Methane Pyrolysis: Innovations in Heat Exchange.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Methane Pyrolysis Background and Objectives
Methane pyrolysis represents a transformative approach to hydrogen production that has gained significant attention in recent decades. This process involves the thermal decomposition of methane (CH₄) into hydrogen (H₂) and solid carbon, without direct CO₂ emissions. The evolution of this technology dates back to the early 20th century, but has seen renewed interest as the world seeks cleaner energy alternatives and carbon-neutral industrial processes.
The historical development of methane pyrolysis has progressed through several distinct phases. Initially explored as a theoretical concept in chemical engineering, it remained largely academic until the 1960s when industrial applications began to emerge. By the 1990s, environmental concerns drove further research, and the 21st century has witnessed accelerated development as hydrogen economy initiatives gain momentum globally.
Current technological trends in methane pyrolysis focus on improving energy efficiency, reaction kinetics, and heat exchange mechanisms. Traditional methods require temperatures exceeding 1000°C, creating significant challenges for reactor materials and energy consumption. Recent innovations have explored catalytic approaches, plasma-assisted decomposition, and novel reactor designs to reduce these energy requirements and improve conversion rates.
The primary technical objective of innovations in heat exchange for methane pyrolysis is to develop systems that can efficiently transfer and recover thermal energy within the reaction environment. This includes designing heat exchangers capable of withstanding high temperatures while maintaining structural integrity, minimizing energy losses, and optimizing heat distribution throughout the reactor.
Secondary objectives include reducing the carbon footprint of the overall process, enhancing scalability for industrial applications, and developing cost-effective solutions that can compete economically with conventional hydrogen production methods such as steam methane reforming.
The long-term vision for methane pyrolysis technology encompasses its integration into broader hydrogen infrastructure, contributing to decarbonization efforts across multiple sectors including transportation, power generation, and industrial manufacturing. Additionally, the solid carbon byproduct presents opportunities for value-added applications in materials science, potentially creating a circular economy approach to carbon utilization.
As global energy systems transition toward lower carbon intensities, methane pyrolysis stands at a critical juncture where innovations in heat exchange technology could determine its commercial viability and widespread adoption. The technical challenges are substantial but surmountable, with significant research efforts underway across academic institutions, national laboratories, and private industry to overcome these barriers.
The historical development of methane pyrolysis has progressed through several distinct phases. Initially explored as a theoretical concept in chemical engineering, it remained largely academic until the 1960s when industrial applications began to emerge. By the 1990s, environmental concerns drove further research, and the 21st century has witnessed accelerated development as hydrogen economy initiatives gain momentum globally.
Current technological trends in methane pyrolysis focus on improving energy efficiency, reaction kinetics, and heat exchange mechanisms. Traditional methods require temperatures exceeding 1000°C, creating significant challenges for reactor materials and energy consumption. Recent innovations have explored catalytic approaches, plasma-assisted decomposition, and novel reactor designs to reduce these energy requirements and improve conversion rates.
The primary technical objective of innovations in heat exchange for methane pyrolysis is to develop systems that can efficiently transfer and recover thermal energy within the reaction environment. This includes designing heat exchangers capable of withstanding high temperatures while maintaining structural integrity, minimizing energy losses, and optimizing heat distribution throughout the reactor.
Secondary objectives include reducing the carbon footprint of the overall process, enhancing scalability for industrial applications, and developing cost-effective solutions that can compete economically with conventional hydrogen production methods such as steam methane reforming.
The long-term vision for methane pyrolysis technology encompasses its integration into broader hydrogen infrastructure, contributing to decarbonization efforts across multiple sectors including transportation, power generation, and industrial manufacturing. Additionally, the solid carbon byproduct presents opportunities for value-added applications in materials science, potentially creating a circular economy approach to carbon utilization.
As global energy systems transition toward lower carbon intensities, methane pyrolysis stands at a critical juncture where innovations in heat exchange technology could determine its commercial viability and widespread adoption. The technical challenges are substantial but surmountable, with significant research efforts underway across academic institutions, national laboratories, and private industry to overcome these barriers.
Market Analysis for Hydrogen Production Technologies
The global hydrogen production market is experiencing significant growth, projected to reach $160 billion by 2030, with a compound annual growth rate exceeding 9.2% during the forecast period. This expansion is primarily driven by increasing industrial applications, growing demand for clean energy solutions, and supportive government policies worldwide. Currently, hydrogen production is dominated by conventional methods, with steam methane reforming (SMR) accounting for approximately 76% of global production, followed by coal gasification at 22%, and electrolysis at merely 2%.
Methane pyrolysis represents a disruptive technology within this landscape, offering a potentially cleaner alternative to traditional hydrogen production methods. The market segment for low-carbon hydrogen production technologies is expected to grow at a faster rate than the overall hydrogen market, with projections suggesting a 25% CAGR through 2035. This acceleration is largely attributed to increasing carbon pricing mechanisms and stringent emissions regulations across major economies.
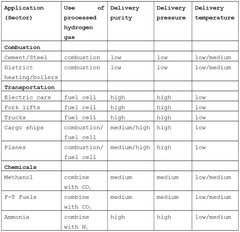
The demand for hydrogen is diversifying across multiple sectors. While traditional industrial applications in ammonia production and petroleum refining remain strong, emerging applications in transportation, power generation, and energy storage are creating new market opportunities. The transportation sector, particularly fuel cell electric vehicles (FCEVs), represents a high-growth segment with potential hydrogen demand increasing by 40% annually in certain regions.
Regional analysis reveals significant market variations. Asia-Pacific currently leads hydrogen consumption, with China, Japan, and South Korea making substantial investments in hydrogen infrastructure. Europe follows closely, with Germany, the Netherlands, and the UK establishing ambitious hydrogen strategies. North America, particularly the United States, is experiencing renewed interest in hydrogen technologies, supported by recent policy initiatives like the Inflation Reduction Act.
The economic competitiveness of methane pyrolysis compared to other hydrogen production methods varies by region and depends heavily on natural gas prices, carbon pricing mechanisms, and technological maturity. Current production costs range from $1.50-3.00/kg H₂, positioning it between conventional SMR ($1.00-1.80/kg) and electrolysis ($3.00-6.00/kg). However, innovations in heat exchange technology could potentially reduce production costs by 15-30%, significantly enhancing market competitiveness.
Market barriers include high capital expenditure requirements, technological uncertainties, and infrastructure limitations. Nevertheless, the solid carbon co-product from methane pyrolysis offers additional revenue streams, potentially improving the overall economics and creating synergies with materials industries.
Methane pyrolysis represents a disruptive technology within this landscape, offering a potentially cleaner alternative to traditional hydrogen production methods. The market segment for low-carbon hydrogen production technologies is expected to grow at a faster rate than the overall hydrogen market, with projections suggesting a 25% CAGR through 2035. This acceleration is largely attributed to increasing carbon pricing mechanisms and stringent emissions regulations across major economies.
The demand for hydrogen is diversifying across multiple sectors. While traditional industrial applications in ammonia production and petroleum refining remain strong, emerging applications in transportation, power generation, and energy storage are creating new market opportunities. The transportation sector, particularly fuel cell electric vehicles (FCEVs), represents a high-growth segment with potential hydrogen demand increasing by 40% annually in certain regions.
Regional analysis reveals significant market variations. Asia-Pacific currently leads hydrogen consumption, with China, Japan, and South Korea making substantial investments in hydrogen infrastructure. Europe follows closely, with Germany, the Netherlands, and the UK establishing ambitious hydrogen strategies. North America, particularly the United States, is experiencing renewed interest in hydrogen technologies, supported by recent policy initiatives like the Inflation Reduction Act.
The economic competitiveness of methane pyrolysis compared to other hydrogen production methods varies by region and depends heavily on natural gas prices, carbon pricing mechanisms, and technological maturity. Current production costs range from $1.50-3.00/kg H₂, positioning it between conventional SMR ($1.00-1.80/kg) and electrolysis ($3.00-6.00/kg). However, innovations in heat exchange technology could potentially reduce production costs by 15-30%, significantly enhancing market competitiveness.
Market barriers include high capital expenditure requirements, technological uncertainties, and infrastructure limitations. Nevertheless, the solid carbon co-product from methane pyrolysis offers additional revenue streams, potentially improving the overall economics and creating synergies with materials industries.
Current Challenges in Methane Pyrolysis Heat Exchange
Methane pyrolysis represents a promising pathway for hydrogen production with significantly reduced carbon emissions compared to traditional steam methane reforming. However, the process faces substantial heat exchange challenges that currently limit its commercial viability and widespread adoption. The primary challenge lies in achieving and maintaining the high temperatures (800-1200°C) required for efficient methane decomposition while managing energy consumption and system durability.
Thermal management presents a critical obstacle, as heat distribution within pyrolysis reactors often suffers from non-uniformity, creating hot and cold spots that reduce conversion efficiency and accelerate catalyst deactivation. This temperature gradient challenge is particularly pronounced in scaled-up systems where maintaining consistent heat transfer becomes increasingly difficult across larger reactor volumes.
Catalyst performance degradation due to thermal stress represents another significant hurdle. Most catalytic systems experience rapid deactivation under the extreme thermal conditions required for methane pyrolysis, necessitating frequent replacement and increasing operational costs. The carbon deposition on heat exchange surfaces further exacerbates this problem by forming insulating layers that progressively reduce heat transfer efficiency.
Material limitations constitute a fundamental constraint in advancing methane pyrolysis technology. Current reactor materials struggle to withstand the combination of high temperatures and carbon-rich environments without suffering from carburization, metal dusting, or thermal fatigue. These material failures not only compromise system integrity but also introduce contamination into the produced hydrogen and carbon streams.
Energy recovery inefficiencies significantly impact the overall process economics. The inability to effectively recover and reuse thermal energy from the hot product streams results in excessive energy consumption, undermining the environmental benefits of the technology. Current heat exchanger designs fail to optimize this energy recovery, particularly when dealing with carbon-laden gas streams.
Scale-up challenges persist as laboratory-proven concepts face difficulties in industrial implementation. The heat exchange solutions that work effectively at small scales often encounter unforeseen complications when scaled to commercial production levels, including pressure drop issues, flow distribution problems, and mechanical stress failures.
Addressing these interconnected challenges requires innovative approaches to heat exchanger design, novel material development, and advanced process integration strategies. Recent research has begun exploring molten metal reactors, fluidized bed systems, and plasma-assisted processes as potential solutions, but each approach introduces its own set of heat exchange complexities that must be resolved before methane pyrolysis can achieve widespread commercial adoption.
Thermal management presents a critical obstacle, as heat distribution within pyrolysis reactors often suffers from non-uniformity, creating hot and cold spots that reduce conversion efficiency and accelerate catalyst deactivation. This temperature gradient challenge is particularly pronounced in scaled-up systems where maintaining consistent heat transfer becomes increasingly difficult across larger reactor volumes.
Catalyst performance degradation due to thermal stress represents another significant hurdle. Most catalytic systems experience rapid deactivation under the extreme thermal conditions required for methane pyrolysis, necessitating frequent replacement and increasing operational costs. The carbon deposition on heat exchange surfaces further exacerbates this problem by forming insulating layers that progressively reduce heat transfer efficiency.
Material limitations constitute a fundamental constraint in advancing methane pyrolysis technology. Current reactor materials struggle to withstand the combination of high temperatures and carbon-rich environments without suffering from carburization, metal dusting, or thermal fatigue. These material failures not only compromise system integrity but also introduce contamination into the produced hydrogen and carbon streams.
Energy recovery inefficiencies significantly impact the overall process economics. The inability to effectively recover and reuse thermal energy from the hot product streams results in excessive energy consumption, undermining the environmental benefits of the technology. Current heat exchanger designs fail to optimize this energy recovery, particularly when dealing with carbon-laden gas streams.
Scale-up challenges persist as laboratory-proven concepts face difficulties in industrial implementation. The heat exchange solutions that work effectively at small scales often encounter unforeseen complications when scaled to commercial production levels, including pressure drop issues, flow distribution problems, and mechanical stress failures.
Addressing these interconnected challenges requires innovative approaches to heat exchanger design, novel material development, and advanced process integration strategies. Recent research has begun exploring molten metal reactors, fluidized bed systems, and plasma-assisted processes as potential solutions, but each approach introduces its own set of heat exchange complexities that must be resolved before methane pyrolysis can achieve widespread commercial adoption.
Current Heat Exchange Solutions for Methane Pyrolysis
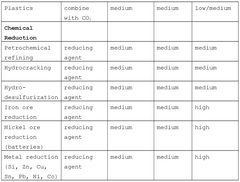
01 Reactor designs for methane pyrolysis
Various reactor designs have been developed for methane pyrolysis that optimize heat exchange. These include fluidized bed reactors, molten metal reactors, and plasma reactors that efficiently transfer heat to methane molecules. The reactor designs incorporate specialized heat exchange mechanisms to maintain optimal pyrolysis temperatures while managing energy consumption. Some designs feature integrated cooling systems to recover heat from the process, improving overall energy efficiency.- Reactor design for methane pyrolysis: Specialized reactor designs are crucial for efficient methane pyrolysis processes. These designs focus on optimizing heat transfer while maintaining the high temperatures required for pyrolysis reactions. Key features include specialized chambers, catalytic beds, and structural elements that enhance reaction efficiency while managing the carbon byproduct formation. Advanced reactor configurations help improve conversion rates and energy efficiency in the methane decomposition process.
- Heat recovery systems in pyrolysis processes: Heat recovery systems play a vital role in methane pyrolysis by capturing and reusing thermal energy from the high-temperature reaction. These systems typically incorporate heat exchangers that transfer heat from reaction products to incoming feedstock or other process streams. Efficient heat recovery significantly reduces overall energy consumption, improves process economics, and enhances the sustainability of hydrogen production through methane pyrolysis.
- Catalytic methods for methane decomposition: Catalytic approaches enhance methane pyrolysis efficiency by lowering the activation energy required for decomposition. Various catalysts, including metal-based and carbon-based materials, facilitate the breaking of carbon-hydrogen bonds at lower temperatures than thermal-only processes. Catalyst selection and design significantly impact reaction kinetics, energy requirements, and the morphology of the solid carbon byproduct, ultimately affecting the heat exchange dynamics of the system.
- Integrated cooling and heat exchange mechanisms: Integrated cooling and heat exchange mechanisms are essential components in methane pyrolysis systems to manage the extreme temperatures involved. These systems often employ multiple heat exchange stages with different cooling media to gradually reduce temperatures while recovering thermal energy. Advanced designs incorporate specialized materials resistant to high temperatures and carbon deposition, ensuring efficient heat transfer while maintaining system integrity during continuous operation.
- Carbon handling and separation technologies: Effective carbon handling and separation technologies are critical in methane pyrolysis heat exchange systems. As solid carbon is a primary byproduct, specialized mechanisms are needed to continuously remove carbon deposits that can impair heat transfer efficiency. These technologies include mechanical separation systems, fluidized bed arrangements, and innovative reactor designs that facilitate carbon removal while maintaining optimal thermal conditions for the pyrolysis reaction.
02 Heat recovery systems in methane pyrolysis
Heat recovery systems are crucial in methane pyrolysis processes to improve energy efficiency. These systems capture and reuse thermal energy from hot reaction products and exhaust gases. Advanced heat exchangers transfer this recovered heat to incoming methane feed or other process streams. Some systems incorporate regenerative heat exchange where solid materials temporarily store and release heat. These recovery methods significantly reduce the external energy requirements for the pyrolysis process.Expand Specific Solutions03 Catalytic systems for enhanced heat transfer
Catalytic systems play an important role in methane pyrolysis by lowering activation energy and improving heat transfer efficiency. These catalysts, often metal-based, facilitate the breakdown of methane at lower temperatures than non-catalytic processes. Some catalytic systems are designed with high surface area structures that maximize contact between the catalyst and methane while optimizing heat transfer. The integration of catalysts with specialized heat exchange surfaces creates more energy-efficient pyrolysis processes.Expand Specific Solutions04 Innovative heat exchanger designs
Novel heat exchanger designs specifically for methane pyrolysis applications focus on handling the unique challenges of the process, including carbon deposition and high temperatures. These include micro-channel heat exchangers, ceramic heat exchangers resistant to high temperatures, and specialized tube arrangements that minimize fouling. Some designs incorporate self-cleaning mechanisms to address carbon buildup that can reduce heat transfer efficiency. Advanced materials selection ensures durability under the harsh conditions of pyrolysis reactions.Expand Specific Solutions05 Process integration and thermal management
Comprehensive process integration approaches optimize the overall thermal management of methane pyrolysis systems. These include strategic coupling of exothermic and endothermic process steps, cascading heat utilization across different temperature requirements, and intelligent control systems that dynamically adjust heat distribution. Some integrated systems combine methane pyrolysis with other processes like hydrogen purification or carbon processing to maximize energy efficiency. Advanced thermal management strategies minimize energy losses and improve the economic viability of methane pyrolysis.Expand Specific Solutions
Leading Companies and Research Institutions in Pyrolysis
Methane pyrolysis for heat exchange innovations is currently in the early growth phase, with the market expected to expand significantly due to increasing focus on hydrogen production and carbon reduction technologies. The global market size is projected to reach substantial value as industries seek cleaner energy alternatives. Technologically, the field shows varying maturity levels across players. Johnson Matthey, Haldor Topsøe, and BASF lead with advanced catalyst technologies, while Sinopec and China Petroleum & Chemical Corp. demonstrate strong process integration capabilities. Research institutions like Korea Research Institute of Chemical Technology and Tianjin University are advancing fundamental innovations. Companies including Electrochaea and SGL Carbon are developing specialized heat exchange materials and reactor designs that promise to overcome efficiency barriers in methane decomposition processes.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed advanced catalytic methane pyrolysis systems that utilize structured metal catalysts to significantly lower the activation energy required for methane decomposition. Their proprietary heat exchange technology incorporates specially designed reactor configurations with integrated heat recovery systems that capture and redistribute thermal energy throughout the process. The company's innovation focuses on a multi-zone reactor design where the endothermic pyrolysis reaction is thermally coupled with exothermic processes, creating a more energy-efficient system. Their catalysts are engineered with specific surface area treatments that enhance carbon deposition patterns while minimizing catalyst deactivation, allowing for extended operational cycles between regenerations. Johnson Matthey has also pioneered molten metal bubble column reactors that combine excellent heat transfer properties with continuous carbon removal capabilities [1][3].
Strengths: Superior catalyst formulations with extended lifetime and selectivity; integrated heat management systems that reduce energy consumption by up to 30% compared to conventional methods; proven scalability from pilot to commercial applications. Weaknesses: Higher initial capital investment requirements; catalyst systems may require specialized handling and periodic replacement; performance sensitivity to feed gas impurities.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive methane pyrolysis technology platform focusing on innovative heat exchange solutions. Their approach utilizes a multi-tubular fixed-bed reactor system with advanced ceramic heat exchange elements that can withstand extreme temperatures (>1000°C) while maintaining structural integrity. Sinopec's technology incorporates a staged heating approach where incoming methane is preheated using the hydrogen product stream before entering the main pyrolysis zone. The company has pioneered the use of specialized high-temperature alloys with enhanced thermal conductivity coatings that improve heat transfer efficiency while resisting carbon fouling. Their system also features a novel fluidized bed design that allows for continuous carbon removal while maintaining optimal heat distribution throughout the reactor. Sinopec has integrated advanced process control systems that dynamically adjust heating parameters based on real-time monitoring of reaction conditions [2][5].
Strengths: Exceptional thermal efficiency with heat recovery systems capturing up to 85% of process heat; robust reactor materials capable of withstanding thermal cycling; integrated carbon handling systems for continuous operation. Weaknesses: High capital expenditure requirements; complex control systems requiring specialized expertise; technology primarily optimized for large-scale operations with limited flexibility for smaller applications.
Key Patents and Research in Pyrolysis Heat Transfer
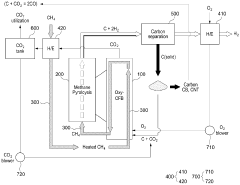
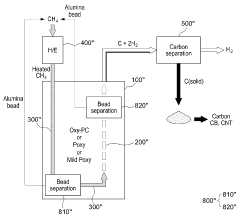
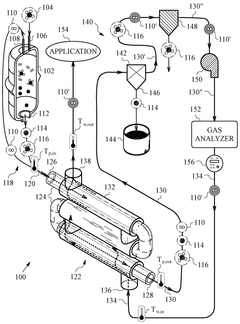
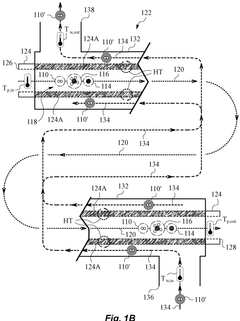
A hydrogen and carbon production system through methane pyrolysis of fluidized bed indirect heat exchange method
PatentActiveKR1020230046347A
Innovation
- A fluidized bed indirect heat exchange method using alumina beads for heat transfer and pure oxygen combustion to pyrolyze methane, eliminating the need for catalysts and separate separation processes, while utilizing carbon as fuel for bead regeneration and producing high-purity carbon monoxide through the Boudouard reaction.
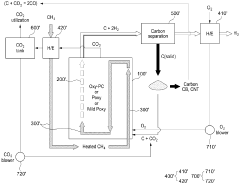
Delivery of high temperature hydrogen via hydrocarbon pyrolysis
PatentWO2025085765A1
Innovation
- A chemical process and system that utilizes a pyrolysis reactor to decompose hydrocarbon feedstocks into hydrogen and solid carbon, followed by a heat exchanger that cools the pyrolysis products and reuses the cooled hydrogen as a working fluid to achieve high temperature hydrogen delivery suitable for industrial use.
Carbon Management and Utilization Strategies
Carbon management and utilization strategies are critical components in the methane pyrolysis ecosystem, particularly when considering innovations in heat exchange. The primary output of methane pyrolysis—solid carbon—represents both an environmental opportunity and a potential revenue stream that can significantly impact the economic viability of the process.
The carbon produced through methane pyrolysis exists in various forms, including carbon black, graphite, and carbon nanotubes, each with distinct market applications. High-quality carbon black can be utilized in rubber manufacturing, printing inks, and plastics, while graphitic carbon finds applications in battery electrodes and construction materials. The most valuable form, carbon nanotubes, commands premium prices in electronics, composite materials, and energy storage applications.
Effective carbon management begins with precise control of the pyrolysis conditions through advanced heat exchange technologies. Temperature profiles, residence time, and cooling rates directly influence the morphology and quality of the carbon produced. Innovations in heat exchange systems that enable rapid temperature adjustments can facilitate the selective production of specific carbon allotropes, thereby maximizing value creation.
Post-production processing represents another crucial aspect of carbon management. Separation technologies that efficiently isolate carbon from reactor systems without contamination are essential for maintaining product quality. Recent innovations include magnetic separation techniques for catalyst recovery and advanced filtration systems that preserve the structural integrity of high-value carbon forms.
Market-driven utilization strategies must consider regional demand variations and transportation logistics. Locating pyrolysis facilities near industrial centers that consume carbon products can significantly reduce distribution costs and carbon footprint. Additionally, vertical integration opportunities exist where pyrolysis operations can directly supply carbon to manufacturing processes within the same industrial complex.
Emerging applications for pyrolysis-derived carbon include soil amendment (biochar), construction materials (carbon-reinforced concrete), and advanced energy storage systems. These applications not only provide carbon sequestration benefits but also create value-added products that improve the economics of methane pyrolysis operations.
Certification and standardization of carbon products represent a developing frontier in carbon utilization. As markets for climate-friendly materials expand, verification systems that authenticate the low-carbon provenance of pyrolysis-derived carbon can command premium pricing in environmentally conscious markets, further enhancing the economic case for methane pyrolysis technologies with innovative heat exchange systems.
The carbon produced through methane pyrolysis exists in various forms, including carbon black, graphite, and carbon nanotubes, each with distinct market applications. High-quality carbon black can be utilized in rubber manufacturing, printing inks, and plastics, while graphitic carbon finds applications in battery electrodes and construction materials. The most valuable form, carbon nanotubes, commands premium prices in electronics, composite materials, and energy storage applications.
Effective carbon management begins with precise control of the pyrolysis conditions through advanced heat exchange technologies. Temperature profiles, residence time, and cooling rates directly influence the morphology and quality of the carbon produced. Innovations in heat exchange systems that enable rapid temperature adjustments can facilitate the selective production of specific carbon allotropes, thereby maximizing value creation.
Post-production processing represents another crucial aspect of carbon management. Separation technologies that efficiently isolate carbon from reactor systems without contamination are essential for maintaining product quality. Recent innovations include magnetic separation techniques for catalyst recovery and advanced filtration systems that preserve the structural integrity of high-value carbon forms.
Market-driven utilization strategies must consider regional demand variations and transportation logistics. Locating pyrolysis facilities near industrial centers that consume carbon products can significantly reduce distribution costs and carbon footprint. Additionally, vertical integration opportunities exist where pyrolysis operations can directly supply carbon to manufacturing processes within the same industrial complex.
Emerging applications for pyrolysis-derived carbon include soil amendment (biochar), construction materials (carbon-reinforced concrete), and advanced energy storage systems. These applications not only provide carbon sequestration benefits but also create value-added products that improve the economics of methane pyrolysis operations.
Certification and standardization of carbon products represent a developing frontier in carbon utilization. As markets for climate-friendly materials expand, verification systems that authenticate the low-carbon provenance of pyrolysis-derived carbon can command premium pricing in environmentally conscious markets, further enhancing the economic case for methane pyrolysis technologies with innovative heat exchange systems.
Economic Viability and Scaling Considerations
The economic viability of methane pyrolysis technology hinges on several critical factors that determine its commercial feasibility and potential for widespread adoption. Current cost analyses indicate that methane pyrolysis processes require significant capital investment, with reactor systems representing approximately 40-50% of total installation costs. Operating expenses are dominated by energy requirements for maintaining high temperatures (typically 700-1200°C), which constitute 30-35% of ongoing costs.
Market competitiveness remains challenging when compared to conventional hydrogen production methods. Steam methane reforming (SMR) currently produces hydrogen at $1.50-2.00/kg, while methane pyrolysis processes range from $2.80-4.50/kg depending on technology configuration and scale. However, when carbon pricing mechanisms are factored in, the economic equation shifts favorably toward pyrolysis due to its significantly lower CO2 emissions profile.
Scaling considerations present both opportunities and obstacles for industrial implementation. Laboratory-scale reactors demonstrating high conversion efficiencies often encounter performance degradation when scaled to commercial volumes. Heat transfer limitations become particularly pronounced in larger reactors, where maintaining uniform temperature distribution presents significant engineering challenges. The surface-to-volume ratio decreases with scaling, requiring innovative heat exchanger designs to maintain reaction efficiency.
Energy integration strategies offer promising pathways to economic viability. Systems that recover and repurpose thermal energy from the high-temperature process can reduce operating costs by 15-25%. Additionally, the solid carbon byproduct represents a potential revenue stream, with high-quality carbon black commanding market prices of $1,000-2,500 per ton, though quality consistency remains a challenge in continuous production systems.
Infrastructure requirements present another scaling consideration. Methane pyrolysis facilities require integration with natural gas supply networks and hydrogen distribution systems. The solid carbon handling and processing infrastructure adds complexity not present in gas-only hydrogen production methods. Regional variations in energy costs significantly impact economic feasibility, with locations having access to low-cost renewable electricity showing more favorable economics for electrically heated pyrolysis systems.
Investment timelines and risk profiles must also be considered. The technology's relative immaturity compared to established hydrogen production methods necessitates higher risk premiums for financing. However, policy support mechanisms, including carbon credits, renewable hydrogen incentives, and research grants, are increasingly available to offset these challenges and accelerate commercial deployment.
Market competitiveness remains challenging when compared to conventional hydrogen production methods. Steam methane reforming (SMR) currently produces hydrogen at $1.50-2.00/kg, while methane pyrolysis processes range from $2.80-4.50/kg depending on technology configuration and scale. However, when carbon pricing mechanisms are factored in, the economic equation shifts favorably toward pyrolysis due to its significantly lower CO2 emissions profile.
Scaling considerations present both opportunities and obstacles for industrial implementation. Laboratory-scale reactors demonstrating high conversion efficiencies often encounter performance degradation when scaled to commercial volumes. Heat transfer limitations become particularly pronounced in larger reactors, where maintaining uniform temperature distribution presents significant engineering challenges. The surface-to-volume ratio decreases with scaling, requiring innovative heat exchanger designs to maintain reaction efficiency.
Energy integration strategies offer promising pathways to economic viability. Systems that recover and repurpose thermal energy from the high-temperature process can reduce operating costs by 15-25%. Additionally, the solid carbon byproduct represents a potential revenue stream, with high-quality carbon black commanding market prices of $1,000-2,500 per ton, though quality consistency remains a challenge in continuous production systems.
Infrastructure requirements present another scaling consideration. Methane pyrolysis facilities require integration with natural gas supply networks and hydrogen distribution systems. The solid carbon handling and processing infrastructure adds complexity not present in gas-only hydrogen production methods. Regional variations in energy costs significantly impact economic feasibility, with locations having access to low-cost renewable electricity showing more favorable economics for electrically heated pyrolysis systems.
Investment timelines and risk profiles must also be considered. The technology's relative immaturity compared to established hydrogen production methods necessitates higher risk premiums for financing. However, policy support mechanisms, including carbon credits, renewable hydrogen incentives, and research grants, are increasingly available to offset these challenges and accelerate commercial deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!