How Organoid Culture Systems Influence Drug Screening
OCT 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organoid Technology Background and Objectives
Organoid technology represents a revolutionary advancement in biomedical research, emerging from the convergence of stem cell biology, developmental biology, and tissue engineering over the past decade. These three-dimensional cellular structures self-organize to mimic the architecture and functionality of native organs, providing unprecedented opportunities for modeling human physiology and disease in vitro. The evolution of organoid technology has progressed from simple cell aggregates to complex, multi-cellular structures that recapitulate key aspects of organ development, structure, and function.
The primary objective of organoid technology in drug screening applications is to bridge the gap between traditional two-dimensional cell cultures and animal models, addressing the limitations of both approaches. Traditional cell cultures fail to replicate the complex cellular interactions and tissue architecture found in vivo, while animal models often poorly predict human responses due to species-specific differences. Organoids aim to provide more physiologically relevant platforms for evaluating drug efficacy, toxicity, and mechanisms of action in human tissues.
Recent technological advancements have significantly enhanced organoid culture systems, including the development of specialized extracellular matrices, defined media formulations, and bioengineering approaches that improve reproducibility and physiological relevance. These innovations have expanded the range of organs that can be modeled, now encompassing intestinal, hepatic, pancreatic, renal, cardiac, and cerebral organoids, among others.
The integration of organoid technology into drug screening workflows represents a paradigm shift in pharmaceutical research and development. By providing more accurate models of human tissue responses, organoids hold the potential to increase the success rate of drug candidates in clinical trials, thereby reducing the enormous costs associated with drug development failures. Furthermore, patient-derived organoids enable personalized medicine approaches, allowing for the testing of drug responses in tissue models derived from individual patients.
Looking forward, the field is moving toward increasing complexity and functionality in organoid systems. Current research focuses on incorporating immune components, vascularization, and innervation to better recapitulate organ physiology. Additionally, efforts are underway to standardize organoid production methods and characterization criteria to enhance reproducibility across laboratories and enable high-throughput screening applications.
The ultimate goal of organoid technology in drug screening is to establish validated, standardized platforms that can reliably predict human responses to pharmaceutical compounds, thereby accelerating drug discovery while reducing reliance on animal testing. This technology promises to revolutionize preclinical drug development by providing more translatable models of human biology and disease.
The primary objective of organoid technology in drug screening applications is to bridge the gap between traditional two-dimensional cell cultures and animal models, addressing the limitations of both approaches. Traditional cell cultures fail to replicate the complex cellular interactions and tissue architecture found in vivo, while animal models often poorly predict human responses due to species-specific differences. Organoids aim to provide more physiologically relevant platforms for evaluating drug efficacy, toxicity, and mechanisms of action in human tissues.
Recent technological advancements have significantly enhanced organoid culture systems, including the development of specialized extracellular matrices, defined media formulations, and bioengineering approaches that improve reproducibility and physiological relevance. These innovations have expanded the range of organs that can be modeled, now encompassing intestinal, hepatic, pancreatic, renal, cardiac, and cerebral organoids, among others.
The integration of organoid technology into drug screening workflows represents a paradigm shift in pharmaceutical research and development. By providing more accurate models of human tissue responses, organoids hold the potential to increase the success rate of drug candidates in clinical trials, thereby reducing the enormous costs associated with drug development failures. Furthermore, patient-derived organoids enable personalized medicine approaches, allowing for the testing of drug responses in tissue models derived from individual patients.
Looking forward, the field is moving toward increasing complexity and functionality in organoid systems. Current research focuses on incorporating immune components, vascularization, and innervation to better recapitulate organ physiology. Additionally, efforts are underway to standardize organoid production methods and characterization criteria to enhance reproducibility across laboratories and enable high-throughput screening applications.
The ultimate goal of organoid technology in drug screening is to establish validated, standardized platforms that can reliably predict human responses to pharmaceutical compounds, thereby accelerating drug discovery while reducing reliance on animal testing. This technology promises to revolutionize preclinical drug development by providing more translatable models of human biology and disease.
Market Analysis for Organoid-Based Drug Screening
The organoid-based drug screening market has experienced remarkable growth in recent years, driven by increasing demand for more physiologically relevant models in pharmaceutical research. The global market for organoid technology was valued at approximately $1.3 billion in 2022 and is projected to reach $3.4 billion by 2027, representing a compound annual growth rate (CAGR) of 21.2%. Within this broader market, drug screening applications account for the largest segment, comprising nearly 40% of the total market value.
Pharmaceutical companies are increasingly adopting organoid-based screening platforms to reduce the high attrition rates in drug development. Traditional 2D cell cultures and animal models have shown limited predictive value for human responses, with only about 10% of drug candidates that enter clinical trials ultimately receiving regulatory approval. Organoid models have demonstrated superior predictive capabilities, potentially increasing success rates by 15-20% and reducing development costs by up to $300 million per approved drug.
The oncology segment currently dominates the organoid-based drug screening market, accounting for approximately 35% of applications. This is followed by neurology (20%), gastroenterology (15%), and hepatology (10%). Emerging application areas include cardiology, nephrology, and respiratory medicine, which collectively represent significant growth opportunities as the technology matures.
Regionally, North America leads the market with a 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the fastest growth rate of 25% annually through 2027, driven by increasing R&D investments and favorable government initiatives supporting precision medicine.
Contract research organizations (CROs) specializing in organoid services have emerged as key market players, offering end-to-end solutions from organoid development to high-throughput screening. This segment is growing at 28% annually, outpacing the overall market as pharmaceutical companies increasingly outsource specialized screening activities.
Key market challenges include high costs associated with organoid culture systems, with average per-sample testing costs ranging from $1,000 to $5,000 depending on complexity. Standardization issues and reproducibility concerns also remain significant barriers to wider adoption. However, technological advancements in automation, microfluidics, and AI-driven analysis are gradually addressing these limitations, potentially reducing costs by 30-40% over the next five years.
The market is witnessing increasing integration of organoid technology with complementary platforms such as organ-on-chip devices and AI-powered predictive analytics, creating comprehensive drug discovery ecosystems that offer enhanced predictive value and operational efficiency.
Pharmaceutical companies are increasingly adopting organoid-based screening platforms to reduce the high attrition rates in drug development. Traditional 2D cell cultures and animal models have shown limited predictive value for human responses, with only about 10% of drug candidates that enter clinical trials ultimately receiving regulatory approval. Organoid models have demonstrated superior predictive capabilities, potentially increasing success rates by 15-20% and reducing development costs by up to $300 million per approved drug.
The oncology segment currently dominates the organoid-based drug screening market, accounting for approximately 35% of applications. This is followed by neurology (20%), gastroenterology (15%), and hepatology (10%). Emerging application areas include cardiology, nephrology, and respiratory medicine, which collectively represent significant growth opportunities as the technology matures.
Regionally, North America leads the market with a 45% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China, South Korea, and Japan, is expected to witness the fastest growth rate of 25% annually through 2027, driven by increasing R&D investments and favorable government initiatives supporting precision medicine.
Contract research organizations (CROs) specializing in organoid services have emerged as key market players, offering end-to-end solutions from organoid development to high-throughput screening. This segment is growing at 28% annually, outpacing the overall market as pharmaceutical companies increasingly outsource specialized screening activities.
Key market challenges include high costs associated with organoid culture systems, with average per-sample testing costs ranging from $1,000 to $5,000 depending on complexity. Standardization issues and reproducibility concerns also remain significant barriers to wider adoption. However, technological advancements in automation, microfluidics, and AI-driven analysis are gradually addressing these limitations, potentially reducing costs by 30-40% over the next five years.
The market is witnessing increasing integration of organoid technology with complementary platforms such as organ-on-chip devices and AI-powered predictive analytics, creating comprehensive drug discovery ecosystems that offer enhanced predictive value and operational efficiency.
Current Challenges in Organoid Culture Systems
Despite significant advancements in organoid technology, several critical challenges persist that limit the widespread adoption and reliability of organoid culture systems for drug screening applications. The heterogeneity of organoids represents a major obstacle, as variations in size, shape, and cellular composition can lead to inconsistent drug responses, complicating data interpretation and reducing reproducibility across experiments. This variability stems from both intrinsic biological factors and technical inconsistencies in culture protocols.
Scalability remains another significant hurdle, as current methods for organoid generation and maintenance are labor-intensive, time-consuming, and expensive. The manual nature of many protocols creates bottlenecks that impede high-throughput screening capabilities essential for pharmaceutical applications. Additionally, the low throughput of traditional organoid culture systems makes them less attractive for industrial drug discovery pipelines compared to conventional 2D cell cultures.
The extracellular matrix (ECM) components, particularly Matrigel, introduce further complications. Matrigel's undefined composition, batch-to-batch variability, and animal origin raise concerns regarding experimental reproducibility and regulatory compliance. The search for chemically defined, synthetic alternatives has progressed but has not yet yielded widely adopted solutions that fully recapitulate the complex cell-matrix interactions necessary for proper organoid development.
Vascularization deficiency in current organoid models presents another critical limitation. The absence of functional blood vessels restricts nutrient and oxygen diffusion to approximately 200-300 μm, resulting in necrotic cores in larger organoids. This limitation not only affects organoid viability but also fails to model drug distribution dynamics accurately, potentially leading to misleading pharmacokinetic assessments during drug screening.
The integration of immune components remains underdeveloped in most organoid systems. Given the crucial role of immune responses in drug efficacy and toxicity, this absence represents a significant gap in predictive capability. Current co-culture systems with immune cells are still in early developmental stages and face challenges in maintaining long-term stability and physiological relevance.
Standardization across the field presents perhaps the most pervasive challenge. The lack of universally accepted protocols for organoid generation, maintenance, and drug testing hampers cross-laboratory comparisons and validation studies. This absence of standardization extends to quality control metrics, maturation assessments, and functional readouts, creating barriers to establishing organoids as reliable drug screening platforms that can generate consistent, translatable results across different research settings.
Scalability remains another significant hurdle, as current methods for organoid generation and maintenance are labor-intensive, time-consuming, and expensive. The manual nature of many protocols creates bottlenecks that impede high-throughput screening capabilities essential for pharmaceutical applications. Additionally, the low throughput of traditional organoid culture systems makes them less attractive for industrial drug discovery pipelines compared to conventional 2D cell cultures.
The extracellular matrix (ECM) components, particularly Matrigel, introduce further complications. Matrigel's undefined composition, batch-to-batch variability, and animal origin raise concerns regarding experimental reproducibility and regulatory compliance. The search for chemically defined, synthetic alternatives has progressed but has not yet yielded widely adopted solutions that fully recapitulate the complex cell-matrix interactions necessary for proper organoid development.
Vascularization deficiency in current organoid models presents another critical limitation. The absence of functional blood vessels restricts nutrient and oxygen diffusion to approximately 200-300 μm, resulting in necrotic cores in larger organoids. This limitation not only affects organoid viability but also fails to model drug distribution dynamics accurately, potentially leading to misleading pharmacokinetic assessments during drug screening.
The integration of immune components remains underdeveloped in most organoid systems. Given the crucial role of immune responses in drug efficacy and toxicity, this absence represents a significant gap in predictive capability. Current co-culture systems with immune cells are still in early developmental stages and face challenges in maintaining long-term stability and physiological relevance.
Standardization across the field presents perhaps the most pervasive challenge. The lack of universally accepted protocols for organoid generation, maintenance, and drug testing hampers cross-laboratory comparisons and validation studies. This absence of standardization extends to quality control metrics, maturation assessments, and functional readouts, creating barriers to establishing organoids as reliable drug screening platforms that can generate consistent, translatable results across different research settings.
Current Organoid Culture Methodologies
01 3D organoid culture systems for drug screening
Three-dimensional organoid culture systems provide more physiologically relevant models for drug screening compared to traditional 2D cell cultures. These systems better mimic the in vivo microenvironment, cellular organization, and functionality of organs, allowing for more accurate prediction of drug efficacy and toxicity. The 3D structure enables proper cell-cell interactions and extracellular matrix formation, which are crucial for maintaining tissue-specific functions and responses to pharmaceutical compounds.- 3D organoid culture systems for drug screening: Three-dimensional organoid culture systems provide more physiologically relevant models for drug screening compared to traditional 2D cell cultures. These systems better mimic the in vivo microenvironment, cellular organization, and functionality of organs, allowing for more accurate prediction of drug efficacy and toxicity. The 3D structure enables proper cell-cell interactions and extracellular matrix formation, which are crucial for maintaining tissue-specific functions and responses to pharmaceutical compounds.
- Specialized equipment and devices for organoid culture: Various specialized equipment and devices have been developed to facilitate organoid culture for drug screening applications. These include microfluidic platforms, bioreactors, and specialized culture vessels designed to maintain optimal growth conditions for organoids. Such equipment allows for controlled nutrient delivery, waste removal, and physical stimulation that more accurately replicate physiological conditions, enhancing the reliability of drug screening results.
- Patient-derived organoids for personalized medicine: Patient-derived organoids enable personalized drug screening approaches by using cells obtained directly from individual patients. These organoids retain the genetic and phenotypic characteristics of the original patient tissue, allowing for the evaluation of drug responses that reflect individual variability. This approach facilitates personalized treatment selection and can predict patient-specific drug efficacy and toxicity, potentially improving clinical outcomes in various diseases including cancer.
- High-throughput screening methods using organoids: High-throughput screening methods have been adapted for organoid-based drug discovery platforms. These methods involve automated culture, treatment, and analysis of multiple organoids simultaneously, allowing for rapid screening of large compound libraries. Advanced imaging techniques, biomarkers, and analytical tools are employed to assess drug effects on organoid growth, morphology, and function, significantly accelerating the drug discovery process while maintaining physiological relevance.
- Disease-specific organoid models for targeted drug development: Disease-specific organoid models have been developed to recapitulate particular pathological conditions for targeted drug development. These models incorporate genetic modifications or patient-derived cells that exhibit disease phenotypes, allowing researchers to study disease mechanisms and test therapeutic interventions in a relevant context. Examples include cancer organoids, neurodegenerative disease models, and infectious disease models, which enable more precise evaluation of drug candidates targeting specific disease pathways.
02 Patient-derived organoids for personalized medicine
Patient-derived organoids can be used for personalized drug screening and treatment selection. These organoids are developed from patient tissue samples and maintain the genetic and phenotypic characteristics of the original tissue, including disease-specific features. This approach allows for testing drug responses on patient-specific organoids before administering treatments to patients, potentially improving therapeutic outcomes and reducing adverse effects by identifying the most effective drugs for individual patients.Expand Specific Solutions03 High-throughput screening platforms for organoid cultures
Advanced high-throughput screening platforms have been developed specifically for organoid culture systems to facilitate efficient drug discovery and development. These platforms incorporate automated handling, imaging, and analysis technologies to screen large libraries of compounds against organoid models. The integration of microfluidic systems, robotics, and computational tools enables rapid assessment of drug effects on organoids, accelerating the identification of potential therapeutic candidates and reducing the time and cost of drug development.Expand Specific Solutions04 Specialized culture media and matrices for organoid development
Specialized culture media formulations and extracellular matrices are essential for successful organoid development and maintenance during drug screening. These media contain specific growth factors, hormones, and supplements that support the growth and differentiation of organoids while maintaining their tissue-specific characteristics. Advanced matrices provide structural support and biochemical cues that mimic the native tissue environment, ensuring organoid stability and functionality throughout the drug screening process.Expand Specific Solutions05 Disease modeling using organoids for targeted drug discovery
Organoids can be engineered to model specific diseases, enabling targeted drug discovery for conditions such as cancer, neurodegenerative disorders, and genetic diseases. By incorporating disease-specific genetic mutations or exposing organoids to pathological conditions, researchers can create accurate disease models for studying disease mechanisms and identifying potential therapeutic interventions. These disease-specific organoid models provide valuable platforms for screening compounds that target particular pathological pathways, accelerating the development of novel treatments for challenging diseases.Expand Specific Solutions
Leading Organizations in Organoid Research
The organoid culture systems market is rapidly evolving, currently in its growth phase with increasing adoption for drug screening applications. The global market is expanding significantly as pharmaceutical companies seek more physiologically relevant models than traditional 2D cell cultures. Technologically, the field shows varying maturity levels across different companies. Industry leaders like Takeda Pharmaceutical and Xilis are advancing high-throughput organoid screening platforms, while research institutions such as Duke University and EPFL are developing innovative methodologies. Companies like Cell Microsystems and eNUVIO are creating specialized microfluidic platforms for organoid culture, while Hefei Zhongke Presheng and D1 Medical Technology focus on patient-derived organoid models for personalized medicine applications. This competitive landscape reflects a dynamic ecosystem where academic-industry partnerships are driving technological innovation and clinical translation.
Cincinnati Children's Hospital Medical Center
Technical Solution: Cincinnati Children's Hospital Medical Center has established a pioneering organoid program focused on pediatric disease modeling and drug screening applications. Their technology centers on patient-derived intestinal organoids (enteroids) and has expanded to include multiple organ systems including liver, pancreas, and brain organoids. Their approach incorporates specialized media formulations that promote long-term organoid stability while maintaining tissue-specific functions critical for accurate drug response modeling. The center has developed high-content imaging protocols specifically optimized for pediatric tumor organoids, allowing for detection of subtle drug-induced changes in organoid morphology and function. Their platform includes innovative cryopreservation methods that maintain organoid viability and drug response profiles after thawing, enabling the creation of living biobanks for longitudinal studies. Cincinnati Children's researchers have demonstrated successful application of their organoid technology in cystic fibrosis drug screening, identifying patient-specific responses to CFTR modulators that correlated with subsequent clinical outcomes[8][9]. Their system incorporates automated liquid handling for standardized drug administration across multiple organoid lines simultaneously, increasing throughput while reducing technical variability.
Strengths: Specialized expertise in pediatric applications addresses an underserved area in drug development; established biobanking capabilities enable repeated testing on the same patient samples; demonstrated clinical translation in cystic fibrosis. Weaknesses: Primary focus on pediatric applications may limit broader applicability; academic setting may present challenges for commercial scaling; potential limitations in throughput compared to industrial platforms.
Xilis, Inc.
Technical Solution: Xilis has developed a proprietary Micro-Organosphere (MOS) technology platform that enables high-throughput patient-derived organoid screening for precision medicine applications. Their system creates thousands of 3D tumor micro-organoids that maintain the original tumor microenvironment, including immune cells and stromal components. This technology significantly reduces the time required for organoid generation from weeks to days, allowing for rapid drug sensitivity testing. Xilis's platform incorporates advanced imaging analytics and AI-driven predictive algorithms to analyze drug responses across multiple parameters simultaneously. The company has demonstrated clinical validation in colorectal cancer studies where their organoid-based predictions showed over 85% concordance with actual patient outcomes to standard-of-care treatments[1][2]. Their integrated workflow includes automated culture systems that standardize organoid production, reducing variability between samples and increasing reproducibility of drug screening results.
Strengths: Rapid turnaround time (days vs weeks) enables clinically actionable results; preservation of tumor microenvironment provides more physiologically relevant drug responses; high-throughput capability allows testing of numerous drug combinations. Weaknesses: Still requires primary patient tissue samples; technology may be less established for certain rare cancer types; higher cost compared to traditional 2D cell culture screening methods.
Key Innovations in Organoid-Drug Interaction Studies
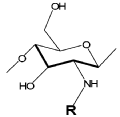
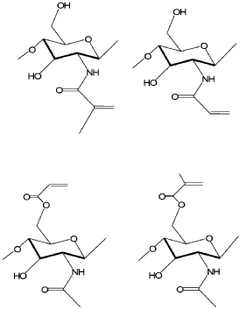
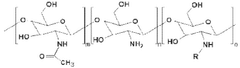
Organoid produced using carrier for cell culture, and method for evaluating drug toxicity using same
PatentWO2020101461A1
Innovation
- The development of organoids cultured using microcapsules containing gelatin, natural polymers, and oil thickener as a cell culture carrier, which allows for the creation of 3D organoids that mimic organ functions and respond to drug toxicity, enabling more accurate drug testing and personalized treatment approaches.
Multi-functional oxygenating microparticle loaded cell aggregates
PatentWO2019200197A1
Innovation
- The development of multicellular cell aggregates loaded with oxygenating microparticles, specifically fluorinated polymer or chitosan microparticles with immobilized perfluorocarbons, which enhance oxygen transport and reduce hypoxia by sequestering oxygen and maintaining high local oxygen concentrations.
Regulatory Considerations for Organoid-Based Drug Testing
The regulatory landscape for organoid-based drug testing is evolving rapidly as these advanced 3D culture systems gain prominence in pharmaceutical research. Currently, there exists a significant regulatory gap, as most frameworks were designed for traditional 2D cell cultures or animal models, not fully accounting for the unique characteristics of organoids. This creates uncertainty for pharmaceutical companies seeking to incorporate organoid data in regulatory submissions.
The FDA and EMA have begun acknowledging organoid models in their guidance documents, particularly for toxicity testing and early-stage drug development. However, specific validation requirements and standardization protocols remain under development. Both agencies have initiated pilot programs to evaluate how organoid data can complement or potentially replace certain animal studies, especially for organ-specific toxicity assessments.
Standardization represents a critical regulatory challenge. Without established protocols for organoid generation, maintenance, and characterization, reproducibility concerns persist across different laboratories. Industry consortia and regulatory bodies are collaborating to develop reference standards and quality control metrics specifically for organoid systems used in drug screening applications.
Data interpretation guidelines present another regulatory consideration. The complex nature of organoids—containing multiple cell types with intricate spatial arrangements—requires specialized approaches to data analysis that differ from traditional in vitro models. Regulatory agencies are working to establish appropriate benchmarks for determining when organoid responses can be considered predictive of human outcomes.
Patient-derived organoids introduce additional regulatory complexities regarding informed consent, data privacy, and genetic information handling. These considerations become particularly important when organoids are used for personalized medicine applications, where patient-specific drug responses may guide treatment decisions.
Validation requirements for organoid-based assays are gradually being formalized. Regulatory bodies increasingly expect demonstration of physiological relevance, reproducibility across batches, and correlation with clinical outcomes. Companies must develop comprehensive validation packages that address these aspects when submitting organoid data as part of regulatory filings.
Looking forward, a risk-based regulatory approach is emerging, where organoid data may initially supplement rather than replace established testing methods. As confidence in these systems grows through accumulated evidence and improved standardization, their regulatory acceptance is expected to expand, potentially reducing animal testing requirements and accelerating drug development timelines.
The FDA and EMA have begun acknowledging organoid models in their guidance documents, particularly for toxicity testing and early-stage drug development. However, specific validation requirements and standardization protocols remain under development. Both agencies have initiated pilot programs to evaluate how organoid data can complement or potentially replace certain animal studies, especially for organ-specific toxicity assessments.
Standardization represents a critical regulatory challenge. Without established protocols for organoid generation, maintenance, and characterization, reproducibility concerns persist across different laboratories. Industry consortia and regulatory bodies are collaborating to develop reference standards and quality control metrics specifically for organoid systems used in drug screening applications.
Data interpretation guidelines present another regulatory consideration. The complex nature of organoids—containing multiple cell types with intricate spatial arrangements—requires specialized approaches to data analysis that differ from traditional in vitro models. Regulatory agencies are working to establish appropriate benchmarks for determining when organoid responses can be considered predictive of human outcomes.
Patient-derived organoids introduce additional regulatory complexities regarding informed consent, data privacy, and genetic information handling. These considerations become particularly important when organoids are used for personalized medicine applications, where patient-specific drug responses may guide treatment decisions.
Validation requirements for organoid-based assays are gradually being formalized. Regulatory bodies increasingly expect demonstration of physiological relevance, reproducibility across batches, and correlation with clinical outcomes. Companies must develop comprehensive validation packages that address these aspects when submitting organoid data as part of regulatory filings.
Looking forward, a risk-based regulatory approach is emerging, where organoid data may initially supplement rather than replace established testing methods. As confidence in these systems grows through accumulated evidence and improved standardization, their regulatory acceptance is expected to expand, potentially reducing animal testing requirements and accelerating drug development timelines.
Cost-Benefit Analysis of Organoid vs Traditional Screening
The economic implications of adopting organoid culture systems for drug screening represent a critical consideration for pharmaceutical companies and research institutions. When comparing organoid-based screening with traditional cell line and animal model approaches, several cost factors must be evaluated against potential benefits to determine overall value.
Initial establishment of organoid culture systems requires significant investment in specialized equipment, culture media, and technical expertise. The per-sample cost of organoid development ranges from $200-1,500 depending on complexity, compared to $5-50 for traditional cell lines. Additionally, organoid maintenance demands specialized growth factors and matrices that can increase operational expenses by 3-5 times compared to conventional methods.
Labor costs present another substantial consideration, as organoid systems require highly skilled technicians and longer culture periods (typically 2-4 weeks versus 2-7 days for cell lines). This extended timeline translates to higher personnel expenses and reduced throughput in initial screening phases.
However, cost-benefit analysis must account for downstream savings. Studies indicate that organoid models demonstrate 60-85% predictive accuracy for clinical outcomes compared to 30-50% for traditional 2D cultures. This improved predictive capacity can significantly reduce late-stage clinical trial failures, where costs can exceed $100 million per failed compound.
The economic equation also benefits from reduced animal testing requirements. A comprehensive screening program utilizing organoids can decrease animal model usage by 30-50%, representing savings of $50,000-250,000 per drug candidate while addressing ethical concerns and regulatory pressures to minimize animal experimentation.
Scale and automation represent critical inflection points in the cost curve. Recent technological advances have reduced per-sample costs by approximately 40% over the past five years. Industry projections suggest that with continued automation and standardization, organoid screening costs could approach traditional methods within 3-5 years while maintaining superior predictive value.
Return on investment calculations indicate that despite higher initial costs, organoid systems can deliver net positive economic returns for drug development programs exceeding $50 million in total budget. The financial advantage becomes particularly evident when factoring in the reduced time-to-market potential of 6-18 months that more predictive screening can facilitate.
Initial establishment of organoid culture systems requires significant investment in specialized equipment, culture media, and technical expertise. The per-sample cost of organoid development ranges from $200-1,500 depending on complexity, compared to $5-50 for traditional cell lines. Additionally, organoid maintenance demands specialized growth factors and matrices that can increase operational expenses by 3-5 times compared to conventional methods.
Labor costs present another substantial consideration, as organoid systems require highly skilled technicians and longer culture periods (typically 2-4 weeks versus 2-7 days for cell lines). This extended timeline translates to higher personnel expenses and reduced throughput in initial screening phases.
However, cost-benefit analysis must account for downstream savings. Studies indicate that organoid models demonstrate 60-85% predictive accuracy for clinical outcomes compared to 30-50% for traditional 2D cultures. This improved predictive capacity can significantly reduce late-stage clinical trial failures, where costs can exceed $100 million per failed compound.
The economic equation also benefits from reduced animal testing requirements. A comprehensive screening program utilizing organoids can decrease animal model usage by 30-50%, representing savings of $50,000-250,000 per drug candidate while addressing ethical concerns and regulatory pressures to minimize animal experimentation.
Scale and automation represent critical inflection points in the cost curve. Recent technological advances have reduced per-sample costs by approximately 40% over the past five years. Industry projections suggest that with continued automation and standardization, organoid screening costs could approach traditional methods within 3-5 years while maintaining superior predictive value.
Return on investment calculations indicate that despite higher initial costs, organoid systems can deliver net positive economic returns for drug development programs exceeding $50 million in total budget. The financial advantage becomes particularly evident when factoring in the reduced time-to-market potential of 6-18 months that more predictive screening can facilitate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!