the carbon footprint of hydrogen production.
The technological trajectory suggests that future methane pyrolysis reactors will likely incorporate advanced materials capable of withstanding extreme conditions, intelligent control systems for process optimization, and modular designs that facilitate distributed hydrogen production at various scales.
In the 1960s, early methane pyrolysis reactors were primarily focused on carbon black production rather than hydrogen generation. These initial designs utilized thermal decomposition in furnaces with limited control over reaction parameters and product characteristics. The 1980s marked a shift toward more sophisticated reactor concepts, including fluidized bed reactors that improved heat transfer efficiency and reaction rates.
The 1990s witnessed the emergence of plasma-based reactors, which enabled methane decomposition at lower temperatures through the use of non-thermal plasma. This period also saw increased interest in catalytic pyrolysis approaches, with various transition metals being explored as potential catalysts to reduce the energy requirements of the process.
The early 2000s brought significant advancements in molten metal reactor designs, particularly those utilizing liquid metals like tin, nickel, and various alloys. These reactors offered superior heat transfer characteristics and continuous carbon separation capabilities, addressing key challenges in scaling methane pyrolysis technology.
Recent innovations have focused on hybrid reactor designs that combine multiple approaches, such as plasma-catalytic systems and concentrated solar-thermal reactors. These hybrid systems aim to optimize energy efficiency while maximizing hydrogen yield and facilitating continuous carbon removal.
The primary objectives driving methane pyrolysis reactor development include reducing the energy intensity of the process, enhancing reaction selectivity toward hydrogen production, enabling continuous operation without catalyst deactivation or carbon buildup, and developing cost-effective scaling strategies for industrial implementation.
Current research goals focus on achieving thermal efficiency exceeding 70%, hydrogen production costs below $2/kg, reactor lifespans of 10+ years under continuous operation, and carbon products with market value to offset production costs. Additionally, there is growing emphasis on reactor designs compatible with renewable energy sources to further reduce the carbon footprint of hydrogen production.
The technological trajectory suggests that future methane pyrolysis reactors will likely incorporate advanced materials capable of withstanding extreme conditions, intelligent control systems for process optimization, and modular designs that facilitate distributed hydrogen production at various scales.
Market Demand for Clean Hydrogen Production
The global hydrogen market is experiencing a significant shift towards clean production methods, driven by increasing environmental concerns and stringent carbon emission regulations. Currently, the hydrogen market is valued at approximately $150 billion annually, with projections indicating growth to $600 billion by 2050. Traditional hydrogen production methods, primarily steam methane reforming (SMR), account for over 95% of current production but generate substantial CO2 emissions—approximately 9-12 kg CO2 per kg of hydrogen produced.
Methane pyrolysis offers a compelling alternative for clean hydrogen production, addressing the growing demand for low-carbon hydrogen across multiple sectors. Industrial applications represent the largest current market segment, with refineries and chemical manufacturing consuming about 70% of globally produced hydrogen. However, emerging applications in transportation, power generation, and energy storage are expected to drive significant future demand growth.
The transportation sector shows particularly promising growth potential, with fuel cell electric vehicles (FCEVs) gaining traction in heavy-duty transport, long-haul trucking, and public transportation. Major automotive manufacturers have announced substantial investments in hydrogen fuel cell technology, with Toyota, Hyundai, and Honda leading commercial deployments.
Energy industry stakeholders are increasingly recognizing hydrogen's role in decarbonization strategies. Several countries have established national hydrogen strategies with ambitious targets—Japan aims to have 800,000 FCEVs by 2030, while the European Union's Hydrogen Strategy targets 40 GW of renewable hydrogen electrolyzers by 2030. These policy frameworks are creating favorable market conditions for clean hydrogen technologies like methane pyrolysis.
Cost considerations remain critical for market adoption. Current hydrogen production costs via SMR range from $1-2/kg, while conventional clean hydrogen production methods like electrolysis typically cost $3-7/kg. Methane pyrolysis potentially offers hydrogen at $1.5-3/kg with significantly lower carbon emissions, positioning it competitively in the evolving hydrogen economy.
Market analysis indicates that industrial clusters with existing natural gas infrastructure present the most immediate opportunities for methane pyrolysis deployment. Regions with strong decarbonization policies, access to natural gas, and established carbon pricing mechanisms—such as the European Union, Japan, South Korea, and parts of North America—represent primary target markets.
The solid carbon byproduct from methane pyrolysis creates additional value streams, with potential applications in construction materials, soil enhancement, and advanced materials manufacturing. This carbon market opportunity, estimated at several billion dollars annually, enhances the overall economic proposition of methane pyrolysis technology.
Methane pyrolysis offers a compelling alternative for clean hydrogen production, addressing the growing demand for low-carbon hydrogen across multiple sectors. Industrial applications represent the largest current market segment, with refineries and chemical manufacturing consuming about 70% of globally produced hydrogen. However, emerging applications in transportation, power generation, and energy storage are expected to drive significant future demand growth.
The transportation sector shows particularly promising growth potential, with fuel cell electric vehicles (FCEVs) gaining traction in heavy-duty transport, long-haul trucking, and public transportation. Major automotive manufacturers have announced substantial investments in hydrogen fuel cell technology, with Toyota, Hyundai, and Honda leading commercial deployments.
Energy industry stakeholders are increasingly recognizing hydrogen's role in decarbonization strategies. Several countries have established national hydrogen strategies with ambitious targets—Japan aims to have 800,000 FCEVs by 2030, while the European Union's Hydrogen Strategy targets 40 GW of renewable hydrogen electrolyzers by 2030. These policy frameworks are creating favorable market conditions for clean hydrogen technologies like methane pyrolysis.
Cost considerations remain critical for market adoption. Current hydrogen production costs via SMR range from $1-2/kg, while conventional clean hydrogen production methods like electrolysis typically cost $3-7/kg. Methane pyrolysis potentially offers hydrogen at $1.5-3/kg with significantly lower carbon emissions, positioning it competitively in the evolving hydrogen economy.
Market analysis indicates that industrial clusters with existing natural gas infrastructure present the most immediate opportunities for methane pyrolysis deployment. Regions with strong decarbonization policies, access to natural gas, and established carbon pricing mechanisms—such as the European Union, Japan, South Korea, and parts of North America—represent primary target markets.
The solid carbon byproduct from methane pyrolysis creates additional value streams, with potential applications in construction materials, soil enhancement, and advanced materials manufacturing. This carbon market opportunity, estimated at several billion dollars annually, enhances the overall economic proposition of methane pyrolysis technology.
Technical Barriers in Methane Pyrolysis Reactors
Methane pyrolysis represents a promising pathway for hydrogen production with significantly reduced carbon emissions compared to traditional steam methane reforming. However, several technical barriers impede the widespread implementation of this technology. The primary challenge lies in reactor design, where maintaining stable operation at high temperatures (800-1200°C) necessary for efficient methane decomposition presents significant materials science challenges. Conventional reactor materials often suffer from thermal degradation, reducing operational lifespan and increasing maintenance costs.
Carbon management within reactors constitutes another major technical hurdle. As methane decomposes into hydrogen and solid carbon, the accumulation of carbon deposits on reactor surfaces leads to catalyst deactivation, reduced heat transfer efficiency, and eventual reactor clogging. Current carbon removal systems require frequent maintenance interventions, compromising continuous operation capabilities essential for industrial-scale implementation.
Catalyst development presents additional complexities. While metal catalysts (Ni, Fe, Co) can lower reaction temperatures and improve conversion rates, they suffer from rapid deactivation due to carbon encapsulation. Carbon-based catalysts show promising stability but typically demonstrate lower activity. The ideal catalyst combining high activity, selectivity, and durability remains elusive despite extensive research efforts.
Heat management systems represent another significant barrier. The endothermic nature of methane pyrolysis requires substantial energy input, and achieving uniform temperature distribution throughout the reactor volume presents considerable engineering challenges. Inefficient heat transfer results in temperature gradients that reduce conversion efficiency and create localized hotspots that accelerate material degradation.
Scale-up challenges further complicate commercial deployment. Laboratory-scale reactors demonstrating promising performance often encounter unforeseen complications when scaled to industrial capacities. Issues include non-linear scaling of heat and mass transfer phenomena, increased carbon management difficulties, and reduced conversion efficiencies at larger scales.
Process integration barriers also exist, particularly regarding hydrogen purification systems. The product gas stream contains trace impurities that must be removed before downstream applications, requiring additional separation technologies that increase system complexity and operational costs.
Economic viability remains constrained by high capital expenditure requirements and energy intensity. Current reactor designs typically demand expensive high-temperature materials and sophisticated control systems, while energy requirements for maintaining reaction temperatures significantly impact operational economics, particularly when renewable energy sources are not available.
Carbon management within reactors constitutes another major technical hurdle. As methane decomposes into hydrogen and solid carbon, the accumulation of carbon deposits on reactor surfaces leads to catalyst deactivation, reduced heat transfer efficiency, and eventual reactor clogging. Current carbon removal systems require frequent maintenance interventions, compromising continuous operation capabilities essential for industrial-scale implementation.
Catalyst development presents additional complexities. While metal catalysts (Ni, Fe, Co) can lower reaction temperatures and improve conversion rates, they suffer from rapid deactivation due to carbon encapsulation. Carbon-based catalysts show promising stability but typically demonstrate lower activity. The ideal catalyst combining high activity, selectivity, and durability remains elusive despite extensive research efforts.
Heat management systems represent another significant barrier. The endothermic nature of methane pyrolysis requires substantial energy input, and achieving uniform temperature distribution throughout the reactor volume presents considerable engineering challenges. Inefficient heat transfer results in temperature gradients that reduce conversion efficiency and create localized hotspots that accelerate material degradation.
Scale-up challenges further complicate commercial deployment. Laboratory-scale reactors demonstrating promising performance often encounter unforeseen complications when scaled to industrial capacities. Issues include non-linear scaling of heat and mass transfer phenomena, increased carbon management difficulties, and reduced conversion efficiencies at larger scales.
Process integration barriers also exist, particularly regarding hydrogen purification systems. The product gas stream contains trace impurities that must be removed before downstream applications, requiring additional separation technologies that increase system complexity and operational costs.
Economic viability remains constrained by high capital expenditure requirements and energy intensity. Current reactor designs typically demand expensive high-temperature materials and sophisticated control systems, while energy requirements for maintaining reaction temperatures significantly impact operational economics, particularly when renewable energy sources are not available.
Current Reactor Designs for Methane Decomposition
01 Fluidized bed reactor designs for methane pyrolysis
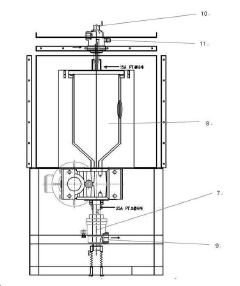
Fluidized bed reactors are commonly used for methane pyrolysis due to their excellent heat and mass transfer characteristics. These reactors typically contain solid particles (often catalysts or heat carriers) suspended in a gas stream, creating a fluid-like behavior. The design allows for continuous operation with efficient carbon removal and improved reaction kinetics. Key design considerations include bed material selection, gas velocity control, and temperature distribution management to optimize methane conversion while preventing catalyst deactivation.- Fluidized bed reactor designs for methane pyrolysis: Fluidized bed reactors are commonly used for methane pyrolysis due to their excellent heat and mass transfer characteristics. These designs typically incorporate solid particles (such as catalysts or heat carriers) suspended in a gas flow, creating efficient contact between methane and catalytic surfaces. The fluidization helps maintain uniform temperature distribution and prevents carbon deposition from hindering the reaction. Advanced fluidized bed designs may include multiple stages, specialized gas distribution systems, or integrated heat recovery mechanisms to optimize the pyrolysis process.
- Catalytic reactor systems for enhanced methane conversion: Catalytic reactor systems employ specialized materials to lower the activation energy required for methane pyrolysis, enabling the reaction to proceed at lower temperatures. These reactors incorporate various catalyst configurations including fixed beds, moving beds, or dispersed catalysts. Key design considerations include catalyst selection (metals, metal oxides, or carbon-based materials), catalyst support structures, and mechanisms to manage catalyst deactivation due to carbon deposition. Advanced catalytic systems may feature regeneration capabilities or novel catalyst delivery methods to maintain conversion efficiency over extended operation periods.
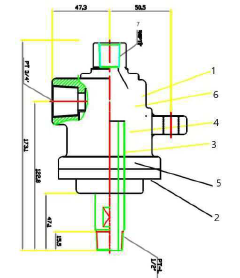
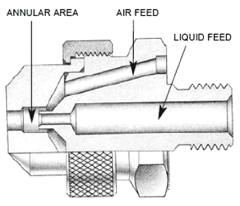
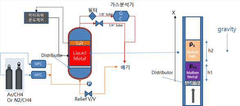
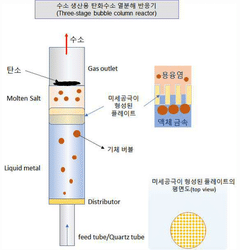
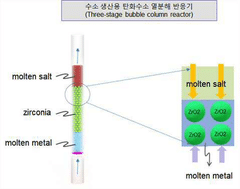
- Molten metal reactor technology for methane decomposition: Molten metal reactors represent an innovative approach to methane pyrolysis, using liquid metals (such as tin, bismuth, lead, or their alloys) as both heat transfer medium and catalyst. The design typically features a vessel containing the molten metal through which methane is bubbled. This configuration offers advantages including excellent heat transfer, continuous carbon separation as solid carbon floats to the surface, and prevention of carbon deposition on reactor walls. Key design considerations include materials of construction to withstand high temperatures and corrosive molten metals, gas injection systems, and mechanisms for continuous carbon removal.
- Plasma-assisted reactor designs for methane pyrolysis: Plasma-assisted reactors utilize electrical energy to generate plasma that can decompose methane at lower bulk temperatures than conventional thermal processes. These designs may incorporate various plasma generation methods including microwave, radio frequency, or arc discharge systems. The high-energy plasma environment creates reactive species that facilitate methane decomposition pathways not available in conventional thermal reactors. Key design features include electrode configurations, power supply systems, plasma containment structures, and mechanisms to manage the high temperatures at plasma-material interfaces while maintaining overall process efficiency.
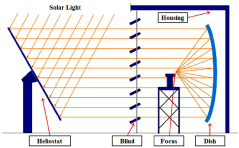
- Heat management and recovery systems for pyrolysis reactors: Effective heat management is critical in methane pyrolysis reactor design due to the highly endothermic nature of the reaction. Advanced reactor designs incorporate sophisticated heat recovery systems to capture and reuse thermal energy, improving overall process efficiency. These may include regenerative heat exchangers, product gas heat recovery, or integration with complementary exothermic processes. Reactor wall design often features specialized materials and insulation systems to minimize heat loss while withstanding the high temperatures required for pyrolysis. Some designs implement novel heating approaches such as induction heating, concentrated solar energy, or combustion of process by-products to provide the necessary reaction energy.
02 Molten metal reactor systems for methane decomposition
Molten metal reactors utilize liquid metals (such as tin, bismuth, or lead) as reaction media for methane pyrolysis. These systems offer advantages including excellent heat transfer, continuous carbon separation, and catalyst-free operation. The molten metal serves as both heat transfer medium and carbon collector, with solid carbon floating to the surface for easy removal. Key design elements include metal selection based on melting point and reactivity, gas injection systems, and mechanisms for continuous carbon harvesting without interrupting the process.Expand Specific Solutions03 Plasma-assisted reactor technologies for methane pyrolysis
Plasma reactors utilize electrical energy to create high-temperature plasma zones that enable methane decomposition at accelerated rates. These designs can achieve higher conversion efficiencies at lower bulk temperatures compared to conventional thermal reactors. The plasma environment creates reactive species that enhance reaction kinetics while reducing energy requirements. Design considerations include electrode configuration, power supply systems, plasma stabilization mechanisms, and methods for managing carbon deposition on reactor surfaces to ensure continuous operation.Expand Specific Solutions04 Catalytic reactor designs with carbon management systems
Specialized catalytic reactor designs incorporate innovative carbon management systems to address the challenge of catalyst deactivation due to carbon deposition. These reactors feature mechanisms for continuous or periodic carbon removal while maintaining reaction efficiency. Design approaches include moving bed reactors, catalyst regeneration systems, and specialized reactor geometries that facilitate carbon separation. Materials selection focuses on catalysts with high activity and resistance to coking, while reactor configuration optimizes gas flow patterns to enhance methane conversion and extend catalyst lifetime.Expand Specific Solutions05 Heat management and energy integration in pyrolysis reactors
Advanced thermal management systems are critical for methane pyrolysis reactor design, as the reaction is highly endothermic. These designs incorporate innovative heat transfer mechanisms, energy recovery systems, and thermal integration with other process units. Key features include specialized heating elements, heat exchanger configurations, and thermal insulation systems to maintain optimal temperature profiles. Some designs utilize the hydrogen product or other process streams for heat recovery, while others implement novel heating methods such as induction or microwave heating to deliver energy precisely where needed in the reaction zone.Expand Specific Solutions
Leading Companies in Methane Pyrolysis Industry
The methane pyrolysis reactor design innovation landscape is currently in a growth phase, with increasing market size driven by clean hydrogen production demands. The technology is approaching commercial maturity, with major energy companies leading development. ExxonMobil Chemical Patents and TotalEnergies OneTech are pioneering Western innovations, while Asian players like China Petroleum & Chemical Corp., SK Innovation, and RIST are rapidly advancing their technologies. Research institutions including Korea Institute of Energy Research and Industrial Technology Research Institute provide critical R&D support. Technology specialists like Haldor Topsøe, UOP LLC, and Hazer Group are developing proprietary catalytic processes, while industrial giants BASF and Linde are leveraging their engineering expertise to scale solutions for industrial implementation.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has developed an advanced catalytic methane pyrolysis reactor system that operates at moderate temperatures between 700-900°C. Their innovation centers on proprietary nickel-based catalysts supported on specialized ceramic structures that significantly lower the activation energy required for methane decomposition. The reactor design features a structured packed bed configuration with optimized gas flow distribution to maximize catalyst utilization and minimize pressure drop. Topsøe's system incorporates a novel catalyst regeneration approach where periodic oxidation-reduction cycles restore catalyst activity without requiring physical removal from the reactor. Their technology includes advanced heat integration systems that recover thermal energy from product gases to preheat incoming methane, improving overall energy efficiency by approximately 35% compared to conventional designs. The company has demonstrated successful operation at demonstration scale with methane conversion rates consistently above 75% and hydrogen yields approaching theoretical limits. Recent innovations include catalyst modifications that selectively produce specific carbon morphologies (nanotubes, graphene, etc.) as valuable by-products, potentially improving the economics of the process through carbon valorization.
Strengths: Moderate operating temperatures reduce energy requirements and material constraints; proprietary catalysts significantly enhance reaction kinetics; in-situ catalyst regeneration enables extended operation periods. Weaknesses: Catalyst deactivation still occurs despite regeneration capabilities; carbon removal from the reactor remains challenging; catalyst production involves some expensive materials increasing overall costs.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has pioneered advanced reactor designs for methane pyrolysis focusing on molten metal technology. Their approach utilizes liquid metals (primarily tin or bismuth-tin alloys) as both heat transfer medium and catalyst surface. Methane bubbles through the molten metal bath at temperatures of 1000-1200°C, where it decomposes into hydrogen gas and solid carbon. Shell's innovation lies in their reactor configuration that enables continuous carbon removal from the metal bath, preventing catalyst deactivation. Their design incorporates specialized gas injection systems that optimize bubble size and residence time, enhancing methane conversion efficiency. The company has developed proprietary methods for carbon separation and extraction from the metal bath, addressing one of the key challenges in molten metal pyrolysis. Shell's reactor also features advanced heat recovery systems that significantly improve energy efficiency by recapturing thermal energy from the high-temperature process, reducing overall energy requirements by approximately 25% compared to conventional designs.
Strengths: Achieves high methane conversion rates (>90%); continuous operation capability with carbon removal systems; excellent heat transfer properties of molten metals enable efficient temperature control. Weaknesses: High operating temperatures require specialized materials increasing capital costs; safety concerns related to handling molten metals; energy intensity remains significant despite heat recovery improvements.
Breakthrough Patents in Methane Pyrolysis Systems
Methane pyrolysis solar rotary reactor and method for producing hydrogen and carbon black using the same
PatentActiveKR1020210059142A
Innovation
- A methane pyrolysis solar thermal rotary reactor using a steam injector with a gas-liquid atomizing nozzle to inject steam and methane into a porous reaction layer, rotating at controlled speeds to suppress carbon deposition and maintain temperature, employing solar heat for thermal decomposition.
Methane pyrolysis reactor for hydrogen production
PatentActiveKR1020210096362A
Innovation
- A hydrocarbon pyrolysis reactor with a bubble column design incorporating a molten metal layer, a molten salt layer, and an interfacial property control unit, such as a laminated bead or porous material, to control gas bubble size and residence time, reducing the need for molten salt and enhancing thermal efficiency.
Carbon Management Strategies in Pyrolysis Processes
Carbon management represents a critical aspect of methane pyrolysis processes, particularly as this technology gains prominence as a potential pathway for clean hydrogen production. The primary advantage of methane pyrolysis lies in its ability to produce solid carbon instead of CO2 emissions, creating opportunities for carbon sequestration and utilization that significantly enhance the environmental profile of hydrogen production.
Current carbon management strategies in methane pyrolysis focus on three main approaches: carbon capture, carbon storage, and carbon utilization. The quality and morphology of carbon produced during pyrolysis largely depend on reactor design parameters, including temperature profiles, residence time, and catalyst selection. Advanced reactor designs now incorporate specialized carbon collection systems that minimize carbon deposition on reactor walls and catalyst surfaces, which has traditionally been a major operational challenge.
Continuous carbon removal systems represent a significant innovation in reactor design, allowing for uninterrupted operation while maintaining process efficiency. These systems employ mechanical scrapers, fluidized bed configurations, or molten metal media that facilitate the separation and extraction of solid carbon. The integration of these removal mechanisms directly impacts reactor longevity and maintenance requirements, with some designs achieving operational periods exceeding 1,000 hours without shutdown for carbon removal.
The carbon produced through methane pyrolysis varies in quality from amorphous carbon to highly structured forms like carbon nanotubes or graphene, depending on process conditions. High-value carbon products can significantly improve the economics of hydrogen production through pyrolysis. Recent reactor innovations specifically target the controlled production of these premium carbon allotropes by precisely managing temperature gradients and introducing selective catalytic surfaces within the reaction zone.
Life cycle assessment studies indicate that effective carbon management strategies can result in negative carbon footprints for hydrogen production when the solid carbon is permanently sequestered or used in long-lasting applications. This potential for carbon-negative hydrogen production represents a compelling advantage over conventional hydrogen production methods, even those equipped with carbon capture technologies.
Emerging reactor designs are increasingly incorporating in-situ carbon functionalization capabilities, allowing for immediate modification of carbon products to enhance their value and application potential. These integrated approaches to carbon management not only address operational challenges but also transform what was once considered a waste product into a valuable co-product, fundamentally altering the economic proposition of methane pyrolysis technology.
Current carbon management strategies in methane pyrolysis focus on three main approaches: carbon capture, carbon storage, and carbon utilization. The quality and morphology of carbon produced during pyrolysis largely depend on reactor design parameters, including temperature profiles, residence time, and catalyst selection. Advanced reactor designs now incorporate specialized carbon collection systems that minimize carbon deposition on reactor walls and catalyst surfaces, which has traditionally been a major operational challenge.
Continuous carbon removal systems represent a significant innovation in reactor design, allowing for uninterrupted operation while maintaining process efficiency. These systems employ mechanical scrapers, fluidized bed configurations, or molten metal media that facilitate the separation and extraction of solid carbon. The integration of these removal mechanisms directly impacts reactor longevity and maintenance requirements, with some designs achieving operational periods exceeding 1,000 hours without shutdown for carbon removal.
The carbon produced through methane pyrolysis varies in quality from amorphous carbon to highly structured forms like carbon nanotubes or graphene, depending on process conditions. High-value carbon products can significantly improve the economics of hydrogen production through pyrolysis. Recent reactor innovations specifically target the controlled production of these premium carbon allotropes by precisely managing temperature gradients and introducing selective catalytic surfaces within the reaction zone.
Life cycle assessment studies indicate that effective carbon management strategies can result in negative carbon footprints for hydrogen production when the solid carbon is permanently sequestered or used in long-lasting applications. This potential for carbon-negative hydrogen production represents a compelling advantage over conventional hydrogen production methods, even those equipped with carbon capture technologies.
Emerging reactor designs are increasingly incorporating in-situ carbon functionalization capabilities, allowing for immediate modification of carbon products to enhance their value and application potential. These integrated approaches to carbon management not only address operational challenges but also transform what was once considered a waste product into a valuable co-product, fundamentally altering the economic proposition of methane pyrolysis technology.
Techno-economic Analysis of Pyrolysis Reactor Designs
The techno-economic analysis of methane pyrolysis reactor designs reveals significant variations in economic viability across different technological approaches. Molten metal reactors, particularly those utilizing liquid tin or nickel as catalysts, demonstrate operational costs ranging from $1.2-1.8/kg H₂, with capital expenditures between $800-1,200/kW. These systems benefit from excellent heat transfer properties but face challenges with metal carryover and containment material compatibility, increasing maintenance expenses by approximately 15-20% compared to conventional systems.
Plasma-based reactors show promising economics at scale, with production costs potentially reaching $1.5-2.0/kg H₂ when operating at multi-megawatt capacity. However, their high electricity requirements (4.5-5.5 kWh/m³ of methane processed) create significant operational expense sensitivity to electricity pricing, making them economically viable primarily in regions with electricity costs below $0.04/kWh.
Moving bed reactors utilizing carbon catalysts present the lowest capital intensity at $600-900/kW, with operational costs competitive at $1.3-1.7/kg H₂. Their simplified design reduces maintenance requirements by up to 30% compared to molten metal systems, though catalyst replacement cycles (typically 500-1,000 hours) introduce recurring operational expenses that must be carefully managed.
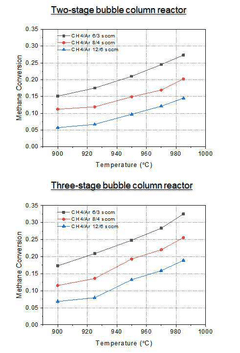
Fluidized bed designs offer excellent scalability with economies of scale reducing production costs by approximately 25% when scaling from 100 kg/day to 1 ton/day of hydrogen production. Their thermal efficiency ranges from 65-75%, with heat recovery systems potentially improving overall system efficiency by an additional 10-15%.
Sensitivity analysis indicates that natural gas pricing remains the dominant factor in economic viability across all reactor designs, accounting for 45-60% of production costs. Carbon credit mechanisms significantly impact economic calculations, with carbon prices above $50/ton potentially reducing hydrogen production costs by $0.3-0.5/kg through valorization of solid carbon byproducts.
The levelized cost of hydrogen (LCOH) analysis demonstrates that methane pyrolysis reactors can achieve cost parity with steam methane reforming plus carbon capture (SMR+CCS) when carbon prices exceed $70/ton or natural gas prices fall below $3/MMBtu. Most reactor designs show payback periods of 5-8 years under current market conditions, with molten metal and moving bed designs demonstrating the most favorable economics in small to medium-scale applications.
Plasma-based reactors show promising economics at scale, with production costs potentially reaching $1.5-2.0/kg H₂ when operating at multi-megawatt capacity. However, their high electricity requirements (4.5-5.5 kWh/m³ of methane processed) create significant operational expense sensitivity to electricity pricing, making them economically viable primarily in regions with electricity costs below $0.04/kWh.
Moving bed reactors utilizing carbon catalysts present the lowest capital intensity at $600-900/kW, with operational costs competitive at $1.3-1.7/kg H₂. Their simplified design reduces maintenance requirements by up to 30% compared to molten metal systems, though catalyst replacement cycles (typically 500-1,000 hours) introduce recurring operational expenses that must be carefully managed.
Fluidized bed designs offer excellent scalability with economies of scale reducing production costs by approximately 25% when scaling from 100 kg/day to 1 ton/day of hydrogen production. Their thermal efficiency ranges from 65-75%, with heat recovery systems potentially improving overall system efficiency by an additional 10-15%.
Sensitivity analysis indicates that natural gas pricing remains the dominant factor in economic viability across all reactor designs, accounting for 45-60% of production costs. Carbon credit mechanisms significantly impact economic calculations, with carbon prices above $50/ton potentially reducing hydrogen production costs by $0.3-0.5/kg through valorization of solid carbon byproducts.
The levelized cost of hydrogen (LCOH) analysis demonstrates that methane pyrolysis reactors can achieve cost parity with steam methane reforming plus carbon capture (SMR+CCS) when carbon prices exceed $70/ton or natural gas prices fall below $3/MMBtu. Most reactor designs show payback periods of 5-8 years under current market conditions, with molten metal and moving bed designs demonstrating the most favorable economics in small to medium-scale applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!