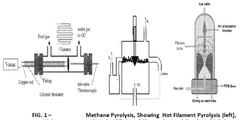
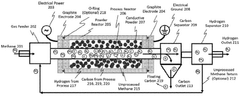
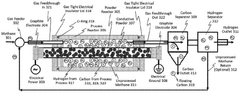
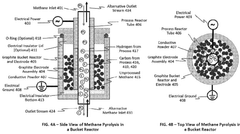
Methane Pyrolysis: Energy Efficiency Optimization.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Background and Objectives
Methane pyrolysis represents a transformative approach to hydrogen production that has gained significant attention in recent decades. This process involves the thermal decomposition of methane (CH₄) into hydrogen (H₂) and solid carbon in the absence of oxygen. Unlike conventional hydrogen production methods such as steam methane reforming (SMR), methane pyrolysis produces no direct CO₂ emissions, positioning it as a potentially cleaner pathway for hydrogen generation in a carbon-constrained world.
The historical development of methane pyrolysis dates back to the early 20th century, but significant research acceleration occurred in the 1990s when environmental concerns about greenhouse gas emissions became more prominent. The technology has evolved through several generations, from basic thermal decomposition approaches to more sophisticated catalytic and plasma-assisted methods that aim to reduce the energy requirements and improve conversion efficiencies.
Current technological trajectories indicate a growing focus on novel reactor designs, advanced catalysts, and innovative heating methods to overcome the inherent thermodynamic challenges of methane decomposition. The endothermic nature of the reaction (requiring approximately 74.6 kJ/mol of energy input) presents a fundamental challenge that researchers are addressing through various approaches including molten metal catalysts, carbon-based catalysts, and concentrated solar heating systems.
The primary objective of energy efficiency optimization in methane pyrolysis is to reduce the overall energy consumption per unit of hydrogen produced, thereby improving the economic viability and environmental benefits of the process. This involves minimizing heat losses, maximizing heat recovery, optimizing reaction conditions, and developing more effective catalysts that can lower the activation energy required for methane decomposition.
Secondary objectives include enhancing the value proposition of the solid carbon co-product, which can potentially offset production costs if high-quality carbon materials (such as carbon nanotubes or graphene) can be reliably produced. Additionally, process intensification aims to reduce capital costs through more compact and efficient reactor designs.
The technology evolution trend points toward hybrid systems that combine methane pyrolysis with renewable energy sources, particularly concentrated solar power, to provide the necessary heat input without fossil fuel combustion. This approach could potentially lead to truly zero-emission hydrogen production pathways, aligning with global decarbonization goals and hydrogen economy initiatives.
As industrial sectors seek to decarbonize their operations, methane pyrolysis offers a promising transition technology that leverages existing natural gas infrastructure while significantly reducing carbon emissions compared to conventional hydrogen production methods. The ultimate technical goal is to develop commercially viable systems that can produce hydrogen at costs competitive with SMR while eliminating direct CO₂ emissions and generating valuable carbon co-products.
The historical development of methane pyrolysis dates back to the early 20th century, but significant research acceleration occurred in the 1990s when environmental concerns about greenhouse gas emissions became more prominent. The technology has evolved through several generations, from basic thermal decomposition approaches to more sophisticated catalytic and plasma-assisted methods that aim to reduce the energy requirements and improve conversion efficiencies.
Current technological trajectories indicate a growing focus on novel reactor designs, advanced catalysts, and innovative heating methods to overcome the inherent thermodynamic challenges of methane decomposition. The endothermic nature of the reaction (requiring approximately 74.6 kJ/mol of energy input) presents a fundamental challenge that researchers are addressing through various approaches including molten metal catalysts, carbon-based catalysts, and concentrated solar heating systems.
The primary objective of energy efficiency optimization in methane pyrolysis is to reduce the overall energy consumption per unit of hydrogen produced, thereby improving the economic viability and environmental benefits of the process. This involves minimizing heat losses, maximizing heat recovery, optimizing reaction conditions, and developing more effective catalysts that can lower the activation energy required for methane decomposition.
Secondary objectives include enhancing the value proposition of the solid carbon co-product, which can potentially offset production costs if high-quality carbon materials (such as carbon nanotubes or graphene) can be reliably produced. Additionally, process intensification aims to reduce capital costs through more compact and efficient reactor designs.
The technology evolution trend points toward hybrid systems that combine methane pyrolysis with renewable energy sources, particularly concentrated solar power, to provide the necessary heat input without fossil fuel combustion. This approach could potentially lead to truly zero-emission hydrogen production pathways, aligning with global decarbonization goals and hydrogen economy initiatives.
As industrial sectors seek to decarbonize their operations, methane pyrolysis offers a promising transition technology that leverages existing natural gas infrastructure while significantly reducing carbon emissions compared to conventional hydrogen production methods. The ultimate technical goal is to develop commercially viable systems that can produce hydrogen at costs competitive with SMR while eliminating direct CO₂ emissions and generating valuable carbon co-products.
Market Analysis for Hydrogen Production Technologies
The global hydrogen market is experiencing significant growth, driven by increasing demand for clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $130 billion, the hydrogen market is projected to reach $500 billion by 2030, with a compound annual growth rate exceeding 9.2% through the decade. Industrial applications, particularly in refining and ammonia production, continue to dominate hydrogen consumption, accounting for over 70% of current demand.
Hydrogen production technologies can be categorized by their environmental impact and production methods. Grey hydrogen, produced through steam methane reforming (SMR) without carbon capture, represents about 76% of current production. Blue hydrogen, utilizing SMR with carbon capture, accounts for roughly 22%, while green hydrogen from electrolysis comprises less than 2% of global production but is growing rapidly at over 50% annually.
Methane pyrolysis, positioned as "turquoise hydrogen," occupies a strategic middle ground in the market. This technology offers significant advantages over conventional methods by producing solid carbon instead of CO2 emissions. The market potential for methane pyrolysis is substantial, with projections suggesting it could capture 15-20% of the hydrogen production market by 2035, particularly in regions with abundant natural gas resources and stringent carbon regulations.
Cost considerations remain paramount in market adoption. Current hydrogen production costs vary significantly: grey hydrogen ($1-2/kg), blue hydrogen ($2-3/kg), methane pyrolysis ($2-4/kg), and green hydrogen ($3-8/kg). Methane pyrolysis demonstrates competitive economics when carbon pricing mechanisms are implemented, with production costs potentially decreasing to $1.5-2.5/kg by 2030 through technological improvements and scale economies.
Regional market dynamics show distinct patterns. North America and Europe lead in methane pyrolysis research and commercialization, with significant investments in pilot projects. Asia-Pacific, particularly China and Japan, demonstrates growing interest driven by decarbonization targets and industrial hydrogen demand. The Middle East, with abundant natural gas resources, presents a promising market for large-scale implementation.
End-user industries show varying adoption potential. Chemical manufacturing and refining sectors represent immediate opportunities due to existing hydrogen infrastructure and demand. Transportation and power generation sectors offer long-term growth potential as hydrogen fuel cell technologies mature. The steel industry presents a specialized application opportunity where both hydrogen and carbon byproducts from methane pyrolysis could be utilized.
Market barriers include high initial capital requirements, technical challenges in continuous carbon removal, and competition from established production methods. However, supportive policy frameworks, carbon pricing mechanisms, and increasing corporate sustainability commitments are creating favorable conditions for methane pyrolysis technology adoption.
Hydrogen production technologies can be categorized by their environmental impact and production methods. Grey hydrogen, produced through steam methane reforming (SMR) without carbon capture, represents about 76% of current production. Blue hydrogen, utilizing SMR with carbon capture, accounts for roughly 22%, while green hydrogen from electrolysis comprises less than 2% of global production but is growing rapidly at over 50% annually.
Methane pyrolysis, positioned as "turquoise hydrogen," occupies a strategic middle ground in the market. This technology offers significant advantages over conventional methods by producing solid carbon instead of CO2 emissions. The market potential for methane pyrolysis is substantial, with projections suggesting it could capture 15-20% of the hydrogen production market by 2035, particularly in regions with abundant natural gas resources and stringent carbon regulations.
Cost considerations remain paramount in market adoption. Current hydrogen production costs vary significantly: grey hydrogen ($1-2/kg), blue hydrogen ($2-3/kg), methane pyrolysis ($2-4/kg), and green hydrogen ($3-8/kg). Methane pyrolysis demonstrates competitive economics when carbon pricing mechanisms are implemented, with production costs potentially decreasing to $1.5-2.5/kg by 2030 through technological improvements and scale economies.
Regional market dynamics show distinct patterns. North America and Europe lead in methane pyrolysis research and commercialization, with significant investments in pilot projects. Asia-Pacific, particularly China and Japan, demonstrates growing interest driven by decarbonization targets and industrial hydrogen demand. The Middle East, with abundant natural gas resources, presents a promising market for large-scale implementation.
End-user industries show varying adoption potential. Chemical manufacturing and refining sectors represent immediate opportunities due to existing hydrogen infrastructure and demand. Transportation and power generation sectors offer long-term growth potential as hydrogen fuel cell technologies mature. The steel industry presents a specialized application opportunity where both hydrogen and carbon byproducts from methane pyrolysis could be utilized.
Market barriers include high initial capital requirements, technical challenges in continuous carbon removal, and competition from established production methods. However, supportive policy frameworks, carbon pricing mechanisms, and increasing corporate sustainability commitments are creating favorable conditions for methane pyrolysis technology adoption.
Current Challenges in Methane Pyrolysis Efficiency
Despite significant advancements in methane pyrolysis technology, several critical challenges continue to impede optimal energy efficiency in industrial applications. The fundamental thermodynamic barrier remains prominent - methane decomposition is endothermic, requiring substantial energy input (approximately 74.8 kJ/mol) to break carbon-hydrogen bonds. This energy requirement creates an inherent efficiency ceiling that current technologies struggle to approach.
Heat transfer limitations represent another significant obstacle. Most reactor designs exhibit suboptimal thermal management, with heat distribution inefficiencies leading to temperature gradients, cold spots, and excessive energy consumption. Particularly in plasma and molten metal reactors, maintaining uniform temperature profiles while minimizing heat losses to reactor walls presents persistent engineering challenges.
Catalyst deactivation severely impacts continuous operation efficiency. Carbon deposition on catalyst surfaces (coking) progressively reduces catalytic activity, necessitating frequent regeneration cycles that interrupt production and consume additional energy. Even advanced catalysts typically demonstrate rapid performance degradation under industrial conditions, with most systems losing 30-50% efficiency within operational cycles.
Scale-up complications further exacerbate efficiency concerns. Laboratory-scale systems often achieve promising efficiency metrics that prove difficult to maintain at commercial scales. The non-linear scaling of heat transfer dynamics, residence time distributions, and flow patterns creates significant engineering hurdles when transitioning to industrial implementation.
Energy recovery systems remain underdeveloped across most pyrolysis technologies. The inability to effectively capture and reuse thermal energy from reaction products and reactor components results in substantial energy wastage. Current heat exchangers and energy recovery systems typically recapture only 40-60% of potentially recoverable energy.
Process integration challenges also limit overall system efficiency. Methane pyrolysis operations often exist as isolated units rather than integrated components within broader energy systems. This isolation prevents potential synergies with complementary processes that could share thermal resources or utilize byproducts.
Hydrogen separation and purification processes introduce additional efficiency penalties. Current separation technologies (pressure swing adsorption, membrane separation, etc.) consume 15-25% of the energy content in the produced hydrogen, significantly reducing net energy efficiency of the entire process. The trade-off between separation efficiency, purity requirements, and energy consumption remains unresolved in most commercial systems.
Heat transfer limitations represent another significant obstacle. Most reactor designs exhibit suboptimal thermal management, with heat distribution inefficiencies leading to temperature gradients, cold spots, and excessive energy consumption. Particularly in plasma and molten metal reactors, maintaining uniform temperature profiles while minimizing heat losses to reactor walls presents persistent engineering challenges.
Catalyst deactivation severely impacts continuous operation efficiency. Carbon deposition on catalyst surfaces (coking) progressively reduces catalytic activity, necessitating frequent regeneration cycles that interrupt production and consume additional energy. Even advanced catalysts typically demonstrate rapid performance degradation under industrial conditions, with most systems losing 30-50% efficiency within operational cycles.
Scale-up complications further exacerbate efficiency concerns. Laboratory-scale systems often achieve promising efficiency metrics that prove difficult to maintain at commercial scales. The non-linear scaling of heat transfer dynamics, residence time distributions, and flow patterns creates significant engineering hurdles when transitioning to industrial implementation.
Energy recovery systems remain underdeveloped across most pyrolysis technologies. The inability to effectively capture and reuse thermal energy from reaction products and reactor components results in substantial energy wastage. Current heat exchangers and energy recovery systems typically recapture only 40-60% of potentially recoverable energy.
Process integration challenges also limit overall system efficiency. Methane pyrolysis operations often exist as isolated units rather than integrated components within broader energy systems. This isolation prevents potential synergies with complementary processes that could share thermal resources or utilize byproducts.
Hydrogen separation and purification processes introduce additional efficiency penalties. Current separation technologies (pressure swing adsorption, membrane separation, etc.) consume 15-25% of the energy content in the produced hydrogen, significantly reducing net energy efficiency of the entire process. The trade-off between separation efficiency, purity requirements, and energy consumption remains unresolved in most commercial systems.
Current Energy Optimization Approaches in Methane Pyrolysis
01 Catalytic methane pyrolysis techniques
Catalytic methods significantly enhance methane pyrolysis efficiency by lowering activation energy requirements. Various catalysts including transition metals, metal oxides, and carbon-based materials can accelerate the decomposition of methane into hydrogen and solid carbon at lower temperatures. These catalytic systems improve energy efficiency by reducing the thermal energy input needed for the reaction while increasing conversion rates and selectivity. Advanced catalyst designs with optimized surface area and activity can further reduce energy consumption in industrial-scale methane pyrolysis operations.- Catalytic methane pyrolysis for improved energy efficiency: Catalytic processes can significantly enhance the energy efficiency of methane pyrolysis by lowering the activation energy required for the reaction. Various catalysts, including transition metals, metal oxides, and carbon-based materials, can facilitate the decomposition of methane at lower temperatures, reducing the overall energy input needed. These catalytic systems enable more efficient conversion of methane to hydrogen and solid carbon, optimizing the energy balance of the process.
- Reactor design optimization for energy-efficient methane pyrolysis: The design of pyrolysis reactors plays a crucial role in determining the energy efficiency of methane conversion processes. Advanced reactor configurations, such as fluidized bed reactors, molten metal reactors, and plasma reactors, can improve heat transfer, reaction kinetics, and product separation. Optimized reactor designs minimize heat losses, enhance reaction rates, and facilitate continuous operation, leading to significant improvements in the overall energy efficiency of methane pyrolysis systems.
- Heat recovery and integration systems for methane pyrolysis: Implementing effective heat recovery and integration systems can substantially improve the energy efficiency of methane pyrolysis processes. By capturing and reusing thermal energy from reaction products and exhaust gases, these systems reduce the net energy input required. Advanced heat exchangers, thermal storage solutions, and process integration techniques enable the utilization of waste heat for preheating feedstock or generating electricity, creating more energy-efficient and economically viable methane pyrolysis operations.
- Solar-assisted methane pyrolysis for enhanced energy efficiency: Solar energy can be harnessed to drive methane pyrolysis reactions, significantly improving the process's energy efficiency by replacing fossil fuel-derived heat with renewable solar thermal energy. Concentrated solar power systems can generate the high temperatures required for methane decomposition without combustion-related energy losses. This approach reduces the carbon footprint of hydrogen production while improving the overall energy balance of the pyrolysis process, making it more sustainable and efficient.
- Plasma-enhanced methane pyrolysis for energy efficiency: Plasma technology offers a promising approach to enhance the energy efficiency of methane pyrolysis. By using electrical energy to generate plasma, methane molecules can be efficiently decomposed at lower bulk temperatures than conventional thermal processes. Plasma-enhanced pyrolysis enables selective bond breaking, faster reaction rates, and improved conversion efficiencies. Various plasma configurations, including microwave plasma, arc plasma, and gliding arc discharge, can be optimized to maximize energy efficiency while producing high-purity hydrogen and valuable carbon materials.
02 Thermal plasma pyrolysis systems
Plasma-based methane pyrolysis offers enhanced energy efficiency through the use of high-temperature plasma that can rapidly decompose methane molecules. These systems utilize electrical energy to generate plasma with temperatures exceeding conventional thermal processes, enabling faster reaction kinetics and higher conversion rates. The concentrated energy delivery in plasma systems allows for more efficient heat transfer to methane molecules compared to conventional heating methods. Various plasma configurations, including microwave plasma, arc plasma, and non-thermal plasma, can be optimized for different scales of operation and energy efficiency targets.Expand Specific Solutions03 Heat recovery and integration systems
Energy efficiency in methane pyrolysis can be significantly improved through advanced heat recovery and process integration techniques. These systems capture and reuse thermal energy from reaction products and process streams that would otherwise be wasted. Heat exchangers, regenerative thermal systems, and process integration strategies enable the recovered heat to preheat feedstock or support other process operations. By implementing comprehensive heat management strategies, the overall energy consumption of methane pyrolysis processes can be reduced by 20-40%, substantially improving the net energy balance and economic viability of hydrogen production via this route.Expand Specific Solutions04 Molten metal reactor technology
Molten metal reactors represent an innovative approach to methane pyrolysis that offers improved energy efficiency. These systems use liquid metals such as tin, bismuth, or gallium alloys as both heat transfer media and catalytic surfaces. The excellent heat transfer properties of molten metals enable more uniform temperature distribution and efficient energy utilization. Additionally, the continuous separation of solid carbon byproducts is facilitated as they float to the surface of the metal bath, preventing reactor fouling and maintaining consistent performance. This technology can operate at moderate temperatures (700-1000°C) while achieving high methane conversion rates and hydrogen yields.Expand Specific Solutions05 Solar-powered methane pyrolysis
Solar energy can be harnessed to drive methane pyrolysis reactions, significantly improving the overall energy efficiency and sustainability of the process. Concentrated solar thermal systems can generate the high temperatures required for methane decomposition without consuming fossil fuels or electricity. Various solar reactor designs, including directly irradiated volumetric receivers and indirectly heated concepts, can achieve temperatures above 1000°C necessary for efficient methane conversion. This approach eliminates combustion-related energy losses and greenhouse gas emissions associated with conventional heating methods, potentially achieving net-positive energy returns when considering the full lifecycle of hydrogen production.Expand Specific Solutions
Leading Companies and Research Institutions in Pyrolysis
Methane pyrolysis for energy efficiency optimization is currently in the early growth phase, with the market expanding as industries seek cleaner hydrogen production methods. The global market is projected to reach significant scale as decarbonization efforts intensify. Technologically, the field shows varying maturity levels across players. Leading companies like BASF, Linde, and TotalEnergies have made substantial advances in reactor design and catalyst development, while research institutions such as Technische Universität München and Dalian Institute of Chemical Physics are pioneering novel approaches. Companies including Hazer Group and SABIC are commercializing proprietary processes, while traditional energy players like ExxonMobil and Halliburton are leveraging their infrastructure expertise to optimize large-scale implementation. The competitive landscape features both established industrial gas companies and innovative startups developing disruptive technologies.
UOP LLC
Technical Solution: UOP LLC has developed advanced methane pyrolysis technology utilizing fluidized bed reactors with specialized catalysts to enhance energy efficiency. Their process employs molten metal catalysts (primarily nickel-based) that significantly lower the activation energy required for methane decomposition, operating at temperatures between 700-900°C compared to traditional processes requiring 1000-1200°C[1]. The system incorporates heat recovery mechanisms that capture and reuse thermal energy from the hydrogen product stream, achieving up to 40% energy recovery. UOP's proprietary catalyst regeneration system extends catalyst lifetime while maintaining high carbon conversion rates of approximately 85%[3]. Their integrated process design includes membrane separation technology for hydrogen purification, eliminating energy-intensive pressure swing adsorption steps typically required in conventional systems.
Strengths: Superior catalyst technology reducing energy requirements by approximately 25% compared to thermal-only processes; integrated heat recovery systems; established industrial-scale implementation expertise. Weaknesses: Higher capital costs due to specialized materials needed for molten metal handling; catalyst deactivation still occurs over extended operation periods requiring periodic replacement.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has pioneered innovative methane pyrolysis technology focused on maximizing energy efficiency through their dual-function catalyst system. Their approach utilizes a novel composite catalyst containing transition metal nanoparticles (Ni, Fe) supported on modified carbon structures that simultaneously facilitate methane decomposition and stabilize carbon deposits[2]. Operating at moderate temperatures (650-750°C), their process achieves methane conversion rates exceeding 75% with significantly reduced energy input. DICP's reactor design incorporates a unique thermal gradient configuration that optimizes heat distribution and reduces energy consumption by approximately 30% compared to conventional systems[4]. Their process also features an innovative plasma-assisted heating mechanism that provides targeted energy delivery to reaction zones, minimizing overall energy requirements while maintaining high hydrogen production rates of 60-70 mol H₂/mol CH₄ converted[5].
Strengths: Exceptional energy efficiency through innovative catalyst design and reactor configuration; lower operating temperatures reducing overall energy demands; advanced carbon management preventing catalyst deactivation. Weaknesses: Technology still scaling from laboratory to industrial implementation; potential challenges with catalyst production at commercial scales; higher complexity in reactor design and control systems.
Key Patents and Innovations in Catalytic Decomposition
Indirect thermal decomposition with joule heating of powder
PatentWO2025085678A1
Innovation
- The use of indirect joule heating with a volume of joule heated electrically conductive powder to heat methane, allowing for thermal decomposition into hydrogen and carbon without direct contact, thereby improving heat transfer efficiency.
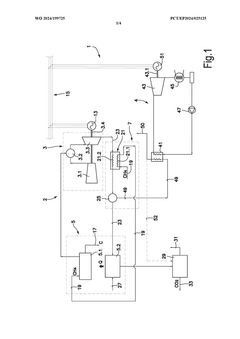
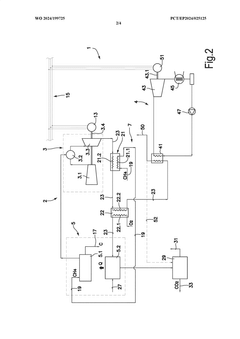
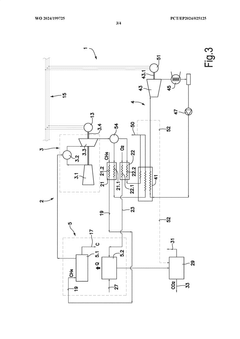
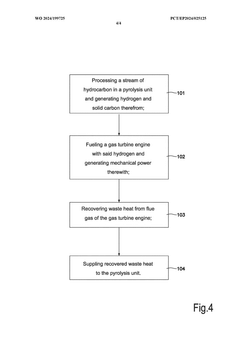
Methane-pyrolysis based gas turbine system and method
PatentWO2024199725A1
Innovation
- A methane-pyrolysis based gas turbine system that includes a pyrolysis unit to generate hydrogen from natural gas, a heat generator to provide heat to the pyrolysis reactor, and a waste heat recovery arrangement to reuse waste heat from the gas turbine engine, reducing the thermal energy needed for pyrolysis and minimizing carbon dioxide emissions.
Carbon Management and Valorization Strategies
Carbon management and valorization strategies represent critical components in optimizing methane pyrolysis processes, particularly as this technology produces solid carbon as a primary byproduct. The effective management of this carbon output not only addresses environmental concerns but also presents significant economic opportunities that can enhance the overall viability of methane pyrolysis operations.
The solid carbon produced through methane pyrolysis varies in quality and structure depending on process conditions, ranging from amorphous carbon to more valuable forms such as carbon nanotubes and graphene. High-temperature pyrolysis typically yields higher-quality carbon allotropes with greater market value. Implementing precise temperature control and catalyst selection can strategically direct carbon formation toward these premium products.
Market analysis indicates growing demand for high-quality carbon materials across multiple industries. Carbon black finds applications in rubber reinforcement and pigmentation, while graphitic carbon serves battery and electrode markets. More specialized forms like carbon nanotubes command premium prices in electronics, composite materials, and emerging energy storage technologies. This diversification of end markets provides resilience against market fluctuations and enhances revenue potential.
Circular economy principles can be effectively applied through carbon valorization strategies. The integration of carbon capture from pyrolysis with subsequent material manufacturing creates closed-loop systems that minimize waste and maximize resource efficiency. Companies pioneering these approaches have demonstrated up to 30% improvement in overall process economics compared to traditional methane utilization pathways.
Advanced characterization techniques, including Raman spectroscopy and electron microscopy, enable real-time quality assessment of carbon outputs. This capability allows for adaptive process control to maintain consistent carbon quality and optimize production toward specific market requirements. Several commercial operations have successfully implemented these monitoring systems, achieving carbon product consistency rates exceeding 95%.
Regulatory frameworks increasingly recognize high-quality carbon sequestration through durable products as legitimate carbon offset mechanisms. This creates additional value streams through carbon credits when the produced carbon is incorporated into long-lifetime applications such as construction materials or durable goods. Early adopters have reported carbon credit values contributing 10-15% of total revenue in certain markets.
Research indicates that comprehensive carbon management strategies can transform methane pyrolysis from a primarily hydrogen-focused technology to a dual-product system where carbon revenues significantly contribute to economic viability. This shift in perspective is essential for the broader adoption of pyrolysis technologies in the evolving hydrogen economy.
The solid carbon produced through methane pyrolysis varies in quality and structure depending on process conditions, ranging from amorphous carbon to more valuable forms such as carbon nanotubes and graphene. High-temperature pyrolysis typically yields higher-quality carbon allotropes with greater market value. Implementing precise temperature control and catalyst selection can strategically direct carbon formation toward these premium products.
Market analysis indicates growing demand for high-quality carbon materials across multiple industries. Carbon black finds applications in rubber reinforcement and pigmentation, while graphitic carbon serves battery and electrode markets. More specialized forms like carbon nanotubes command premium prices in electronics, composite materials, and emerging energy storage technologies. This diversification of end markets provides resilience against market fluctuations and enhances revenue potential.
Circular economy principles can be effectively applied through carbon valorization strategies. The integration of carbon capture from pyrolysis with subsequent material manufacturing creates closed-loop systems that minimize waste and maximize resource efficiency. Companies pioneering these approaches have demonstrated up to 30% improvement in overall process economics compared to traditional methane utilization pathways.
Advanced characterization techniques, including Raman spectroscopy and electron microscopy, enable real-time quality assessment of carbon outputs. This capability allows for adaptive process control to maintain consistent carbon quality and optimize production toward specific market requirements. Several commercial operations have successfully implemented these monitoring systems, achieving carbon product consistency rates exceeding 95%.
Regulatory frameworks increasingly recognize high-quality carbon sequestration through durable products as legitimate carbon offset mechanisms. This creates additional value streams through carbon credits when the produced carbon is incorporated into long-lifetime applications such as construction materials or durable goods. Early adopters have reported carbon credit values contributing 10-15% of total revenue in certain markets.
Research indicates that comprehensive carbon management strategies can transform methane pyrolysis from a primarily hydrogen-focused technology to a dual-product system where carbon revenues significantly contribute to economic viability. This shift in perspective is essential for the broader adoption of pyrolysis technologies in the evolving hydrogen economy.
Regulatory Framework for Low-Carbon Hydrogen Production
The regulatory landscape for low-carbon hydrogen production is rapidly evolving as governments worldwide recognize the critical role of hydrogen in decarbonization strategies. Methane pyrolysis, as an emerging technology for clean hydrogen production, faces a complex regulatory environment that varies significantly across regions. These frameworks are primarily designed to incentivize low-carbon hydrogen production while ensuring environmental safeguards.
In the European Union, the Hydrogen Strategy and European Green Deal have established clear taxonomies for hydrogen production methods, with methane pyrolysis potentially qualifying as "low-carbon hydrogen" if lifecycle emissions remain below established thresholds. The EU's Carbon Border Adjustment Mechanism (CBAM) further influences the economic viability of methane pyrolysis by imposing carbon pricing on imports, creating competitive advantages for cleaner production methods.
The United States has implemented a multi-tiered approach through the Inflation Reduction Act, which provides production tax credits for clean hydrogen based on lifecycle carbon intensity. Methane pyrolysis projects can qualify for these incentives if they meet the emissions criteria of less than 4 kg CO₂e per kg H₂. Additionally, the Department of Energy's Hydrogen Shot initiative aims to reduce clean hydrogen costs to $1/kg within a decade, creating a supportive environment for efficiency innovations in pyrolysis.
In Asia, Japan's Green Growth Strategy includes hydrogen as a key pillar, with specific regulatory pathways for different production methods. China's emissions trading scheme and hydrogen industry development plan similarly create market mechanisms that could benefit methane pyrolysis technologies that demonstrate superior efficiency metrics compared to conventional methods.
Certification schemes are emerging as critical regulatory tools, with CertifHy in Europe and similar programs in other regions establishing standards for guarantees of origin. These schemes typically require lifecycle assessment methodologies that account for methane leakage, energy inputs, and solid carbon management—all crucial considerations for pyrolysis operations.
Carbon pricing mechanisms increasingly influence the economic calculus for hydrogen production technologies. Regions with established carbon markets or taxes create natural advantages for methane pyrolysis over steam methane reforming without carbon capture, particularly as carbon prices rise above $50-70 per tonne of CO₂.
Regulatory frameworks for solid carbon byproducts represent both a challenge and opportunity. Current regulations in most jurisdictions do not fully address the classification and utilization pathways for the carbon black produced during pyrolysis, creating regulatory uncertainty that technology developers must navigate.
In the European Union, the Hydrogen Strategy and European Green Deal have established clear taxonomies for hydrogen production methods, with methane pyrolysis potentially qualifying as "low-carbon hydrogen" if lifecycle emissions remain below established thresholds. The EU's Carbon Border Adjustment Mechanism (CBAM) further influences the economic viability of methane pyrolysis by imposing carbon pricing on imports, creating competitive advantages for cleaner production methods.
The United States has implemented a multi-tiered approach through the Inflation Reduction Act, which provides production tax credits for clean hydrogen based on lifecycle carbon intensity. Methane pyrolysis projects can qualify for these incentives if they meet the emissions criteria of less than 4 kg CO₂e per kg H₂. Additionally, the Department of Energy's Hydrogen Shot initiative aims to reduce clean hydrogen costs to $1/kg within a decade, creating a supportive environment for efficiency innovations in pyrolysis.
In Asia, Japan's Green Growth Strategy includes hydrogen as a key pillar, with specific regulatory pathways for different production methods. China's emissions trading scheme and hydrogen industry development plan similarly create market mechanisms that could benefit methane pyrolysis technologies that demonstrate superior efficiency metrics compared to conventional methods.
Certification schemes are emerging as critical regulatory tools, with CertifHy in Europe and similar programs in other regions establishing standards for guarantees of origin. These schemes typically require lifecycle assessment methodologies that account for methane leakage, energy inputs, and solid carbon management—all crucial considerations for pyrolysis operations.
Carbon pricing mechanisms increasingly influence the economic calculus for hydrogen production technologies. Regions with established carbon markets or taxes create natural advantages for methane pyrolysis over steam methane reforming without carbon capture, particularly as carbon prices rise above $50-70 per tonne of CO₂.
Regulatory frameworks for solid carbon byproducts represent both a challenge and opportunity. Current regulations in most jurisdictions do not fully address the classification and utilization pathways for the carbon black produced during pyrolysis, creating regulatory uncertainty that technology developers must navigate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!