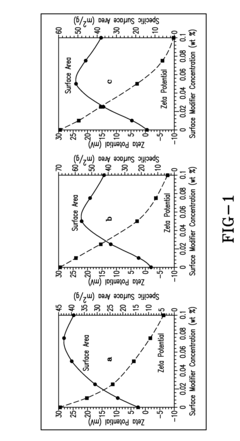
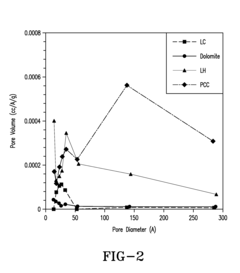
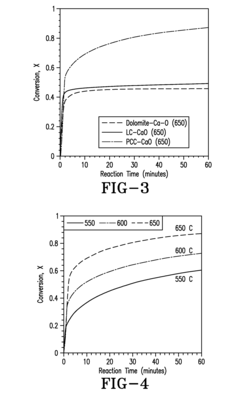
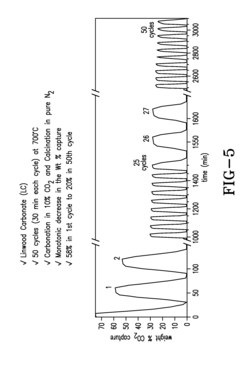
Hydrogen Purity Standards in Methane Pyrolysis.
SEP 5, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Hydrogen Purity Background and Objectives
Methane pyrolysis has emerged as a promising pathway for hydrogen production, offering a potentially cleaner alternative to conventional methods such as steam methane reforming. The evolution of this technology dates back to the early 20th century, but significant advancements have only materialized in recent decades with the growing emphasis on decarbonization and sustainable energy solutions. The trajectory of methane pyrolysis development has been characterized by incremental improvements in catalytic systems, reactor designs, and carbon management strategies.
The current technological landscape is witnessing a convergence of thermal, catalytic, and plasma-assisted pyrolysis approaches, each with distinct implications for hydrogen purity. Traditional thermal pyrolysis operates at temperatures exceeding 1000°C, while catalytic methods utilize transition metals to reduce activation energy and operating temperatures. Plasma-assisted techniques represent the cutting edge, employing non-equilibrium plasma to enhance reaction kinetics at lower temperatures.
Hydrogen purity standards in methane pyrolysis are fundamentally driven by end-use requirements across various sectors. Fuel cell applications, particularly in transportation, demand ultra-high purity hydrogen (99.999+%) to prevent catalyst poisoning and ensure optimal performance. Industrial applications such as semiconductor manufacturing and metallurgical processes similarly require stringent purity specifications, albeit with different impurity tolerance profiles.
The technical objectives for advancing hydrogen purity in methane pyrolysis are multifaceted. Primary goals include developing in-situ purification techniques that can selectively remove contaminants during the pyrolysis process, thereby reducing downstream purification requirements. Additionally, there is a pressing need to establish standardized analytical methods for accurately quantifying trace impurities specific to pyrolytic hydrogen, as current methods are largely adapted from other hydrogen production pathways.
Another critical objective involves understanding the relationship between process parameters and impurity formation mechanisms. This includes mapping how variables such as temperature profiles, residence time, pressure conditions, and catalyst properties influence the formation of contaminants like carbon monoxide, methane slip, sulfur compounds, and aromatic hydrocarbons. Such knowledge is essential for designing targeted purification strategies and optimizing process conditions.
The evolution of regulatory frameworks and industry standards for hydrogen purity represents another dimension of this technological landscape. As hydrogen economies scale globally, harmonization of purity specifications across different regions and applications becomes increasingly important for market development and technology deployment. The technical community aims to establish evidence-based standards that balance purity requirements with economic feasibility.
The current technological landscape is witnessing a convergence of thermal, catalytic, and plasma-assisted pyrolysis approaches, each with distinct implications for hydrogen purity. Traditional thermal pyrolysis operates at temperatures exceeding 1000°C, while catalytic methods utilize transition metals to reduce activation energy and operating temperatures. Plasma-assisted techniques represent the cutting edge, employing non-equilibrium plasma to enhance reaction kinetics at lower temperatures.
Hydrogen purity standards in methane pyrolysis are fundamentally driven by end-use requirements across various sectors. Fuel cell applications, particularly in transportation, demand ultra-high purity hydrogen (99.999+%) to prevent catalyst poisoning and ensure optimal performance. Industrial applications such as semiconductor manufacturing and metallurgical processes similarly require stringent purity specifications, albeit with different impurity tolerance profiles.
The technical objectives for advancing hydrogen purity in methane pyrolysis are multifaceted. Primary goals include developing in-situ purification techniques that can selectively remove contaminants during the pyrolysis process, thereby reducing downstream purification requirements. Additionally, there is a pressing need to establish standardized analytical methods for accurately quantifying trace impurities specific to pyrolytic hydrogen, as current methods are largely adapted from other hydrogen production pathways.
Another critical objective involves understanding the relationship between process parameters and impurity formation mechanisms. This includes mapping how variables such as temperature profiles, residence time, pressure conditions, and catalyst properties influence the formation of contaminants like carbon monoxide, methane slip, sulfur compounds, and aromatic hydrocarbons. Such knowledge is essential for designing targeted purification strategies and optimizing process conditions.
The evolution of regulatory frameworks and industry standards for hydrogen purity represents another dimension of this technological landscape. As hydrogen economies scale globally, harmonization of purity specifications across different regions and applications becomes increasingly important for market development and technology deployment. The technical community aims to establish evidence-based standards that balance purity requirements with economic feasibility.
Market Analysis for High-Purity Hydrogen Demand
The global market for high-purity hydrogen is experiencing significant growth, driven primarily by increasing applications in various industrial sectors. The demand for hydrogen with purity levels exceeding 99.99% has been steadily rising, with particular emphasis on applications requiring ultra-high purity standards of 99.9999% (6N) or higher. This trend is especially relevant for methane pyrolysis processes, which are gaining attention as a cleaner alternative to traditional hydrogen production methods.
Industrial sectors including semiconductor manufacturing, electronics, specialty chemicals, and advanced materials processing represent the largest consumers of high-purity hydrogen. The semiconductor industry alone accounts for a substantial portion of the market, requiring hydrogen with minimal contaminants for critical manufacturing processes. Additionally, the pharmaceutical industry demands high-purity hydrogen for hydrogenation reactions and other specialized applications where impurities could compromise product quality.
The transportation sector presents an emerging market for high-purity hydrogen, particularly in fuel cell electric vehicles (FCEVs). Current industry standards for automotive fuel cells typically require hydrogen with 99.97% purity, though this specification is becoming more stringent as fuel cell technology advances. Countries with established hydrogen infrastructure initiatives, such as Japan, South Korea, Germany, and parts of the United States, are driving this segment of demand.
Market analysis indicates that the premium pricing structure for high-purity hydrogen creates significant economic incentives for producers. The price differential between standard industrial-grade hydrogen (99.95%) and ultra-high-purity grades (99.9999%) can range from 3 to 10 times, depending on volume requirements and geographic location. This premium pricing reflects both the technical challenges in achieving higher purity levels and the specialized applications that require them.
Regional demand patterns show Asia-Pacific leading the market for high-purity hydrogen consumption, followed by North America and Europe. This distribution aligns with the concentration of semiconductor manufacturing and electronics industries. However, emerging markets in the Middle East and parts of Africa are showing increased interest in high-purity hydrogen for specialized industrial applications and potential export opportunities.
The market for hydrogen produced specifically through methane pyrolysis is still developing but shows promising growth potential due to its lower carbon footprint compared to traditional methods. End-users increasingly value the environmental benefits of this production pathway, particularly as carbon pricing mechanisms become more widespread globally. This environmental premium is creating new market opportunities for methane pyrolysis-derived hydrogen that meets high-purity standards.
Industrial sectors including semiconductor manufacturing, electronics, specialty chemicals, and advanced materials processing represent the largest consumers of high-purity hydrogen. The semiconductor industry alone accounts for a substantial portion of the market, requiring hydrogen with minimal contaminants for critical manufacturing processes. Additionally, the pharmaceutical industry demands high-purity hydrogen for hydrogenation reactions and other specialized applications where impurities could compromise product quality.
The transportation sector presents an emerging market for high-purity hydrogen, particularly in fuel cell electric vehicles (FCEVs). Current industry standards for automotive fuel cells typically require hydrogen with 99.97% purity, though this specification is becoming more stringent as fuel cell technology advances. Countries with established hydrogen infrastructure initiatives, such as Japan, South Korea, Germany, and parts of the United States, are driving this segment of demand.
Market analysis indicates that the premium pricing structure for high-purity hydrogen creates significant economic incentives for producers. The price differential between standard industrial-grade hydrogen (99.95%) and ultra-high-purity grades (99.9999%) can range from 3 to 10 times, depending on volume requirements and geographic location. This premium pricing reflects both the technical challenges in achieving higher purity levels and the specialized applications that require them.
Regional demand patterns show Asia-Pacific leading the market for high-purity hydrogen consumption, followed by North America and Europe. This distribution aligns with the concentration of semiconductor manufacturing and electronics industries. However, emerging markets in the Middle East and parts of Africa are showing increased interest in high-purity hydrogen for specialized industrial applications and potential export opportunities.
The market for hydrogen produced specifically through methane pyrolysis is still developing but shows promising growth potential due to its lower carbon footprint compared to traditional methods. End-users increasingly value the environmental benefits of this production pathway, particularly as carbon pricing mechanisms become more widespread globally. This environmental premium is creating new market opportunities for methane pyrolysis-derived hydrogen that meets high-purity standards.
Current Hydrogen Purity Standards and Technical Challenges
The global hydrogen market is currently governed by a complex framework of purity standards that vary across regions and applications. For methane pyrolysis-derived hydrogen, the most widely referenced standards include ISO 14687, which categorizes hydrogen into multiple grades based on application, with Grade A (99.97% purity) for industrial applications and Grade D (99.999% purity) for fuel cell vehicles. In the United States, SAE J2719 establishes stringent requirements for fuel cell vehicles, specifying maximum contaminant levels for sulfur compounds (0.004 ppm), carbon monoxide (0.2 ppm), and ammonia (0.1 ppm).
European standards are defined by EN 17124, which closely aligns with ISO requirements but includes region-specific adaptations. The hydrogen produced through methane pyrolysis must meet these standards to be commercially viable, particularly for high-value applications such as fuel cells and semiconductor manufacturing, where even trace impurities can significantly impact performance and durability.
The technical challenges in achieving these purity standards through methane pyrolysis are substantial. The primary challenge stems from the process itself, which generates solid carbon alongside hydrogen. Preventing carbon particle contamination requires sophisticated separation technologies, including membrane filtration systems and pressure swing adsorption (PSA) units specifically designed for pyrolysis outputs.
Catalyst poisoning presents another significant challenge, as sulfur compounds and other contaminants in the feedstock methane can deactivate catalysts used in the pyrolysis process, reducing efficiency and potentially introducing impurities into the hydrogen stream. This necessitates either high-purity methane feedstock or additional purification steps.
Temperature management during pyrolysis creates additional purification challenges, as higher temperatures can lead to the formation of unwanted byproducts including acetylene and ethylene, which must be removed to meet stringent purity standards. The energy requirements for these additional purification steps can significantly impact the overall efficiency and cost-effectiveness of the process.
Scale-up challenges further complicate purity maintenance, as laboratory-scale purification techniques often prove difficult to implement economically at industrial scales. The capital expenditure for high-efficiency purification systems represents a substantial portion of overall plant costs, creating a tension between economic viability and product quality.
Analytical challenges also exist in the real-time monitoring of hydrogen purity at the parts-per-billion level required by certain applications. Current sensor technologies often lack the sensitivity, specificity, or durability needed for continuous industrial monitoring, creating verification challenges for producers and uncertainty for end-users.
European standards are defined by EN 17124, which closely aligns with ISO requirements but includes region-specific adaptations. The hydrogen produced through methane pyrolysis must meet these standards to be commercially viable, particularly for high-value applications such as fuel cells and semiconductor manufacturing, where even trace impurities can significantly impact performance and durability.
The technical challenges in achieving these purity standards through methane pyrolysis are substantial. The primary challenge stems from the process itself, which generates solid carbon alongside hydrogen. Preventing carbon particle contamination requires sophisticated separation technologies, including membrane filtration systems and pressure swing adsorption (PSA) units specifically designed for pyrolysis outputs.
Catalyst poisoning presents another significant challenge, as sulfur compounds and other contaminants in the feedstock methane can deactivate catalysts used in the pyrolysis process, reducing efficiency and potentially introducing impurities into the hydrogen stream. This necessitates either high-purity methane feedstock or additional purification steps.
Temperature management during pyrolysis creates additional purification challenges, as higher temperatures can lead to the formation of unwanted byproducts including acetylene and ethylene, which must be removed to meet stringent purity standards. The energy requirements for these additional purification steps can significantly impact the overall efficiency and cost-effectiveness of the process.
Scale-up challenges further complicate purity maintenance, as laboratory-scale purification techniques often prove difficult to implement economically at industrial scales. The capital expenditure for high-efficiency purification systems represents a substantial portion of overall plant costs, creating a tension between economic viability and product quality.
Analytical challenges also exist in the real-time monitoring of hydrogen purity at the parts-per-billion level required by certain applications. Current sensor technologies often lack the sensitivity, specificity, or durability needed for continuous industrial monitoring, creating verification challenges for producers and uncertainty for end-users.
Existing Purification Methods for Pyrolysis-Derived Hydrogen
01 Purification techniques for hydrogen from methane pyrolysis
Various purification techniques are employed to enhance the purity of hydrogen produced through methane pyrolysis. These methods include pressure swing adsorption (PSA), membrane separation, cryogenic separation, and chemical absorption processes. These techniques effectively remove impurities such as carbon monoxide, carbon dioxide, and unreacted methane from the hydrogen stream, resulting in high-purity hydrogen suitable for various applications including fuel cells and industrial processes.- Purification methods for hydrogen from methane pyrolysis: Various purification techniques are employed to enhance the purity of hydrogen produced through methane pyrolysis. These methods include pressure swing adsorption (PSA), membrane separation, cryogenic separation, and chemical absorption processes. These purification steps are crucial for removing impurities such as carbon monoxide, carbon dioxide, unreacted methane, and other hydrocarbons from the hydrogen stream, resulting in high-purity hydrogen suitable for fuel cell applications and other industrial uses.
- Catalyst systems for improved hydrogen purity: Specialized catalyst systems are developed to enhance the efficiency of methane pyrolysis and directly improve hydrogen purity. These catalysts, including transition metals, metal alloys, and carbon-based materials, facilitate the decomposition of methane while minimizing the formation of byproducts. Advanced catalyst designs incorporate supports and promoters that selectively favor hydrogen production while reducing carbon deposition and catalyst deactivation, thereby increasing the overall purity of the hydrogen product without requiring extensive downstream purification.
- Reactor design for high-purity hydrogen production: Innovative reactor designs specifically engineered for methane pyrolysis focus on maximizing hydrogen purity. These include fluidized bed reactors, molten metal reactors, plasma reactors, and microwave-assisted reactors. The reactor configurations control reaction parameters such as temperature, pressure, and residence time to optimize methane conversion while minimizing side reactions. Advanced heat management systems and continuous carbon removal mechanisms help maintain stable operation conditions that favor the production of high-purity hydrogen with minimal contaminants.
- Integration of separation technologies in methane pyrolysis systems: Integrated separation technologies are incorporated directly into methane pyrolysis systems to achieve higher hydrogen purity. These integrated approaches combine in-situ separation methods with the pyrolysis process, allowing for immediate removal of hydrogen from the reaction zone. Technologies such as hydrogen-selective membranes, temperature swing adsorption, and selective condensation are strategically positioned within the process flow to continuously extract pure hydrogen while leaving carbon and other byproducts behind. This integration reduces contamination risks and energy requirements compared to conventional post-reaction purification.
- Process optimization for enhanced hydrogen purity: Process optimization strategies focus on adjusting operational parameters to maximize hydrogen purity from methane pyrolysis. These approaches include precise temperature control, optimized feed gas composition, staged reaction processes, and innovative cooling methods. Advanced monitoring and control systems enable real-time adjustments to maintain optimal conditions throughout the process. Additionally, hybrid processes that combine pyrolysis with complementary technologies such as reforming or partial oxidation are developed to achieve higher hydrogen purity while improving overall process efficiency and reducing energy consumption.
02 Catalyst systems for improved hydrogen purity
Specialized catalyst systems play a crucial role in methane pyrolysis for producing high-purity hydrogen. Metal-based catalysts (including nickel, iron, and noble metals), carbon-based catalysts, and molten metal catalysts can significantly enhance the conversion efficiency and selectivity of the pyrolysis process. These catalysts minimize the formation of byproducts and increase hydrogen yield and purity by lowering activation energy and promoting specific reaction pathways during the decomposition of methane.Expand Specific Solutions03 Reactor design for high-purity hydrogen production
Advanced reactor designs significantly impact the purity of hydrogen produced through methane pyrolysis. Fluidized bed reactors, molten metal reactors, plasma reactors, and microwave-assisted reactors are engineered to optimize reaction conditions and minimize contamination. These specialized reactor configurations control temperature profiles, residence time, and heat transfer efficiency, leading to more complete methane conversion and reduced formation of impurities in the hydrogen product stream.Expand Specific Solutions04 Process integration and optimization for hydrogen purity
Integrated process systems combine methane pyrolysis with downstream purification steps to achieve high hydrogen purity. These systems incorporate heat recovery, gas separation units, and carbon capture technologies in optimized configurations. Advanced process control strategies, including real-time monitoring and adjustment of operating parameters such as temperature, pressure, and feed rate, ensure consistent hydrogen purity while maximizing energy efficiency and minimizing operational costs.Expand Specific Solutions05 Carbon management strategies affecting hydrogen purity
Effective carbon management during methane pyrolysis directly impacts hydrogen purity. Techniques for solid carbon separation, carbon product valorization, and continuous carbon removal systems prevent carbon contamination in the hydrogen stream. Advanced approaches include in-situ carbon sequestration, carbon filtration systems, and the production of valuable carbon nanomaterials as byproducts. These strategies not only improve hydrogen purity but also enhance the economic and environmental sustainability of the pyrolysis process.Expand Specific Solutions
Leading Companies and Research Institutions in Hydrogen Purification
The methane pyrolysis hydrogen purity standards market is currently in a growth phase, with increasing focus on clean hydrogen production methods. The global market is expanding rapidly, driven by decarbonization initiatives and estimated to reach significant scale as hydrogen economy develops. Technologically, the field shows varying maturity levels across players. Shell Oil and Air Liquide demonstrate advanced capabilities with established purification technologies, while newer entrants like Molten Industries are developing innovative approaches. BASF, ExxonMobil, and Air Products & Chemicals have leveraged their industrial gas expertise to develop proprietary purification methods. Chinese players including CNPC and Sichuan Techairs are rapidly advancing their technical capabilities, particularly in large-scale implementation, positioning this sector for accelerated growth as standards continue to evolve.
Shell Oil Co.
Technical Solution: Shell Oil Co. has developed an integrated hydrogen purification system for methane pyrolysis that combines multiple purification technologies in a modular, scalable platform. Their approach begins with a proprietary high-temperature ceramic filter system that removes solid carbon particles down to sub-micron levels. This is followed by a catalytic purification stage using Shell's patented metal-organic framework (MOF) materials that selectively adsorb contaminants like sulfur compounds and CO2. The core of their technology employs a pressure swing adsorption (PSA) system optimized specifically for pyrolysis-derived hydrogen, featuring rapid cycling and reduced bed sizes compared to conventional PSA. Shell's system achieves hydrogen purities of 99.999+% while maintaining recovery rates above 80%. Their process is distinguished by its energy integration approach, which recovers waste heat from the pyrolysis process to power the purification steps, reducing overall energy consumption by approximately 30% compared to conventional purification methods. The system includes advanced process control algorithms that continuously optimize operating parameters based on feed composition variations.
Strengths: Excellent energy efficiency through heat integration; modular design allowing scalability from small to industrial-scale applications; robust performance with varying feedstock quality. Weaknesses: Complex system requiring sophisticated control systems; higher capital costs for smaller-scale applications; requires periodic replacement of specialized adsorbent materials.
China National Petroleum Corp.
Technical Solution: China National Petroleum Corporation (CNPC) has developed a comprehensive hydrogen purification technology specifically tailored for methane pyrolysis processes. Their system employs a three-stage purification approach beginning with cyclonic separation and electrostatic precipitation to remove solid carbon particles with efficiency exceeding 99.5%. The second stage utilizes CNPC's proprietary mixed metal oxide catalysts that selectively convert trace contaminants like CO and higher hydrocarbons at temperatures between 250-350°C. The final stage incorporates a hybrid membrane-PSA (Pressure Swing Adsorption) system that achieves hydrogen purities exceeding 99.999% while maintaining recovery rates above 85%. CNPC's innovation includes specialized ceramic membrane materials resistant to carbon fouling and optimized for operation in the temperature range typical of pyrolysis off-gases (400-600°C). Their system features advanced process control with real-time gas chromatography monitoring that automatically adjusts operating parameters to maintain consistent purity levels despite variations in feed composition. The technology has been demonstrated at pilot scale (500 kg H2/day) with plans for commercial deployment in CNPC's hydrogen production facilities.
Strengths: Highly effective carbon removal capabilities critical for methane pyrolysis applications; excellent scalability from small to large installations; robust performance with varying feedstock quality. Weaknesses: Higher energy consumption compared to some competing technologies; requires specialized catalyst materials with limited lifetime; complex control systems necessitating skilled operators.
Critical Patents and Innovations in Hydrogen Purification
Separations for methane pyrolysis
PatentPendingUS20250206608A1
Innovation
- Implementing a vacuum pressure swing adsorption (VPSA) cycle with a structured adsorbent bed configuration, such as a monolith with sorbent material coated on interior channels, and optimizing the adsorption and desorption steps to minimize purge streams, thereby enhancing hydrogen recovery and purity.
High purity, high pressure hydrogen production with in-situ co2 and sulfur capture in a single stage reactor
PatentActiveUS20090263316A1
Innovation
- The integration of a calcium looping process that uses calcium oxide (CaO) to remove CO2 and sulfur impurities in a single stage reactor, enhancing the water-gas shift reaction and producing high-purity hydrogen while reducing steam consumption and energy costs.
Environmental Impact Assessment of Purification Processes
The environmental impact of hydrogen purification processes in methane pyrolysis represents a critical consideration for the technology's sustainability credentials. Traditional purification methods often involve energy-intensive processes that can significantly offset the carbon benefits gained from pyrolytic hydrogen production. Current purification technologies such as Pressure Swing Adsorption (PSA), cryogenic separation, and membrane filtration each present distinct environmental footprints that must be carefully evaluated.
PSA systems, while effective at achieving high purity levels (99.999%), require substantial electricity for compression cycles. Life cycle assessments indicate that PSA contributes approximately 0.2-0.4 kg CO2-eq per kg of hydrogen purified, depending on the electricity source. This represents a non-trivial addition to the overall carbon footprint of hydrogen production.
Membrane separation technologies offer potentially lower energy requirements but face durability challenges in industrial settings. Recent advancements in palladium-based and polymer membranes have reduced energy consumption by 15-20% compared to PSA systems, though manufacturing these specialized materials involves environmentally intensive processes and rare metals extraction.
Water consumption presents another significant environmental consideration. Cryogenic separation methods can require 5-7 liters of cooling water per kilogram of hydrogen processed. This water footprint becomes particularly problematic in water-stressed regions where hydrogen production facilities might be located to leverage renewable energy resources.
Solid waste generation from adsorbent replacement and membrane disposal creates additional environmental burdens. Zeolites and activated carbon used in PSA systems require replacement every 3-5 years, generating specialized waste streams that often lack established recycling pathways. The environmental impact assessment must account for these material lifecycle considerations.
Chemical usage in purification processes, particularly amine scrubbing for CO2 removal, introduces potential for toxic releases and wastewater contamination if not properly managed. Modern facilities implement closed-loop systems that reduce chemical consumption by 40-60% compared to earlier generation technologies, significantly mitigating this environmental risk.
Emerging purification technologies show promise for reduced environmental impact. Metal-organic frameworks (MOFs) and electrochemical hydrogen pumping demonstrate 30-50% lower energy requirements in laboratory settings, though commercial-scale validation remains pending. These technologies could potentially reduce the environmental footprint of hydrogen purification by over 40% when fully developed.
The geographical context of purification facilities significantly influences their environmental impact. Facilities powered by renewable electricity can reduce the carbon footprint of purification by 70-90% compared to those using grid electricity in coal-dependent regions, highlighting the importance of integrated energy system planning when deploying methane pyrolysis technologies.
PSA systems, while effective at achieving high purity levels (99.999%), require substantial electricity for compression cycles. Life cycle assessments indicate that PSA contributes approximately 0.2-0.4 kg CO2-eq per kg of hydrogen purified, depending on the electricity source. This represents a non-trivial addition to the overall carbon footprint of hydrogen production.
Membrane separation technologies offer potentially lower energy requirements but face durability challenges in industrial settings. Recent advancements in palladium-based and polymer membranes have reduced energy consumption by 15-20% compared to PSA systems, though manufacturing these specialized materials involves environmentally intensive processes and rare metals extraction.
Water consumption presents another significant environmental consideration. Cryogenic separation methods can require 5-7 liters of cooling water per kilogram of hydrogen processed. This water footprint becomes particularly problematic in water-stressed regions where hydrogen production facilities might be located to leverage renewable energy resources.
Solid waste generation from adsorbent replacement and membrane disposal creates additional environmental burdens. Zeolites and activated carbon used in PSA systems require replacement every 3-5 years, generating specialized waste streams that often lack established recycling pathways. The environmental impact assessment must account for these material lifecycle considerations.
Chemical usage in purification processes, particularly amine scrubbing for CO2 removal, introduces potential for toxic releases and wastewater contamination if not properly managed. Modern facilities implement closed-loop systems that reduce chemical consumption by 40-60% compared to earlier generation technologies, significantly mitigating this environmental risk.
Emerging purification technologies show promise for reduced environmental impact. Metal-organic frameworks (MOFs) and electrochemical hydrogen pumping demonstrate 30-50% lower energy requirements in laboratory settings, though commercial-scale validation remains pending. These technologies could potentially reduce the environmental footprint of hydrogen purification by over 40% when fully developed.
The geographical context of purification facilities significantly influences their environmental impact. Facilities powered by renewable electricity can reduce the carbon footprint of purification by 70-90% compared to those using grid electricity in coal-dependent regions, highlighting the importance of integrated energy system planning when deploying methane pyrolysis technologies.
Regulatory Framework for Hydrogen Quality Certification
The regulatory landscape for hydrogen quality certification is evolving rapidly as methane pyrolysis emerges as a promising hydrogen production method. Currently, most hydrogen purity standards are derived from traditional production methods like steam methane reforming (SMR) and electrolysis, creating a regulatory gap for pyrolysis-specific certification frameworks.
International standards organizations, including ISO and ASTM International, have established baseline hydrogen purity requirements through standards such as ISO 14687 and ASTM D7650. These standards categorize hydrogen by application, with the most stringent requirements for fuel cell vehicles (99.97% purity) and less demanding specifications for industrial applications. However, these standards require adaptation to address the unique impurity profiles resulting from methane pyrolysis processes.
The European Union has taken a leading role in developing hydrogen certification frameworks through initiatives like CertifHy and the Renewable Energy Directive II (RED II). These programs focus on tracking carbon intensity and renewable content but lack specific provisions for pyrolysis-derived hydrogen. The EU Hydrogen Strategy, published in 2020, acknowledges the need for comprehensive certification systems that account for various production pathways, including pyrolysis.
In the United States, regulatory oversight is fragmented across multiple agencies. The Department of Energy's Hydrogen Program and the Environmental Protection Agency provide guidelines, while individual states like California have implemented their own hydrogen quality requirements through programs such as the Low Carbon Fuel Standard. This regulatory patchwork creates compliance challenges for pyrolysis technology developers operating across multiple jurisdictions.
Japan and South Korea have established hydrogen quality certification systems primarily focused on fuel cell applications, with detailed specifications for contaminant limits. These frameworks could serve as models for pyrolysis-specific certification but would require modifications to address carbon solid byproducts and potential trace impurities unique to the pyrolysis process.
Industry consortia and public-private partnerships are increasingly filling regulatory gaps by developing voluntary certification schemes. The Hydrogen Council and the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) are working on harmonized approaches to hydrogen certification that could eventually incorporate pyrolysis-specific parameters.
For methane pyrolysis to achieve commercial viability, a dedicated regulatory framework addressing its unique characteristics is essential. This framework should include standardized testing protocols for solid carbon byproducts, specific impurity thresholds relevant to pyrolysis processes, and lifecycle assessment methodologies that accurately capture the environmental benefits of this production pathway compared to conventional methods.
International standards organizations, including ISO and ASTM International, have established baseline hydrogen purity requirements through standards such as ISO 14687 and ASTM D7650. These standards categorize hydrogen by application, with the most stringent requirements for fuel cell vehicles (99.97% purity) and less demanding specifications for industrial applications. However, these standards require adaptation to address the unique impurity profiles resulting from methane pyrolysis processes.
The European Union has taken a leading role in developing hydrogen certification frameworks through initiatives like CertifHy and the Renewable Energy Directive II (RED II). These programs focus on tracking carbon intensity and renewable content but lack specific provisions for pyrolysis-derived hydrogen. The EU Hydrogen Strategy, published in 2020, acknowledges the need for comprehensive certification systems that account for various production pathways, including pyrolysis.
In the United States, regulatory oversight is fragmented across multiple agencies. The Department of Energy's Hydrogen Program and the Environmental Protection Agency provide guidelines, while individual states like California have implemented their own hydrogen quality requirements through programs such as the Low Carbon Fuel Standard. This regulatory patchwork creates compliance challenges for pyrolysis technology developers operating across multiple jurisdictions.
Japan and South Korea have established hydrogen quality certification systems primarily focused on fuel cell applications, with detailed specifications for contaminant limits. These frameworks could serve as models for pyrolysis-specific certification but would require modifications to address carbon solid byproducts and potential trace impurities unique to the pyrolysis process.
Industry consortia and public-private partnerships are increasingly filling regulatory gaps by developing voluntary certification schemes. The Hydrogen Council and the International Partnership for Hydrogen and Fuel Cells in the Economy (IPHE) are working on harmonized approaches to hydrogen certification that could eventually incorporate pyrolysis-specific parameters.
For methane pyrolysis to achieve commercial viability, a dedicated regulatory framework addressing its unique characteristics is essential. This framework should include standardized testing protocols for solid carbon byproducts, specific impurity thresholds relevant to pyrolysis processes, and lifecycle assessment methodologies that accurately capture the environmental benefits of this production pathway compared to conventional methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!