How PEEK Polymer Properties Facilitate Drug Delivery Systems
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Polymer Evolution and Drug Delivery Objectives
Polyetheretherketone (PEEK) has emerged as a revolutionary polymer in the field of drug delivery systems since its initial development in the late 1970s. Originally engineered for aerospace and automotive applications due to its exceptional thermal stability and mechanical strength, PEEK has undergone significant evolution over the past four decades to become a prominent material in biomedical applications, particularly in drug delivery systems.
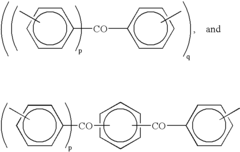
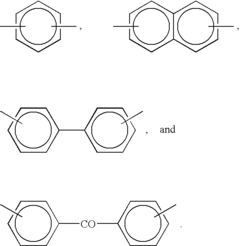
The evolution of PEEK polymer began with its synthesis by Imperial Chemical Industries (ICI) in 1978, featuring a unique molecular structure of aromatic rings connected by ether and ketone linkages. This structure confers remarkable chemical resistance, mechanical properties, and biocompatibility that have proven invaluable for medical applications. The 1990s marked a turning point when researchers began exploring PEEK's potential in biomedical fields, initially focusing on orthopedic and spinal implants.
By the early 2000s, scientists recognized PEEK's potential for drug delivery applications due to its bioinert nature, excellent mechanical properties, and resistance to degradation in biological environments. The polymer's semi-crystalline structure allows for controlled modification of surface properties without compromising its core mechanical integrity, creating an ideal platform for drug encapsulation and release systems.
Recent advancements in PEEK modification techniques have significantly expanded its functionality in drug delivery. Surface treatments including plasma modification, chemical functionalization, and coating technologies have enabled researchers to overcome PEEK's inherent hydrophobicity, allowing for improved drug loading capacity and release kinetics. Additionally, the development of PEEK composites incorporating bioactive materials has further enhanced its utility in targeted drug delivery applications.
The primary objectives for PEEK polymer in drug delivery systems focus on leveraging its unique properties to address current limitations in pharmaceutical delivery. These objectives include developing sustained-release formulations that maintain therapeutic drug concentrations over extended periods, creating targeted delivery systems that minimize systemic side effects, and designing stimuli-responsive PEEK-based carriers that release drugs in response to specific physiological conditions.
Another critical objective involves optimizing PEEK's surface chemistry to enhance drug loading efficiency while maintaining controlled release profiles. Researchers aim to develop scalable manufacturing processes that maintain PEEK's exceptional properties while incorporating pharmaceutical compounds effectively. The ultimate goal is to translate these technological advancements into clinically viable drug delivery platforms that improve patient outcomes through enhanced therapeutic efficacy and reduced administration frequency.
As we look toward future developments, the integration of PEEK with emerging technologies such as 3D printing and nanotechnology presents exciting opportunities for personalized medicine and novel drug delivery approaches, positioning this remarkable polymer at the forefront of pharmaceutical innovation.
The evolution of PEEK polymer began with its synthesis by Imperial Chemical Industries (ICI) in 1978, featuring a unique molecular structure of aromatic rings connected by ether and ketone linkages. This structure confers remarkable chemical resistance, mechanical properties, and biocompatibility that have proven invaluable for medical applications. The 1990s marked a turning point when researchers began exploring PEEK's potential in biomedical fields, initially focusing on orthopedic and spinal implants.
By the early 2000s, scientists recognized PEEK's potential for drug delivery applications due to its bioinert nature, excellent mechanical properties, and resistance to degradation in biological environments. The polymer's semi-crystalline structure allows for controlled modification of surface properties without compromising its core mechanical integrity, creating an ideal platform for drug encapsulation and release systems.
Recent advancements in PEEK modification techniques have significantly expanded its functionality in drug delivery. Surface treatments including plasma modification, chemical functionalization, and coating technologies have enabled researchers to overcome PEEK's inherent hydrophobicity, allowing for improved drug loading capacity and release kinetics. Additionally, the development of PEEK composites incorporating bioactive materials has further enhanced its utility in targeted drug delivery applications.
The primary objectives for PEEK polymer in drug delivery systems focus on leveraging its unique properties to address current limitations in pharmaceutical delivery. These objectives include developing sustained-release formulations that maintain therapeutic drug concentrations over extended periods, creating targeted delivery systems that minimize systemic side effects, and designing stimuli-responsive PEEK-based carriers that release drugs in response to specific physiological conditions.
Another critical objective involves optimizing PEEK's surface chemistry to enhance drug loading efficiency while maintaining controlled release profiles. Researchers aim to develop scalable manufacturing processes that maintain PEEK's exceptional properties while incorporating pharmaceutical compounds effectively. The ultimate goal is to translate these technological advancements into clinically viable drug delivery platforms that improve patient outcomes through enhanced therapeutic efficacy and reduced administration frequency.
As we look toward future developments, the integration of PEEK with emerging technologies such as 3D printing and nanotechnology presents exciting opportunities for personalized medicine and novel drug delivery approaches, positioning this remarkable polymer at the forefront of pharmaceutical innovation.
Market Analysis of PEEK-Based Drug Delivery Systems
The global market for PEEK-based drug delivery systems is experiencing significant growth, driven by increasing demand for advanced biomedical materials with superior mechanical and chemical properties. Currently valued at approximately 320 million USD in 2023, this specialized segment is projected to grow at a compound annual growth rate (CAGR) of 7.8% through 2030, reaching an estimated market size of 540 million USD.
North America dominates the market with approximately 42% share, followed by Europe (31%) and Asia-Pacific (21%). The remaining 6% is distributed across other regions. This regional distribution reflects the concentration of advanced healthcare infrastructure and pharmaceutical research capabilities in developed economies, though emerging markets are showing accelerated adoption rates.
By application segment, orthopedic drug delivery systems represent the largest market share at 38%, followed by dental applications (27%), cardiovascular applications (18%), and other therapeutic areas (17%). The orthopedic segment's dominance stems from PEEK's exceptional mechanical properties that closely mimic human bone, making it ideal for implantable drug delivery systems.
Key market drivers include the rising prevalence of chronic diseases requiring sustained drug release mechanisms, growing demand for minimally invasive therapeutic approaches, and increasing research investments in advanced biomaterials. The aging global population further amplifies market growth as age-related conditions often necessitate implantable drug delivery solutions.
Regulatory considerations significantly impact market dynamics, with stringent approval processes in major markets creating high entry barriers but also ensuring quality standards. The FDA and European Medical Agency's regulatory frameworks for combination products (device-drug combinations) directly influence commercialization timelines and investment requirements for PEEK-based delivery systems.
Pricing analysis reveals premium positioning for PEEK-based systems, with average costs 30-40% higher than conventional polymer alternatives. However, this premium is increasingly justified through clinical outcome data demonstrating superior performance and reduced complication rates.
Market challenges include high manufacturing costs, complex regulatory pathways, and competition from alternative biomaterials. Additionally, reimbursement limitations in some healthcare systems restrict adoption despite clinical benefits. Nevertheless, technological advancements in PEEK surface modifications and manufacturing processes are gradually addressing cost concerns while enhancing drug elution capabilities.
The competitive landscape features both established medical device manufacturers expanding into drug-device combinations and specialized biomaterials companies focusing exclusively on PEEK-based delivery systems. Strategic partnerships between polymer suppliers, pharmaceutical companies, and device manufacturers are becoming increasingly common to leverage complementary expertise.
North America dominates the market with approximately 42% share, followed by Europe (31%) and Asia-Pacific (21%). The remaining 6% is distributed across other regions. This regional distribution reflects the concentration of advanced healthcare infrastructure and pharmaceutical research capabilities in developed economies, though emerging markets are showing accelerated adoption rates.
By application segment, orthopedic drug delivery systems represent the largest market share at 38%, followed by dental applications (27%), cardiovascular applications (18%), and other therapeutic areas (17%). The orthopedic segment's dominance stems from PEEK's exceptional mechanical properties that closely mimic human bone, making it ideal for implantable drug delivery systems.
Key market drivers include the rising prevalence of chronic diseases requiring sustained drug release mechanisms, growing demand for minimally invasive therapeutic approaches, and increasing research investments in advanced biomaterials. The aging global population further amplifies market growth as age-related conditions often necessitate implantable drug delivery solutions.
Regulatory considerations significantly impact market dynamics, with stringent approval processes in major markets creating high entry barriers but also ensuring quality standards. The FDA and European Medical Agency's regulatory frameworks for combination products (device-drug combinations) directly influence commercialization timelines and investment requirements for PEEK-based delivery systems.
Pricing analysis reveals premium positioning for PEEK-based systems, with average costs 30-40% higher than conventional polymer alternatives. However, this premium is increasingly justified through clinical outcome data demonstrating superior performance and reduced complication rates.
Market challenges include high manufacturing costs, complex regulatory pathways, and competition from alternative biomaterials. Additionally, reimbursement limitations in some healthcare systems restrict adoption despite clinical benefits. Nevertheless, technological advancements in PEEK surface modifications and manufacturing processes are gradually addressing cost concerns while enhancing drug elution capabilities.
The competitive landscape features both established medical device manufacturers expanding into drug-device combinations and specialized biomaterials companies focusing exclusively on PEEK-based delivery systems. Strategic partnerships between polymer suppliers, pharmaceutical companies, and device manufacturers are becoming increasingly common to leverage complementary expertise.
Current PEEK Applications and Technical Limitations
Polyetheretherketone (PEEK) has established itself as a versatile high-performance polymer with applications spanning multiple industries. In the medical field, PEEK is primarily utilized in orthopedic implants, dental prosthetics, and spinal fusion devices due to its exceptional mechanical properties and biocompatibility. However, its application in drug delivery systems remains relatively limited despite its promising characteristics.
Current applications of PEEK in drug delivery include experimental drug-eluting implants, where the polymer serves as both a structural component and a drug reservoir. Some research groups have developed PEEK-based microneedles for transdermal drug delivery, leveraging the material's mechanical strength and chemical resistance. Additionally, PEEK has been investigated as a component in controlled-release oral formulations, particularly for drugs requiring protection from harsh gastrointestinal environments.
Despite these applications, PEEK faces several significant technical limitations in drug delivery contexts. The inherent hydrophobicity of PEEK presents a major challenge, as it results in poor wettability and limited interaction with aqueous biological environments. This hydrophobic nature restricts drug loading efficiency and release kinetics, particularly for hydrophilic therapeutic agents. The semi-crystalline structure of PEEK also creates a relatively dense polymer matrix with limited porosity, which impedes drug diffusion and restricts release profiles.
Surface modification techniques have been employed to address these limitations, including plasma treatment, chemical etching, and coating with hydrophilic polymers. However, these modifications often compromise the bulk properties of PEEK or provide only temporary improvements in surface characteristics. The high processing temperatures required for PEEK (typically above 300°C) also present challenges for incorporating temperature-sensitive drugs during manufacturing processes.
Another significant limitation is PEEK's relatively inert chemical structure, which offers limited functional groups for drug conjugation or polymer-drug interactions. This chemical inertness restricts the development of stimuli-responsive drug delivery systems based on PEEK, where drug release would be triggered by specific physiological conditions or external stimuli.
The high cost of medical-grade PEEK compared to conventional polymers used in drug delivery (such as PLGA, PCL, or PEG) represents an economic barrier to widespread adoption. This cost factor becomes particularly significant for disposable or short-term drug delivery applications where the exceptional mechanical properties and durability of PEEK may not provide sufficient added value to justify the increased expense.
Regulatory considerations also present challenges, as novel PEEK-based drug delivery systems require extensive safety and efficacy testing before clinical implementation. While PEEK itself has established biocompatibility, its combinations with various drugs and processing additives necessitate comprehensive evaluation for each specific application.
Current applications of PEEK in drug delivery include experimental drug-eluting implants, where the polymer serves as both a structural component and a drug reservoir. Some research groups have developed PEEK-based microneedles for transdermal drug delivery, leveraging the material's mechanical strength and chemical resistance. Additionally, PEEK has been investigated as a component in controlled-release oral formulations, particularly for drugs requiring protection from harsh gastrointestinal environments.
Despite these applications, PEEK faces several significant technical limitations in drug delivery contexts. The inherent hydrophobicity of PEEK presents a major challenge, as it results in poor wettability and limited interaction with aqueous biological environments. This hydrophobic nature restricts drug loading efficiency and release kinetics, particularly for hydrophilic therapeutic agents. The semi-crystalline structure of PEEK also creates a relatively dense polymer matrix with limited porosity, which impedes drug diffusion and restricts release profiles.
Surface modification techniques have been employed to address these limitations, including plasma treatment, chemical etching, and coating with hydrophilic polymers. However, these modifications often compromise the bulk properties of PEEK or provide only temporary improvements in surface characteristics. The high processing temperatures required for PEEK (typically above 300°C) also present challenges for incorporating temperature-sensitive drugs during manufacturing processes.
Another significant limitation is PEEK's relatively inert chemical structure, which offers limited functional groups for drug conjugation or polymer-drug interactions. This chemical inertness restricts the development of stimuli-responsive drug delivery systems based on PEEK, where drug release would be triggered by specific physiological conditions or external stimuli.
The high cost of medical-grade PEEK compared to conventional polymers used in drug delivery (such as PLGA, PCL, or PEG) represents an economic barrier to widespread adoption. This cost factor becomes particularly significant for disposable or short-term drug delivery applications where the exceptional mechanical properties and durability of PEEK may not provide sufficient added value to justify the increased expense.
Regulatory considerations also present challenges, as novel PEEK-based drug delivery systems require extensive safety and efficacy testing before clinical implementation. While PEEK itself has established biocompatibility, its combinations with various drugs and processing additives necessitate comprehensive evaluation for each specific application.
Existing PEEK Drug Delivery Mechanisms
01 Mechanical properties of PEEK polymers
PEEK (Polyetheretherketone) polymers exhibit exceptional mechanical properties, including high strength, stiffness, and durability. These polymers maintain their mechanical integrity under extreme conditions and demonstrate excellent resistance to wear, fatigue, and creep. The mechanical properties of PEEK make it suitable for high-performance applications in various industries, particularly where components are subjected to high mechanical stress.- Mechanical properties of PEEK polymers: PEEK (Polyether ether ketone) polymers exhibit exceptional mechanical properties, including high strength, stiffness, and impact resistance. These polymers maintain their mechanical integrity at elevated temperatures and show excellent resistance to fatigue and creep. The semi-crystalline structure of PEEK contributes to its superior mechanical performance, making it suitable for high-stress applications in various industries including aerospace, automotive, and medical devices.
- Thermal properties and stability of PEEK: PEEK polymers demonstrate remarkable thermal properties with a high glass transition temperature (around 143°C) and melting point (approximately 343°C). They maintain dimensional stability and mechanical properties at elevated temperatures, making them suitable for high-temperature applications. PEEK also exhibits excellent thermal resistance, low thermal expansion, and good thermal conductivity compared to other high-performance polymers, allowing for use in environments with thermal cycling and extreme temperature conditions.
- Chemical resistance and biocompatibility: PEEK polymers possess outstanding chemical resistance to a wide range of organic and inorganic chemicals, acids, bases, and hydrocarbons. They show minimal absorption of moisture and resist degradation in harsh chemical environments. Additionally, PEEK demonstrates excellent biocompatibility, making it suitable for medical implants and devices. The material is bioinert, does not cause adverse tissue reactions, and can withstand sterilization processes, contributing to its growing use in orthopedic, spinal, and dental applications.
- Processing and manufacturing techniques: PEEK polymers can be processed using various manufacturing techniques including injection molding, extrusion, compression molding, and additive manufacturing. The processing conditions significantly influence the crystallinity and resulting properties of PEEK components. The material requires high processing temperatures (typically 370-400°C) and specialized equipment due to its high melting point. Recent advancements have focused on improving processability through modified grades and composite formulations to enhance flowability while maintaining the polymer's exceptional properties.
- PEEK composites and blends: PEEK can be formulated into composites and blends to enhance specific properties. Carbon fiber reinforced PEEK composites offer improved strength, stiffness, and dimensional stability, while glass fiber reinforced variants provide enhanced electrical insulation properties. PEEK can also be blended with other polymers or filled with additives such as graphene, nanoparticles, or lubricants to modify properties like wear resistance, friction coefficient, and electrical conductivity. These modifications expand the application range of PEEK materials in specialized industrial sectors.
02 Thermal properties and stability of PEEK
PEEK polymers possess remarkable thermal properties, including high heat resistance with a glass transition temperature of approximately 143°C and melting point around 343°C. They maintain structural integrity and mechanical properties at elevated temperatures, making them suitable for high-temperature applications. PEEK also exhibits excellent dimensional stability under thermal cycling and low thermal expansion, which is beneficial for precision components in demanding thermal environments.Expand Specific Solutions03 Chemical resistance of PEEK materials
PEEK polymers demonstrate exceptional resistance to a wide range of chemicals, including acids, bases, hydrocarbons, and organic solvents. This chemical inertness makes PEEK suitable for applications in aggressive chemical environments where other polymers would degrade. The material maintains its properties even after prolonged exposure to harsh chemicals, making it valuable in chemical processing equipment, laboratory apparatus, and medical implants where chemical stability is critical.Expand Specific Solutions04 Electrical and insulating properties of PEEK polymers
PEEK polymers exhibit excellent electrical insulation properties with high dielectric strength and volume resistivity. They maintain these electrical properties across a wide temperature range and in various environmental conditions. The combination of electrical insulation with thermal stability makes PEEK particularly valuable in electrical and electronic applications where reliable performance under thermal stress is required, such as in wire coatings, connectors, and semiconductor processing equipment.Expand Specific Solutions05 Modification and enhancement of PEEK properties
PEEK polymers can be modified through various methods to enhance specific properties. These modifications include reinforcement with fibers (carbon, glass) to improve mechanical properties, addition of fillers to enhance thermal conductivity, and surface treatments to improve adhesion characteristics. Specialized processing techniques can also be employed to optimize crystallinity and molecular orientation, resulting in PEEK variants with tailored property profiles for specific applications in aerospace, automotive, and medical industries.Expand Specific Solutions
Leading Companies in PEEK-Based Medical Solutions
The PEEK polymer drug delivery systems market is in a growth phase, characterized by increasing adoption due to PEEK's exceptional biocompatibility, mechanical strength, and chemical resistance properties. The global market is expanding rapidly, driven by rising demand for controlled-release technologies and implantable devices. Leading companies like Solvay Specialty Polymers and Nitto Denko are advancing PEEK applications through proprietary formulation technologies, while academic institutions including Cornell University, Duke University, and Sichuan University are contributing significant research innovations. Pharmaceutical companies such as Nektar Therapeutics and Intezyne Technologies are leveraging PEEK's unique properties to develop novel drug delivery platforms. The technology is approaching maturity in certain applications but continues to evolve with new composite formulations and surface modifications enhancing drug elution capabilities.
Nektar Therapeutics
Technical Solution: Nektar Therapeutics has pioneered advanced drug delivery systems incorporating modified PEEK polymers through their proprietary PEGylation platform. Their approach involves chemical modification of PEEK to create hybrid polymer systems with enhanced drug loading capacity and controlled release properties. Nektar's technology leverages PEEK's exceptional thermal stability and mechanical strength while addressing its inherent hydrophobicity through surface functionalization with hydrophilic PEG chains. This creates amphiphilic structures capable of encapsulating both hydrophobic and hydrophilic drugs. Their systems demonstrate programmable release kinetics through variations in polymer architecture, crosslinking density, and PEG chain length. Nektar has successfully developed implantable PEEK-based drug reservoirs with zero-order release kinetics for various therapeutic applications, including pain management and hormone therapy. Their technology enables drug release periods ranging from weeks to years with minimal initial burst release, addressing a significant challenge in controlled delivery systems[2][5].
Strengths: Advanced polymer chemistry expertise allowing precise control over drug release kinetics, ability to deliver both small molecules and biologics, and established regulatory pathway with previous FDA approvals for polymer-based delivery systems. Weaknesses: Complex manufacturing processes increasing production costs, potential for immunogenic responses to modified polymer surfaces in some patients, and limitations in scaling up production for certain specialized formulations.
Solvay Specialty Polymers USA LLC
Technical Solution: Solvay has developed advanced PEEK-based drug delivery systems leveraging their proprietary Solviva® PEEK biomaterial platform. Their technology utilizes PEEK's exceptional mechanical properties, biocompatibility, and chemical resistance to create controlled-release implantable devices. Solvay's approach involves surface modification techniques to enhance PEEK's hydrophilicity, allowing for better drug loading capacity and controlled elution profiles. They've engineered micro and nano-structured PEEK surfaces that can be loaded with therapeutic agents through various methods including direct impregnation and coating technologies. Their systems demonstrate sustained drug release over extended periods (up to 12 months in some applications) while maintaining structural integrity in biological environments. Solvay has particularly focused on orthopedic and spinal applications where PEEK's mechanical properties closely match bone, reducing stress shielding while delivering anti-inflammatory or antimicrobial agents locally[1][3].
Strengths: Exceptional mechanical stability matching bone properties, biocompatibility for long-term implantation, and resistance to degradation in biological environments. Their surface modification technology enables tunable drug release profiles without compromising structural integrity. Weaknesses: Higher manufacturing costs compared to conventional polymers, limited drug loading capacity for hydrophilic compounds, and potential challenges in achieving uniform drug distribution throughout the polymer matrix.
Key PEEK Modification Technologies for Controlled Release
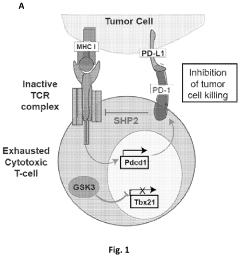
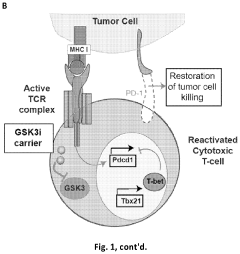
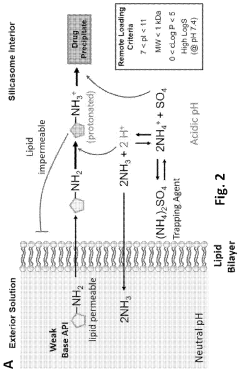
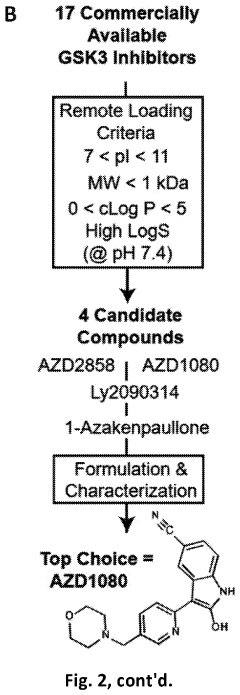
GSK3 inhibitor-loaded NANO formulations as a cancer immunotherapeutic
PatentPendingUS20230241000A1
Innovation
- Development of nanoparticle drug delivery vehicles encapsulating GSK3 inhibitors, coated with a lipid bilayer to enhance delivery and reduce toxicity, allowing for targeted and effective inhibition of PD-1 expression in cancer cells.
Porous beads and process for producing the same
PatentInactiveUS6689465B1
Innovation
- The production method involves mixing the aromatic polyether ketone resin with a solvent to create a resin solution, dispersing it in a non-miscible liquid dispersion medium, cooling to solidify, and separating to form substantially spherical beads with a 50 to 5,000 μm diameter and 40 to 99% porosity, resulting in beads with continuous three-dimensional pores, smooth surfaces, and high mechanical strength.
Biocompatibility and Safety Considerations
PEEK (polyetheretherketone) demonstrates exceptional biocompatibility characteristics that make it highly suitable for drug delivery applications. The polymer's bioinert nature minimizes inflammatory responses when in contact with biological tissues, a critical factor for implantable drug delivery systems. Extensive in vitro and in vivo studies have confirmed PEEK's low cytotoxicity profile, with minimal adverse cellular reactions observed in long-term implantation scenarios.
The safety profile of PEEK is further enhanced by its resistance to degradation in physiological environments. Unlike some polymers that may release potentially harmful degradation products, PEEK maintains structural integrity over extended periods, ensuring consistent drug release kinetics without introducing unexpected compounds into the surrounding tissues. This stability contributes significantly to the predictability and safety of PEEK-based drug delivery systems.
Regulatory bodies, including the FDA and EMA, have recognized PEEK's favorable biocompatibility profile, facilitating approval pathways for medical devices incorporating this polymer. The established safety record of PEEK in orthopedic and spinal implants provides valuable precedent for its application in drug delivery contexts, potentially streamlining regulatory processes for novel delivery systems.
Surface modification techniques can further enhance PEEK's biocompatibility for specific drug delivery applications. Treatments such as plasma modification, coating with bioactive materials, or nanostructuring can modulate tissue interactions while maintaining the polymer's core biocompatibility advantages. These modifications can be tailored to specific anatomical locations or therapeutic requirements without compromising safety.
Sterilization compatibility represents another critical safety consideration for PEEK-based drug delivery systems. The polymer withstands common sterilization methods including gamma irradiation, ethylene oxide, and steam autoclaving without significant degradation of mechanical properties or chemical structure. This versatility ensures that sterility requirements can be met without compromising the integrity of incorporated therapeutic agents.
Long-term safety studies have demonstrated PEEK's minimal potential for systemic toxicity, carcinogenicity, or immunogenicity. The polymer's chemical inertness prevents leaching of potentially harmful compounds into surrounding tissues or the bloodstream, making it particularly valuable for sustained-release drug delivery applications requiring extended implantation periods. This characteristic significantly reduces risks associated with chronic exposure to biomaterials.
When considering the integration of active pharmaceutical ingredients with PEEK, compatibility studies are essential to ensure that drug potency and polymer properties remain unaffected by their combination. Research indicates that PEEK's chemical structure generally resists interactions with most therapeutic compounds, maintaining drug stability while providing the mechanical and physical properties necessary for effective delivery systems.
The safety profile of PEEK is further enhanced by its resistance to degradation in physiological environments. Unlike some polymers that may release potentially harmful degradation products, PEEK maintains structural integrity over extended periods, ensuring consistent drug release kinetics without introducing unexpected compounds into the surrounding tissues. This stability contributes significantly to the predictability and safety of PEEK-based drug delivery systems.
Regulatory bodies, including the FDA and EMA, have recognized PEEK's favorable biocompatibility profile, facilitating approval pathways for medical devices incorporating this polymer. The established safety record of PEEK in orthopedic and spinal implants provides valuable precedent for its application in drug delivery contexts, potentially streamlining regulatory processes for novel delivery systems.
Surface modification techniques can further enhance PEEK's biocompatibility for specific drug delivery applications. Treatments such as plasma modification, coating with bioactive materials, or nanostructuring can modulate tissue interactions while maintaining the polymer's core biocompatibility advantages. These modifications can be tailored to specific anatomical locations or therapeutic requirements without compromising safety.
Sterilization compatibility represents another critical safety consideration for PEEK-based drug delivery systems. The polymer withstands common sterilization methods including gamma irradiation, ethylene oxide, and steam autoclaving without significant degradation of mechanical properties or chemical structure. This versatility ensures that sterility requirements can be met without compromising the integrity of incorporated therapeutic agents.
Long-term safety studies have demonstrated PEEK's minimal potential for systemic toxicity, carcinogenicity, or immunogenicity. The polymer's chemical inertness prevents leaching of potentially harmful compounds into surrounding tissues or the bloodstream, making it particularly valuable for sustained-release drug delivery applications requiring extended implantation periods. This characteristic significantly reduces risks associated with chronic exposure to biomaterials.
When considering the integration of active pharmaceutical ingredients with PEEK, compatibility studies are essential to ensure that drug potency and polymer properties remain unaffected by their combination. Research indicates that PEEK's chemical structure generally resists interactions with most therapeutic compounds, maintaining drug stability while providing the mechanical and physical properties necessary for effective delivery systems.
Regulatory Pathway for PEEK Medical Devices
The regulatory landscape for PEEK (Polyetheretherketone) medical devices is complex and multifaceted, requiring manufacturers to navigate various approval pathways depending on the intended use, risk classification, and geographic market. In the United States, the Food and Drug Administration (FDA) classifies PEEK-based drug delivery systems typically as combination products, necessitating review by multiple centers within the agency.
For PEEK-based implantable drug delivery systems, manufacturers must generally follow the 510(k) pathway if substantially equivalent predicate devices exist, or the more rigorous Premarket Approval (PMA) process for novel applications. The unique chemical stability and biocompatibility of PEEK polymers often facilitate favorable regulatory outcomes, as these properties minimize concerns about material degradation and subsequent toxicity issues.
In the European market, PEEK medical devices fall under the Medical Device Regulation (MDR) or, for drug-device combinations, may be regulated under both MDR and medicinal product directives. The classification depends on the primary mode of action, with PEEK drug delivery systems typically categorized as Class III devices due to their long-term implantable nature and therapeutic intent.
Regulatory bodies worldwide place significant emphasis on the leachables and extractables profile of PEEK polymers when used in drug delivery applications. The material's exceptional chemical resistance provides advantages during this assessment, as PEEK demonstrates minimal interaction with most pharmaceutical compounds, reducing concerns about drug-device compatibility issues that might otherwise complicate regulatory approval.
Quality management systems compliant with ISO 13485 are mandatory for PEEK medical device manufacturers, with additional requirements for design controls and risk management according to ISO 14971. The biocompatibility testing regimen follows ISO 10993 series, with particular attention to parts 1, 5, and 12 for material characterization, cytotoxicity, and sample preparation respectively.
Post-market surveillance requirements for PEEK drug delivery systems are particularly stringent, requiring manufacturers to implement robust systems for adverse event reporting and periodic safety updates. The FDA's unique device identification (UDI) system and the European database on medical devices (EUDAMED) both require specific compliance measures for traceability throughout the product lifecycle.
Emerging regulatory considerations for PEEK drug delivery systems include requirements for real-world evidence generation and patient-reported outcomes, particularly for novel applications where clinical experience may be limited. Manufacturers developing innovative PEEK-based drug delivery technologies should engage with regulatory authorities early through pre-submission consultations to establish appropriate testing protocols and data requirements.
For PEEK-based implantable drug delivery systems, manufacturers must generally follow the 510(k) pathway if substantially equivalent predicate devices exist, or the more rigorous Premarket Approval (PMA) process for novel applications. The unique chemical stability and biocompatibility of PEEK polymers often facilitate favorable regulatory outcomes, as these properties minimize concerns about material degradation and subsequent toxicity issues.
In the European market, PEEK medical devices fall under the Medical Device Regulation (MDR) or, for drug-device combinations, may be regulated under both MDR and medicinal product directives. The classification depends on the primary mode of action, with PEEK drug delivery systems typically categorized as Class III devices due to their long-term implantable nature and therapeutic intent.
Regulatory bodies worldwide place significant emphasis on the leachables and extractables profile of PEEK polymers when used in drug delivery applications. The material's exceptional chemical resistance provides advantages during this assessment, as PEEK demonstrates minimal interaction with most pharmaceutical compounds, reducing concerns about drug-device compatibility issues that might otherwise complicate regulatory approval.
Quality management systems compliant with ISO 13485 are mandatory for PEEK medical device manufacturers, with additional requirements for design controls and risk management according to ISO 14971. The biocompatibility testing regimen follows ISO 10993 series, with particular attention to parts 1, 5, and 12 for material characterization, cytotoxicity, and sample preparation respectively.
Post-market surveillance requirements for PEEK drug delivery systems are particularly stringent, requiring manufacturers to implement robust systems for adverse event reporting and periodic safety updates. The FDA's unique device identification (UDI) system and the European database on medical devices (EUDAMED) both require specific compliance measures for traceability throughout the product lifecycle.
Emerging regulatory considerations for PEEK drug delivery systems include requirements for real-world evidence generation and patient-reported outcomes, particularly for novel applications where clinical experience may be limited. Manufacturers developing innovative PEEK-based drug delivery technologies should engage with regulatory authorities early through pre-submission consultations to establish appropriate testing protocols and data requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!