Material Challenges in Methane Pyrolysis Equipment.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Technology Background and Objectives
Methane pyrolysis represents a transformative approach to hydrogen production that has evolved significantly over the past decades. This process involves the thermal decomposition of methane (CH4) into hydrogen (H2) and solid carbon, offering a potentially low-carbon alternative to traditional steam methane reforming methods which generate substantial CO2 emissions. The technology's development can be traced back to the early 20th century, but significant research acceleration has occurred since the 1990s as environmental concerns and hydrogen economy interests have grown.
The evolution of methane pyrolysis technology has progressed through several distinct phases, beginning with basic thermal decomposition approaches and advancing toward more sophisticated catalytic and plasma-assisted methods. Recent innovations have focused on molten metal reactors, fluidized bed systems, and microwave-assisted pyrolysis, each offering unique advantages in reaction efficiency and carbon handling.
Current technological objectives center on addressing critical material challenges that have historically limited commercial viability. Primary goals include developing reactor materials capable of withstanding extreme operating conditions (temperatures of 800-1500°C), resisting carbon deposition and coking that leads to reactor fouling, and maintaining structural integrity despite thermal cycling and potential metal dusting corrosion.
The industry aims to achieve continuous operation capabilities exceeding 8,000 hours without significant degradation of reactor components, a benchmark necessary for commercial feasibility. Additionally, there is focused effort on materials innovation that can catalyze the pyrolysis reaction at lower temperatures, thereby reducing energy requirements and extending equipment lifespan.
From an environmental perspective, methane pyrolysis technology targets a carbon intensity reduction of over 90% compared to conventional hydrogen production methods. The ultimate objective is creating a commercially viable process that produces hydrogen at costs competitive with steam methane reforming while capturing carbon in solid form for potential valorization in industrial applications.
Research trajectories are increasingly exploring novel material compositions including advanced ceramics, specialized metal alloys, and composite structures that can withstand the harsh operating environment while maintaining process efficiency. The convergence of materials science, chemical engineering, and process design represents the frontier of innovation in this field, with significant potential implications for clean energy transitions and industrial decarbonization pathways.
The evolution of methane pyrolysis technology has progressed through several distinct phases, beginning with basic thermal decomposition approaches and advancing toward more sophisticated catalytic and plasma-assisted methods. Recent innovations have focused on molten metal reactors, fluidized bed systems, and microwave-assisted pyrolysis, each offering unique advantages in reaction efficiency and carbon handling.
Current technological objectives center on addressing critical material challenges that have historically limited commercial viability. Primary goals include developing reactor materials capable of withstanding extreme operating conditions (temperatures of 800-1500°C), resisting carbon deposition and coking that leads to reactor fouling, and maintaining structural integrity despite thermal cycling and potential metal dusting corrosion.
The industry aims to achieve continuous operation capabilities exceeding 8,000 hours without significant degradation of reactor components, a benchmark necessary for commercial feasibility. Additionally, there is focused effort on materials innovation that can catalyze the pyrolysis reaction at lower temperatures, thereby reducing energy requirements and extending equipment lifespan.
From an environmental perspective, methane pyrolysis technology targets a carbon intensity reduction of over 90% compared to conventional hydrogen production methods. The ultimate objective is creating a commercially viable process that produces hydrogen at costs competitive with steam methane reforming while capturing carbon in solid form for potential valorization in industrial applications.
Research trajectories are increasingly exploring novel material compositions including advanced ceramics, specialized metal alloys, and composite structures that can withstand the harsh operating environment while maintaining process efficiency. The convergence of materials science, chemical engineering, and process design represents the frontier of innovation in this field, with significant potential implications for clean energy transitions and industrial decarbonization pathways.
Market Analysis for Hydrogen Production via Methane Pyrolysis
The global hydrogen market is experiencing significant growth, with demand projected to reach 94 million tonnes by 2030, representing a compound annual growth rate (CAGR) of approximately 5.6%. Traditional hydrogen production methods, primarily steam methane reforming (SMR), account for over 76% of current production but face increasing scrutiny due to substantial carbon emissions. This creates a strategic market opportunity for methane pyrolysis as a cleaner alternative.
Methane pyrolysis for hydrogen production represents an emerging segment within the broader hydrogen economy, valued at approximately $130 billion in 2022. The technology offers a compelling value proposition as it produces hydrogen with significantly lower carbon emissions compared to conventional methods, generating solid carbon instead of CO2 as a byproduct. This positions methane pyrolysis as a bridge technology between conventional fossil-based hydrogen production and fully renewable methods.
Market segmentation reveals diverse potential applications across industrial sectors. The chemical industry remains the largest consumer of hydrogen, accounting for roughly 65% of current demand, primarily for ammonia production and petroleum refining. However, emerging applications in transportation, energy storage, and steel manufacturing are expected to drive substantial growth in hydrogen demand over the next decade.
Regional analysis indicates varying market readiness and adoption potential. Europe leads in policy support for low-carbon hydrogen technologies, with Germany and the Netherlands making strategic investments in methane pyrolysis demonstration projects. North America shows strong commercial interest, particularly in regions with abundant natural gas resources and existing hydrogen infrastructure. The Asia-Pacific region, especially Japan and South Korea, presents significant growth opportunities due to national hydrogen strategies and limited domestic energy resources.
Economic analysis of methane pyrolysis reveals promising cost structures compared to other low-carbon hydrogen production methods. Current production costs range between $1.50-2.50/kg H2, positioning it competitively against blue hydrogen ($1.40-2.40/kg) and significantly more affordable than green hydrogen ($3.00-6.00/kg). The potential revenue from carbon byproducts could further improve the economic proposition, with high-quality carbon black commanding market prices of $1,000-2,500 per tonne.
Market barriers include technology scalability challenges, uncertain regulatory frameworks regarding carbon credits, and competition from rapidly advancing electrolysis technologies. However, the increasing corporate commitments to decarbonization and the growing premium market for low-carbon hydrogen provide significant market pull for methane pyrolysis solutions.
Methane pyrolysis for hydrogen production represents an emerging segment within the broader hydrogen economy, valued at approximately $130 billion in 2022. The technology offers a compelling value proposition as it produces hydrogen with significantly lower carbon emissions compared to conventional methods, generating solid carbon instead of CO2 as a byproduct. This positions methane pyrolysis as a bridge technology between conventional fossil-based hydrogen production and fully renewable methods.
Market segmentation reveals diverse potential applications across industrial sectors. The chemical industry remains the largest consumer of hydrogen, accounting for roughly 65% of current demand, primarily for ammonia production and petroleum refining. However, emerging applications in transportation, energy storage, and steel manufacturing are expected to drive substantial growth in hydrogen demand over the next decade.
Regional analysis indicates varying market readiness and adoption potential. Europe leads in policy support for low-carbon hydrogen technologies, with Germany and the Netherlands making strategic investments in methane pyrolysis demonstration projects. North America shows strong commercial interest, particularly in regions with abundant natural gas resources and existing hydrogen infrastructure. The Asia-Pacific region, especially Japan and South Korea, presents significant growth opportunities due to national hydrogen strategies and limited domestic energy resources.
Economic analysis of methane pyrolysis reveals promising cost structures compared to other low-carbon hydrogen production methods. Current production costs range between $1.50-2.50/kg H2, positioning it competitively against blue hydrogen ($1.40-2.40/kg) and significantly more affordable than green hydrogen ($3.00-6.00/kg). The potential revenue from carbon byproducts could further improve the economic proposition, with high-quality carbon black commanding market prices of $1,000-2,500 per tonne.
Market barriers include technology scalability challenges, uncertain regulatory frameworks regarding carbon credits, and competition from rapidly advancing electrolysis technologies. However, the increasing corporate commitments to decarbonization and the growing premium market for low-carbon hydrogen provide significant market pull for methane pyrolysis solutions.
Current Material Limitations and Technical Challenges
Methane pyrolysis equipment faces significant material challenges due to the extreme operating conditions required for the thermal decomposition of methane. Current reactors typically operate at temperatures between 700-1200°C, creating a highly demanding environment for construction materials. Carbon deposition presents one of the most critical issues, as the solid carbon produced during pyrolysis accumulates on reactor surfaces, leading to fouling, reduced heat transfer efficiency, and eventual system failure.
Conventional metal alloys used in high-temperature applications, such as stainless steel and nickel-based superalloys, demonstrate inadequate performance in methane pyrolysis environments. These materials suffer from accelerated thermal degradation, with significant reductions in mechanical strength and creep resistance after extended exposure to operating temperatures. Furthermore, carbon diffusion into metal substrates causes carburization, resulting in embrittlement and increased susceptibility to cracking.
Ceramic materials, while offering superior temperature resistance, present their own set of limitations. Their inherent brittleness makes them vulnerable to thermal shock during operational cycling, and their poor thermal conductivity can create problematic temperature gradients within reactor components. Additionally, manufacturing complex ceramic components with the necessary precision remains technically challenging and economically prohibitive for large-scale industrial applications.
Refractory materials used for reactor linings face degradation through chemical attack from trace impurities in the methane feedstock, particularly sulfur compounds and siloxanes. These contaminants react with refractory components, forming low-melting-point compounds that accelerate material deterioration and reduce operational lifespan.
Molten metal reactors, particularly those utilizing liquid metals like tin or lead as reaction media, encounter challenges with container materials. These materials must simultaneously withstand high temperatures while resisting corrosion from the molten metal and avoiding contamination of the reaction environment. Current containment solutions demonstrate limited durability, with significant degradation observed after relatively short operational periods.
Catalyst materials incorporated into pyrolysis systems face deactivation through multiple mechanisms, including coking, sintering, and poisoning from feedstock impurities. The development of stable, regenerable catalysts capable of maintaining activity under pyrolysis conditions represents a significant technical hurdle that has yet to be fully addressed.
Heat transfer components within pyrolysis systems require materials that combine exceptional thermal conductivity with resistance to carbon fouling and high-temperature stability. Current heat exchanger materials fail to maintain performance over extended operational periods, necessitating frequent maintenance and replacement, which significantly impacts process economics.
Conventional metal alloys used in high-temperature applications, such as stainless steel and nickel-based superalloys, demonstrate inadequate performance in methane pyrolysis environments. These materials suffer from accelerated thermal degradation, with significant reductions in mechanical strength and creep resistance after extended exposure to operating temperatures. Furthermore, carbon diffusion into metal substrates causes carburization, resulting in embrittlement and increased susceptibility to cracking.
Ceramic materials, while offering superior temperature resistance, present their own set of limitations. Their inherent brittleness makes them vulnerable to thermal shock during operational cycling, and their poor thermal conductivity can create problematic temperature gradients within reactor components. Additionally, manufacturing complex ceramic components with the necessary precision remains technically challenging and economically prohibitive for large-scale industrial applications.
Refractory materials used for reactor linings face degradation through chemical attack from trace impurities in the methane feedstock, particularly sulfur compounds and siloxanes. These contaminants react with refractory components, forming low-melting-point compounds that accelerate material deterioration and reduce operational lifespan.
Molten metal reactors, particularly those utilizing liquid metals like tin or lead as reaction media, encounter challenges with container materials. These materials must simultaneously withstand high temperatures while resisting corrosion from the molten metal and avoiding contamination of the reaction environment. Current containment solutions demonstrate limited durability, with significant degradation observed after relatively short operational periods.
Catalyst materials incorporated into pyrolysis systems face deactivation through multiple mechanisms, including coking, sintering, and poisoning from feedstock impurities. The development of stable, regenerable catalysts capable of maintaining activity under pyrolysis conditions represents a significant technical hurdle that has yet to be fully addressed.
Heat transfer components within pyrolysis systems require materials that combine exceptional thermal conductivity with resistance to carbon fouling and high-temperature stability. Current heat exchanger materials fail to maintain performance over extended operational periods, necessitating frequent maintenance and replacement, which significantly impacts process economics.
Current Material Solutions for Pyrolysis Reactors
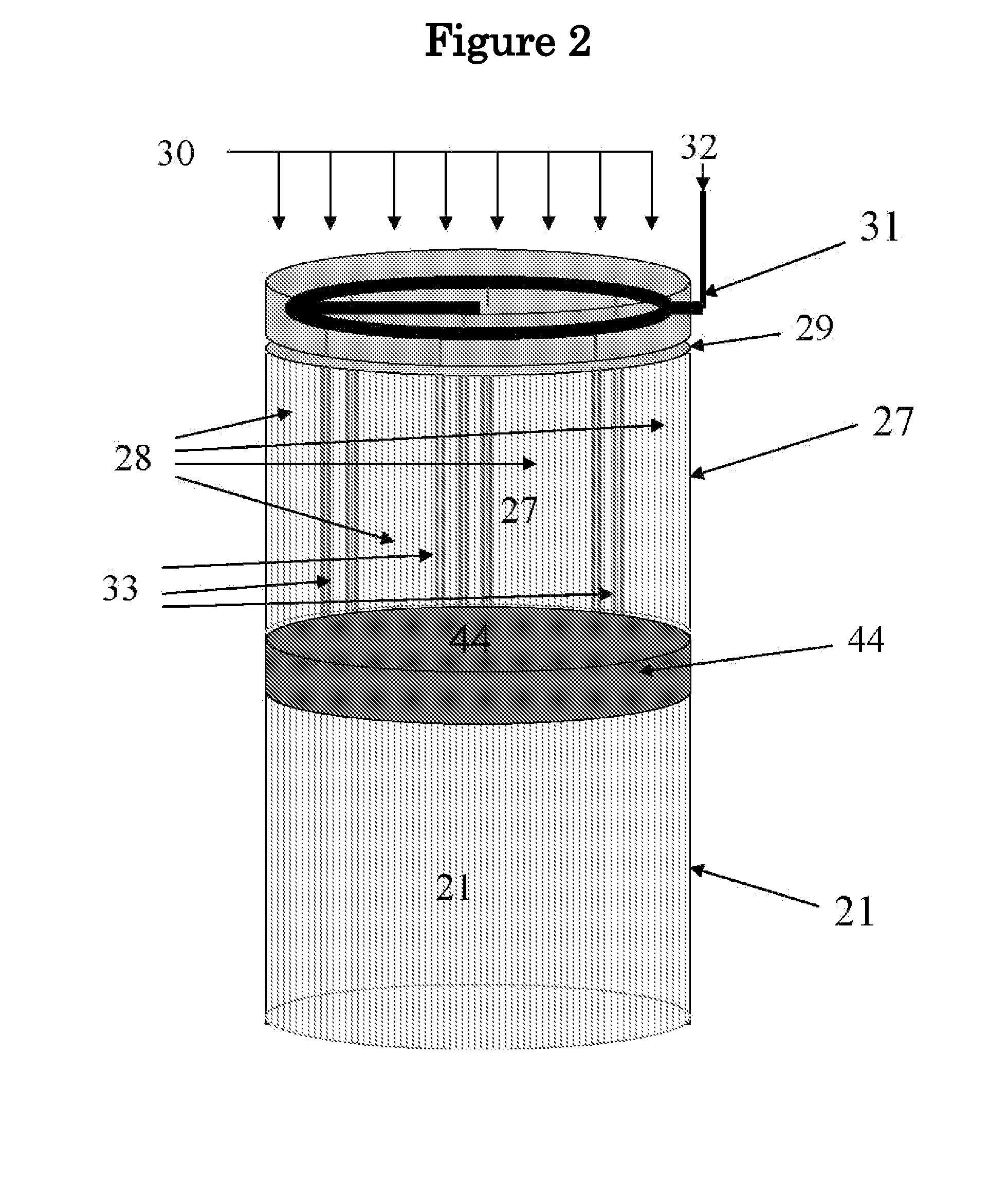
01 Reactor materials for high-temperature methane pyrolysis
Materials used in methane pyrolysis reactors must withstand extreme temperatures and harsh chemical environments. These typically include refractory metals, ceramics, and specialized alloys that maintain structural integrity and catalytic activity at temperatures exceeding 1000°C. The selection of appropriate reactor materials is critical for process efficiency, longevity of equipment, and prevention of carbon fouling during the pyrolysis process.- Reactor materials for high-temperature methane pyrolysis: Materials used in methane pyrolysis reactors must withstand extreme temperatures and harsh chemical environments. Advanced ceramics, refractory metals, and specialized alloys are employed for reactor walls and internal components. These materials exhibit high thermal stability, resistance to carbon deposition, and durability under the reducing atmosphere of methane decomposition. Proper material selection is critical for extending equipment lifespan and maintaining process efficiency in high-temperature pyrolysis operations.
- Catalytic materials for methane conversion: Catalytic materials play a crucial role in methane pyrolysis by lowering activation energy and improving conversion efficiency. Various catalysts including transition metals (Ni, Fe, Co), noble metals, and metal carbides are utilized to enhance reaction kinetics and selectivity. These materials are often supported on high-surface-area substrates like alumina or silica to maximize catalytic activity. The development of novel catalytic materials focuses on improving carbon resistance, thermal stability, and longevity under pyrolysis conditions.
- Molten metal bath technology for methane decomposition: Molten metal bath technology represents an innovative approach to methane pyrolysis, using liquid metals such as tin, bismuth, lead, or gallium as reaction media. These molten metals provide excellent heat transfer properties and facilitate the separation of solid carbon byproducts. The technology allows for continuous operation as carbon floats to the surface for easy removal while hydrogen is released as gas. The selection of appropriate metal or metal alloys depends on factors including melting point, density, and chemical compatibility with methane and hydrogen.
- Carbon handling and collection materials: Materials and systems for handling the solid carbon byproduct of methane pyrolysis are essential components of pyrolysis equipment. Specialized filters, cyclones, and separation systems made from corrosion-resistant materials are employed to collect carbon particles efficiently. Advanced carbon-resistant coatings and materials help prevent fouling and clogging of equipment surfaces. The design of these systems must consider the morphology and properties of the carbon produced, which can range from amorphous carbon to more structured forms depending on process conditions.
- Heat management and insulation materials: Effective thermal management is critical in methane pyrolysis equipment due to the high temperatures involved. Advanced insulation materials including ceramic fibers, refractory bricks, and aerogels are used to minimize heat loss and improve energy efficiency. Heat exchangers constructed from high-temperature alloys recover thermal energy from process streams. The selection of appropriate insulation and heat transfer materials impacts both operational costs and process stability, with modern systems incorporating multilayer insulation designs and specialized thermal barrier coatings to optimize performance.
02 Catalytic materials for methane decomposition
Various catalytic materials are employed to enhance methane decomposition rates and selectivity during pyrolysis. These include transition metals (nickel, iron, cobalt), noble metals (platinum, palladium), metal carbides, and carbon-based catalysts. The catalysts are often supported on high-surface-area materials to maximize contact with methane gas and improve conversion efficiency while minimizing energy requirements.Expand Specific Solutions03 Carbon handling and collection systems
Specialized materials and equipment designs are used for handling and collecting solid carbon produced during methane pyrolysis. These systems include filters, cyclones, and separation chambers made from materials resistant to carbon deposition and abrasion. The design focuses on preventing system clogging while efficiently collecting high-purity carbon products that can be valorized as materials for various applications.Expand Specific Solutions04 Molten media materials for bubble column reactors
Molten metals and salts are used as heat transfer and reaction media in bubble column pyrolysis reactors. Materials such as tin, bismuth, lead alloys, and various molten salts provide excellent heat transfer properties while facilitating the separation of solid carbon products. The selection of appropriate molten media considers melting point, density, viscosity, and chemical compatibility with methane and hydrogen at operating temperatures.Expand Specific Solutions05 Heat management and insulation materials
Advanced thermal management systems employ specialized insulation materials to maintain process temperatures while minimizing energy consumption. These include ceramic fiber insulation, refractory bricks, aerogels, and multi-layer thermal barriers. The materials must withstand high temperatures while providing effective thermal insulation to improve energy efficiency and reduce operating costs of methane pyrolysis equipment.Expand Specific Solutions
Key Industry Players in Methane Pyrolysis Equipment
The methane pyrolysis equipment market is in a growth phase, characterized by increasing investments in clean hydrogen production technologies. The market is expanding due to global decarbonization efforts, with projections suggesting significant growth as industries seek carbon-neutral hydrogen solutions. Technologically, the field shows varying maturity levels across players. Established energy corporations like ExxonMobil Chemical Patents and SABIC are leveraging their extensive materials expertise to address high-temperature reactor challenges, while specialized innovators such as Molten Industries and Haffner Energy are developing novel catalytic approaches and reactor designs. Research institutions including Dalian Institute of Chemical Physics and Korea Research Institute of Chemical Technology are advancing fundamental materials science for methane decomposition. Companies like Coolbrook Oy and Haldor Topsøe are focusing on scaling commercial solutions, particularly addressing material durability issues in carbon formation environments.
Molten Industries Inc.
Technical Solution: Molten Industries has developed an innovative methane pyrolysis technology using molten metal reactors, specifically focusing on liquid metal bubble column reactors. Their approach utilizes molten metals (primarily nickel, copper, and iron alloys) as both catalysts and heat transfer media. The system operates at temperatures between 900-1100°C, where methane bubbles through the molten metal bath, catalytically decomposing into hydrogen and solid carbon. Their proprietary reactor design incorporates specialized ceramic materials for the reactor walls that resist high-temperature degradation and prevent metal infiltration. The carbon produced is continuously separated and harvested using mechanical separation systems, preventing reactor fouling. Molten Industries has also developed advanced heat management systems that utilize the exothermic nature of certain stages of the pyrolysis process to improve overall energy efficiency.
Strengths: Superior heat transfer properties of molten metals enable efficient and uniform heating; continuous carbon removal prevents reactor clogging; and the system produces high-purity hydrogen without CO2 emissions. Weaknesses: Requires specialized high-temperature materials that increase capital costs; metal loss through volatilization and entrainment in carbon product; and challenges in scaling up while maintaining uniform bubble distribution and reaction conditions.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute has developed a novel catalytic methane pyrolysis technology utilizing structured nano-catalysts to address material challenges. Their approach centers on nickel-based catalysts supported on modified ceramic substrates with hierarchical pore structures that enhance catalyst stability and carbon management. The reactor system operates at moderate temperatures (700-850°C) compared to other pyrolysis technologies, reducing thermal stress on materials while achieving high conversion rates through catalytic enhancement. A key innovation is their development of self-regenerating catalyst systems that incorporate secondary metal promoters (including molybdenum and iron) that continuously expose fresh catalytic sites as carbon forms. The reactor design features specialized ceramic-metal composite materials for critical components that resist carburization while maintaining structural integrity. Their system also incorporates innovative carbon management through controlled carbon nanofiber growth that minimizes catalyst deactivation and reactor fouling. The Institute has demonstrated extended catalyst lifetimes exceeding 1000 hours with minimal performance degradation through their proprietary catalyst formulations and reactor design.
Strengths: Lower operating temperatures reduce material stress and energy requirements; specialized catalysts achieve high conversion rates despite moderate temperatures; and the self-regenerating catalyst system extends operational lifetime. Weaknesses: Catalyst production requires precise control of nanostructures, increasing complexity; potential for catalyst poisoning from feed impurities; and carbon management becomes challenging at industrial scales despite innovations.
Critical Materials Research and Patent Analysis
Pyrolysis reactor materials and methods
PatentWO2010135073A2
Innovation
- Development of stabilized zirconia refractory ceramics with high melting points and yttria stabilization, resistant to carbide-oxide interactions and corrosion, maintaining structural integrity and chemical inertness at extreme temperatures.
Pyrolysis Reactor Materials and Methods
PatentActiveUS20100288617A1
Innovation
- Development of stabilized zirconia refractory ceramics with high melting points and yttria stabilization, resistant to carbide-oxide interactions and corrosion, maintaining structural integrity and chemical inertness at extreme temperatures.
Environmental Impact and Carbon Footprint Assessment
Methane pyrolysis represents a promising pathway for hydrogen production with significantly reduced environmental impact compared to conventional methods. The environmental footprint of methane pyrolysis equipment is substantially lower than steam methane reforming (SMR), which currently dominates industrial hydrogen production but generates substantial CO2 emissions.
The carbon footprint assessment of methane pyrolysis reveals compelling advantages. While SMR produces approximately 9-12 kg CO2 per kg of hydrogen, methane pyrolysis can potentially reduce this to near-zero direct emissions. The process generates solid carbon instead of CO2, effectively sequestering carbon in a stable, solid form that can be stored or utilized in various applications.
Life cycle analyses indicate that methane pyrolysis equipment, when powered by renewable electricity, can achieve greenhouse gas emission reductions of up to 85-90% compared to conventional hydrogen production methods. Even when considering upstream methane leakage and energy inputs for equipment manufacturing, the overall environmental impact remains significantly lower than alternative processes.
Material selection plays a crucial role in determining the environmental profile of pyrolysis equipment. High-temperature resistant materials like ceramics and certain alloys typically require energy-intensive manufacturing processes, contributing to the embodied carbon of the equipment. However, these materials also enable longer operational lifetimes and higher efficiency, offsetting initial environmental costs over the equipment's service life.
Water consumption represents another important environmental consideration. Unlike SMR, which requires substantial water inputs, methane pyrolysis operates with minimal water requirements, reducing pressure on local water resources. This advantage becomes particularly significant in water-stressed regions where hydrogen production is being considered.
Land use impacts of methane pyrolysis facilities are generally comparable to or smaller than those of conventional hydrogen production plants, with the additional benefit that carbon capture and storage infrastructure is not required. This translates to a smaller physical footprint for equivalent hydrogen production capacity.
The solid carbon byproduct presents both environmental challenges and opportunities. When properly managed, this carbon can be utilized in construction materials, soil amendments, or advanced materials manufacturing, creating potential for circular economy applications. However, improper handling could lead to particulate emissions or other environmental hazards, necessitating careful management protocols.
Regulatory frameworks are increasingly recognizing the environmental benefits of methane pyrolysis, with several jurisdictions developing specific carbon accounting methodologies for this technology. These frameworks will be crucial for accurately quantifying the environmental advantages and supporting the technology's commercial deployment.
The carbon footprint assessment of methane pyrolysis reveals compelling advantages. While SMR produces approximately 9-12 kg CO2 per kg of hydrogen, methane pyrolysis can potentially reduce this to near-zero direct emissions. The process generates solid carbon instead of CO2, effectively sequestering carbon in a stable, solid form that can be stored or utilized in various applications.
Life cycle analyses indicate that methane pyrolysis equipment, when powered by renewable electricity, can achieve greenhouse gas emission reductions of up to 85-90% compared to conventional hydrogen production methods. Even when considering upstream methane leakage and energy inputs for equipment manufacturing, the overall environmental impact remains significantly lower than alternative processes.
Material selection plays a crucial role in determining the environmental profile of pyrolysis equipment. High-temperature resistant materials like ceramics and certain alloys typically require energy-intensive manufacturing processes, contributing to the embodied carbon of the equipment. However, these materials also enable longer operational lifetimes and higher efficiency, offsetting initial environmental costs over the equipment's service life.
Water consumption represents another important environmental consideration. Unlike SMR, which requires substantial water inputs, methane pyrolysis operates with minimal water requirements, reducing pressure on local water resources. This advantage becomes particularly significant in water-stressed regions where hydrogen production is being considered.
Land use impacts of methane pyrolysis facilities are generally comparable to or smaller than those of conventional hydrogen production plants, with the additional benefit that carbon capture and storage infrastructure is not required. This translates to a smaller physical footprint for equivalent hydrogen production capacity.
The solid carbon byproduct presents both environmental challenges and opportunities. When properly managed, this carbon can be utilized in construction materials, soil amendments, or advanced materials manufacturing, creating potential for circular economy applications. However, improper handling could lead to particulate emissions or other environmental hazards, necessitating careful management protocols.
Regulatory frameworks are increasingly recognizing the environmental benefits of methane pyrolysis, with several jurisdictions developing specific carbon accounting methodologies for this technology. These frameworks will be crucial for accurately quantifying the environmental advantages and supporting the technology's commercial deployment.
Scalability and Cost Analysis of Advanced Materials
The economic viability of methane pyrolysis technology hinges significantly on the scalability and cost-effectiveness of advanced materials used in reactor construction. Current materials like high-grade nickel alloys and specialized ceramics present substantial cost barriers, with reactor vessels often representing 30-40% of total capital expenditure for industrial-scale installations. These materials must withstand extreme conditions while maintaining structural integrity, creating a direct correlation between material performance and economic feasibility.
Scale-up challenges manifest differently across reactor designs. Molten metal reactors require materials resistant to metal infiltration and thermal cycling, with costs increasing disproportionately at industrial scales due to the complexity of containing larger volumes of molten metals. Plasma-based systems face electrode degradation issues that worsen with scale, necessitating more frequent replacement of expensive components in larger installations.
Material cost sensitivity analysis reveals that a 15% reduction in advanced material costs could improve overall project economics by 8-12%, potentially accelerating commercial adoption timelines by 1-3 years. This highlights the critical importance of material innovation pathways that prioritize cost reduction alongside performance improvements.
Emerging manufacturing techniques offer promising cost reduction opportunities. Additive manufacturing approaches for specialized reactor components have demonstrated potential cost savings of 20-35% in pilot applications, though challenges remain in ensuring consistent material properties at scale. Similarly, advanced coating technologies that apply protective layers to less expensive substrate materials show promise for reducing overall material costs by 25-40% while maintaining performance characteristics.
Supply chain considerations further complicate the economic picture. Many advanced materials rely on rare elements with geographically concentrated supply chains, creating price volatility risks. Diversification of material compositions to incorporate more abundant elements could improve long-term cost stability, though often with performance trade-offs that must be carefully evaluated.
The economic threshold for widespread commercial adoption appears to require a 30-50% reduction in advanced material costs from current levels, highlighting the need for continued investment in material science research specifically targeting cost-effective solutions for methane pyrolysis applications.
Scale-up challenges manifest differently across reactor designs. Molten metal reactors require materials resistant to metal infiltration and thermal cycling, with costs increasing disproportionately at industrial scales due to the complexity of containing larger volumes of molten metals. Plasma-based systems face electrode degradation issues that worsen with scale, necessitating more frequent replacement of expensive components in larger installations.
Material cost sensitivity analysis reveals that a 15% reduction in advanced material costs could improve overall project economics by 8-12%, potentially accelerating commercial adoption timelines by 1-3 years. This highlights the critical importance of material innovation pathways that prioritize cost reduction alongside performance improvements.
Emerging manufacturing techniques offer promising cost reduction opportunities. Additive manufacturing approaches for specialized reactor components have demonstrated potential cost savings of 20-35% in pilot applications, though challenges remain in ensuring consistent material properties at scale. Similarly, advanced coating technologies that apply protective layers to less expensive substrate materials show promise for reducing overall material costs by 25-40% while maintaining performance characteristics.
Supply chain considerations further complicate the economic picture. Many advanced materials rely on rare elements with geographically concentrated supply chains, creating price volatility risks. Diversification of material compositions to incorporate more abundant elements could improve long-term cost stability, though often with performance trade-offs that must be carefully evaluated.
The economic threshold for widespread commercial adoption appears to require a 30-50% reduction in advanced material costs from current levels, highlighting the need for continued investment in material science research specifically targeting cost-effective solutions for methane pyrolysis applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!