Regulatory Challenges Facing PEEK Polymer Application in Medical Devices
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Medical Applications Background and Objectives
Polyetheretherketone (PEEK) has emerged as a revolutionary material in the medical device industry over the past three decades. First developed in the 1980s, PEEK gained significant attention in medical applications during the late 1990s due to its exceptional mechanical properties, biocompatibility, and chemical resistance. The evolution of PEEK in medical applications represents a significant advancement in biomaterials science, transitioning from traditional metallic implants to high-performance polymers that better mimic human tissue properties.
The medical applications of PEEK have expanded considerably, from initial use in spine implants to broader applications in orthopedics, dental implants, cardiovascular devices, and cranial implants. This expansion reflects the growing recognition of PEEK's unique combination of properties, including its radiolucency, which allows for unobstructed imaging, and its elastic modulus similar to human bone, reducing stress shielding effects common with metallic implants.
Despite its promising attributes, PEEK faces significant regulatory challenges across global markets. The FDA in the United States, the European Medicines Agency through MDR in Europe, and the NMPA in China each maintain distinct regulatory frameworks for evaluating PEEK-based medical devices. These regulatory bodies have progressively increased scrutiny on biomaterials, particularly regarding long-term biocompatibility, wear characteristics, and potential for adverse reactions.
The primary technical objective in addressing these regulatory challenges involves developing comprehensive testing protocols that satisfy diverse global requirements while demonstrating PEEK's safety and efficacy across different medical applications. This includes establishing standardized methodologies for evaluating biocompatibility, mechanical performance under physiological conditions, and long-term stability in the human body.
Another critical objective is understanding how surface modifications and composite formulations of PEEK affect its regulatory classification and approval pathways. As manufacturers increasingly develop enhanced versions of PEEK with antimicrobial properties, improved osseointegration, or incorporated bioactive materials, the regulatory landscape becomes more complex, requiring sophisticated approaches to compliance.
The technological trajectory suggests a growing need for predictive modeling and simulation tools that can accelerate regulatory approval by providing evidence-based predictions of long-term performance. Additionally, there is increasing emphasis on developing PEEK formulations specifically designed to meet regulatory requirements while maintaining or enhancing clinical performance, representing a shift from adapting existing materials to creating regulation-optimized biomaterials.
The medical applications of PEEK have expanded considerably, from initial use in spine implants to broader applications in orthopedics, dental implants, cardiovascular devices, and cranial implants. This expansion reflects the growing recognition of PEEK's unique combination of properties, including its radiolucency, which allows for unobstructed imaging, and its elastic modulus similar to human bone, reducing stress shielding effects common with metallic implants.
Despite its promising attributes, PEEK faces significant regulatory challenges across global markets. The FDA in the United States, the European Medicines Agency through MDR in Europe, and the NMPA in China each maintain distinct regulatory frameworks for evaluating PEEK-based medical devices. These regulatory bodies have progressively increased scrutiny on biomaterials, particularly regarding long-term biocompatibility, wear characteristics, and potential for adverse reactions.
The primary technical objective in addressing these regulatory challenges involves developing comprehensive testing protocols that satisfy diverse global requirements while demonstrating PEEK's safety and efficacy across different medical applications. This includes establishing standardized methodologies for evaluating biocompatibility, mechanical performance under physiological conditions, and long-term stability in the human body.
Another critical objective is understanding how surface modifications and composite formulations of PEEK affect its regulatory classification and approval pathways. As manufacturers increasingly develop enhanced versions of PEEK with antimicrobial properties, improved osseointegration, or incorporated bioactive materials, the regulatory landscape becomes more complex, requiring sophisticated approaches to compliance.
The technological trajectory suggests a growing need for predictive modeling and simulation tools that can accelerate regulatory approval by providing evidence-based predictions of long-term performance. Additionally, there is increasing emphasis on developing PEEK formulations specifically designed to meet regulatory requirements while maintaining or enhancing clinical performance, representing a shift from adapting existing materials to creating regulation-optimized biomaterials.
Market Demand Analysis for PEEK in Medical Devices
The global market for PEEK (Polyetheretherketone) in medical devices has been experiencing robust growth, driven primarily by the polymer's exceptional mechanical properties, biocompatibility, and resistance to sterilization processes. Current market valuations indicate that the medical-grade PEEK market is growing at a compound annual growth rate of approximately 7% globally, with particularly strong demand in orthopedic implants, dental applications, and spinal fusion devices.
The orthopedic segment represents the largest application area for PEEK, accounting for nearly 40% of the total medical PEEK market. This dominance stems from PEEK's ability to mimic bone's mechanical properties, reducing stress shielding effects that commonly occur with metallic implants. The dental sector follows closely, with increasing adoption of PEEK for removable partial dentures, implant abutments, and frameworks.
Market research indicates that North America currently holds the largest market share at approximately 45%, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. The Asia-Pacific region, particularly China and India, is projected to witness the fastest growth due to expanding healthcare infrastructure, increasing medical tourism, and growing awareness about advanced medical materials.
Demographic trends significantly influence the demand landscape for medical-grade PEEK. The aging global population, with increasing incidence of degenerative joint diseases and dental problems, creates substantial demand for long-lasting implant materials. Additionally, the rising prevalence of sports injuries and road accidents contributes to the growing need for trauma fixation devices, where PEEK offers significant advantages.
Healthcare economics also plays a crucial role in market dynamics. Despite PEEK's higher initial cost compared to traditional materials like titanium or stainless steel, its long-term economic benefits are increasingly recognized. These include reduced revision surgeries, shorter hospital stays, and improved patient outcomes, all contributing to lower overall healthcare costs.
Emerging applications are expanding the market potential for medical PEEK. These include cranio-maxillofacial reconstruction, cardiovascular applications, and drug delivery systems. The development of composite PEEK materials, such as carbon fiber-reinforced PEEK, is opening new avenues for applications requiring enhanced mechanical properties.
Customer preferences are shifting toward personalized medicine, creating demand for patient-specific PEEK implants manufactured using advanced technologies like 3D printing. This trend is expected to accelerate as additive manufacturing technologies mature and regulatory pathways for such devices become more established.
The orthopedic segment represents the largest application area for PEEK, accounting for nearly 40% of the total medical PEEK market. This dominance stems from PEEK's ability to mimic bone's mechanical properties, reducing stress shielding effects that commonly occur with metallic implants. The dental sector follows closely, with increasing adoption of PEEK for removable partial dentures, implant abutments, and frameworks.
Market research indicates that North America currently holds the largest market share at approximately 45%, followed by Europe at 30% and Asia-Pacific at 20%. The remaining 5% is distributed across other regions. The Asia-Pacific region, particularly China and India, is projected to witness the fastest growth due to expanding healthcare infrastructure, increasing medical tourism, and growing awareness about advanced medical materials.
Demographic trends significantly influence the demand landscape for medical-grade PEEK. The aging global population, with increasing incidence of degenerative joint diseases and dental problems, creates substantial demand for long-lasting implant materials. Additionally, the rising prevalence of sports injuries and road accidents contributes to the growing need for trauma fixation devices, where PEEK offers significant advantages.
Healthcare economics also plays a crucial role in market dynamics. Despite PEEK's higher initial cost compared to traditional materials like titanium or stainless steel, its long-term economic benefits are increasingly recognized. These include reduced revision surgeries, shorter hospital stays, and improved patient outcomes, all contributing to lower overall healthcare costs.
Emerging applications are expanding the market potential for medical PEEK. These include cranio-maxillofacial reconstruction, cardiovascular applications, and drug delivery systems. The development of composite PEEK materials, such as carbon fiber-reinforced PEEK, is opening new avenues for applications requiring enhanced mechanical properties.
Customer preferences are shifting toward personalized medicine, creating demand for patient-specific PEEK implants manufactured using advanced technologies like 3D printing. This trend is expected to accelerate as additive manufacturing technologies mature and regulatory pathways for such devices become more established.
PEEK Regulatory Landscape and Technical Barriers
The regulatory landscape for PEEK polymer applications in medical devices is characterized by a complex web of international standards and regional requirements. In the United States, the FDA regulates PEEK-based medical devices primarily through the 510(k) clearance pathway, requiring manufacturers to demonstrate substantial equivalence to legally marketed devices. For novel applications, the more stringent Premarket Approval (PMA) process may be necessary, demanding extensive clinical data to prove safety and efficacy.
In the European Union, the transition from the Medical Device Directive (MDD) to the more stringent Medical Device Regulation (MDR) has significantly impacted PEEK applications. The MDR introduces enhanced requirements for clinical evaluation, post-market surveillance, and technical documentation, particularly for implantable devices where PEEK is commonly used.
Asian markets present varying regulatory approaches, with Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requiring specific biocompatibility testing protocols for PEEK materials, while China's National Medical Products Administration (NMPA) has implemented increasingly stringent requirements for imported medical devices containing advanced polymers.
A significant technical barrier facing PEEK applications is the standardization of manufacturing processes. The polymer's properties can vary substantially depending on processing conditions, creating challenges for consistent regulatory compliance. Manufacturers must validate that their specific PEEK processing methods yield materials with predictable mechanical properties and biocompatibility profiles.
Biocompatibility testing represents another major hurdle, as regulatory bodies increasingly require comprehensive testing beyond ISO 10993 standards. This includes long-term implantation studies and specific tests for wear particles and degradation products, particularly for load-bearing applications where PEEK is replacing traditional materials.
Surface modification technologies for PEEK, such as plasma treatment or hydroxyapatite coating, face additional regulatory scrutiny. These modifications alter the base material's properties and require separate validation studies to demonstrate maintained safety profiles while achieving enhanced functionality.
Sterilization validation presents unique challenges for PEEK devices. Common methods like gamma irradiation can potentially alter the polymer's molecular structure, affecting mechanical properties and long-term stability. Manufacturers must validate that their chosen sterilization methods do not compromise device performance or safety, often requiring accelerated aging studies.
The regulatory pathway becomes particularly complex for composite PEEK materials, such as carbon fiber-reinforced PEEK. These materials must satisfy requirements for both the base polymer and the reinforcement components, with special attention to interface stability and potential particle release in vivo.
In the European Union, the transition from the Medical Device Directive (MDD) to the more stringent Medical Device Regulation (MDR) has significantly impacted PEEK applications. The MDR introduces enhanced requirements for clinical evaluation, post-market surveillance, and technical documentation, particularly for implantable devices where PEEK is commonly used.
Asian markets present varying regulatory approaches, with Japan's Pharmaceuticals and Medical Devices Agency (PMDA) requiring specific biocompatibility testing protocols for PEEK materials, while China's National Medical Products Administration (NMPA) has implemented increasingly stringent requirements for imported medical devices containing advanced polymers.
A significant technical barrier facing PEEK applications is the standardization of manufacturing processes. The polymer's properties can vary substantially depending on processing conditions, creating challenges for consistent regulatory compliance. Manufacturers must validate that their specific PEEK processing methods yield materials with predictable mechanical properties and biocompatibility profiles.
Biocompatibility testing represents another major hurdle, as regulatory bodies increasingly require comprehensive testing beyond ISO 10993 standards. This includes long-term implantation studies and specific tests for wear particles and degradation products, particularly for load-bearing applications where PEEK is replacing traditional materials.
Surface modification technologies for PEEK, such as plasma treatment or hydroxyapatite coating, face additional regulatory scrutiny. These modifications alter the base material's properties and require separate validation studies to demonstrate maintained safety profiles while achieving enhanced functionality.
Sterilization validation presents unique challenges for PEEK devices. Common methods like gamma irradiation can potentially alter the polymer's molecular structure, affecting mechanical properties and long-term stability. Manufacturers must validate that their chosen sterilization methods do not compromise device performance or safety, often requiring accelerated aging studies.
The regulatory pathway becomes particularly complex for composite PEEK materials, such as carbon fiber-reinforced PEEK. These materials must satisfy requirements for both the base polymer and the reinforcement components, with special attention to interface stability and potential particle release in vivo.
Current Regulatory Compliance Strategies
01 PEEK polymer composition and synthesis
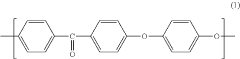
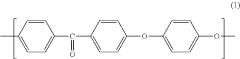
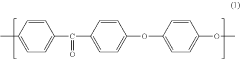
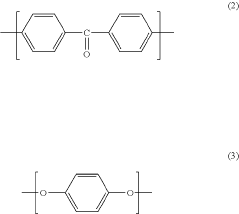
Polyetheretherketone (PEEK) is a high-performance thermoplastic polymer with excellent mechanical properties, chemical resistance, and thermal stability. Various methods for synthesizing PEEK polymers have been developed, including nucleophilic substitution reactions and condensation polymerization. These methods can be optimized to control molecular weight, crystallinity, and other properties that affect the performance of the final material.- PEEK polymer composition and synthesis: Polyetheretherketone (PEEK) is a high-performance thermoplastic polymer with excellent mechanical properties, chemical resistance, and thermal stability. The synthesis of PEEK typically involves nucleophilic aromatic substitution reactions between diphenols and dihalides. Various modifications to the synthesis process can yield PEEK polymers with tailored properties for specific applications. These modifications may include the incorporation of different monomers or the use of specific catalysts to control molecular weight and crystallinity.
- PEEK polymer blends and composites: PEEK can be blended with other polymers or reinforced with various fillers to enhance its properties. Common reinforcements include carbon fibers, glass fibers, and mineral fillers, which significantly improve the mechanical strength, stiffness, and dimensional stability of PEEK. These composites find applications in aerospace, automotive, and industrial sectors where high performance under extreme conditions is required. The processing of these composites often requires specialized techniques due to the high melting point of PEEK.
- PEEK polymer for medical applications: PEEK is widely used in medical applications due to its biocompatibility, radiolucency, and mechanical properties similar to human bone. It is commonly used for implants, including spinal cages, cranial implants, and dental prosthetics. Surface modifications of PEEK for medical applications can enhance osseointegration and reduce bacterial adhesion. These modifications include plasma treatment, coating with bioactive materials, or incorporating antimicrobial agents into the polymer matrix.
- PEEK polymer processing techniques: Processing PEEK requires specialized techniques due to its high melting point (around 343°C) and crystalline nature. Common processing methods include injection molding, extrusion, compression molding, and additive manufacturing. Each technique requires specific processing parameters to achieve optimal mechanical properties and dimensional stability. Recent advancements in processing technologies have enabled the production of complex PEEK parts with improved precision and reduced cycle times.
- PEEK polymer for filtration and separation applications: PEEK membranes and filters are used in harsh chemical environments and high-temperature applications where other polymers would degrade. These filtration systems benefit from PEEK's excellent chemical resistance, thermal stability, and mechanical strength. Applications include pharmaceutical processing, chemical manufacturing, oil and gas production, and wastewater treatment. The pore size and surface properties of PEEK membranes can be tailored to achieve specific separation requirements for different industrial processes.
02 PEEK polymer blends and composites
PEEK can be blended with other polymers or reinforced with various fillers to enhance specific properties. Common reinforcements include carbon fibers, glass fibers, and mineral fillers, which significantly improve mechanical strength, stiffness, and dimensional stability. These composites find applications in aerospace, automotive, and industrial sectors where high performance under extreme conditions is required.Expand Specific Solutions03 PEEK polymer for medical applications
PEEK polymers have gained significant importance in medical and dental applications due to their biocompatibility, radiolucency, and mechanical properties similar to human bone. They are used in implantable medical devices, dental prosthetics, and surgical instruments. Modified PEEK materials with enhanced bioactivity or antimicrobial properties have been developed to improve clinical outcomes in orthopedic and spinal applications.Expand Specific Solutions04 PEEK polymer processing techniques
Various processing techniques have been developed for PEEK polymers, including injection molding, extrusion, compression molding, and additive manufacturing. These techniques require specific processing conditions due to PEEK's high melting temperature and crystallization behavior. Advanced processing methods have been developed to overcome challenges related to the high processing temperatures and to achieve desired microstructures and properties in the final products.Expand Specific Solutions05 PEEK polymer for filtration and separation applications
PEEK polymers are utilized in filtration and separation technologies due to their excellent chemical resistance, thermal stability, and mechanical strength. PEEK membranes and filters can withstand harsh chemical environments and high temperatures, making them suitable for industrial separation processes, water treatment, and pharmaceutical applications. Modified PEEK materials with controlled porosity and surface properties have been developed to enhance separation efficiency and selectivity.Expand Specific Solutions
Key Industry Players in Medical-Grade PEEK
The regulatory landscape for PEEK polymer applications in medical devices is evolving within a growing market estimated at $1 billion annually, currently in its growth phase. Technical maturity varies significantly among key players, with established manufacturers like Solvay Specialty Polymers and Victrex Manufacturing leading innovation with comprehensive regulatory expertise. Medical device companies including DePuy Synthes, Stryker, and Medtronic have developed sophisticated regulatory compliance frameworks for PEEK-based implants. Emerging players like DiFusion and Jilin Joinature Polymer are navigating the complex approval pathways with varying degrees of success. The primary challenges include biocompatibility documentation, manufacturing consistency validation, and navigating different regional regulatory frameworks, particularly between FDA and EU MDR requirements.
Solvay Specialty Polymers USA LLC
Technical Solution: Solvay has implemented a multi-tiered regulatory compliance program for their KetaSpire® PEEK and AvaSpire® PAEK polymers in medical applications. Their approach focuses on addressing the complex regulatory landscape through comprehensive material qualification according to both FDA and EU MDR requirements. Solvay maintains a dedicated Medical Device Regulatory Support Team that assists customers with regulatory submissions by providing detailed documentation packages including biocompatibility testing results, material characterization data, and processing guidelines specific to medical-grade polymers[2]. Their regulatory strategy includes specialized testing protocols for sterilization compatibility (including steam, ethylene oxide, and gamma radiation) and chemical resistance to cleaning agents commonly used in healthcare settings. Solvay has developed specific regulatory pathways for different risk classifications of medical devices, with particular emphasis on addressing the heightened scrutiny for implantable devices containing their high-performance polymers[4]. The company also provides regulatory guidance for the transition from metal to polymer components in medical devices.
Strengths: Comprehensive regulatory support across multiple global markets; specialized expertise in sterilization validation for PEEK components; strong documentation packages that address both US and European regulatory requirements. Weaknesses: Less extensive history with long-term implantable applications compared to some competitors; regulatory pathway for novel surface modifications of PEEK may require additional customer-specific testing.
Victrex Manufacturing Ltd.
Technical Solution: Victrex has developed comprehensive regulatory compliance strategies for their PEEK-OPTIMA™ polymers specifically designed for medical implants. Their approach includes extensive biocompatibility testing according to ISO 10993 standards and master file submissions to the FDA (MAF) to streamline customer device approvals[1]. Victrex maintains Drug Master Files (DMFs) and technical documentation that supports customers through the regulatory pathway for various medical applications including spinal implants, trauma devices, and dental prosthetics. Their regulatory strategy includes comprehensive material characterization, leachables and extractables testing, and long-term implantation studies to address the stringent requirements for permanent implantable devices. Victrex also provides specialized support for customers navigating the transition from metal to PEEK components, addressing the unique regulatory challenges this material substitution presents in medical device submissions[3].
Strengths: Established regulatory pathway with FDA master files that expedite customer approvals; extensive biocompatibility data supporting long-term implantation claims; global regulatory expertise across multiple medical device classifications. Weaknesses: Regulatory support primarily focused on established applications; potential challenges with novel or combination devices requiring additional testing beyond existing documentation.
Critical Patents and Technical Literature Review
Polyether ether ketone and method for producing polyether ether ketone
PatentActiveUS20230265244A1
Innovation
- A method involving the reaction of 4,4′-dichlorobenzophenone and hydroquinone under specific temperature conditions (260° C. to 320° C.) to produce a PEEK with a crystallization temperature Tc of 255° C. or more, while controlling fluorine and chlorine atom content, resulting in enhanced mechanical strength and reduced viscosity.
Polyether ether ketone composite material
PatentInactiveUS20140039127A1
Innovation
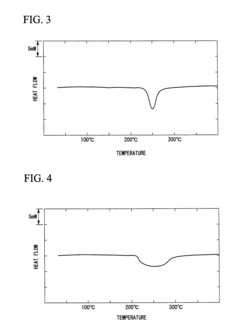
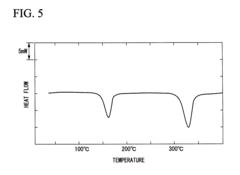
- A PEEK composite material comprising PEEK and polyolefin with a compatible structure, where the polyolefin is dispersed in a matrix of PEEK, achieving a single endothermic peak in DSC, allowing for a lower molding temperature and preventing colorant discoloration, with particle sizes of dispersed parts optimized to 1 μm or smaller for efficient thermal properties.
Biocompatibility Testing Standards and Protocols
Biocompatibility testing for PEEK (Polyetheretherketone) polymer in medical devices follows rigorous international standards to ensure patient safety. The primary framework governing these tests is ISO 10993, which outlines a comprehensive series of evaluations for medical device materials. For PEEK applications, the most relevant sections include ISO 10993-1 (evaluation framework), ISO 10993-5 (cytotoxicity), and ISO 10993-12 (sample preparation). These standards require manufacturers to assess biological risks based on the nature and duration of patient contact.
The FDA has adopted these ISO standards while adding specific guidance through its "Use of International Standard ISO 10993-1" document. This guidance emphasizes a risk-based approach to biocompatibility testing, allowing manufacturers to potentially reduce unnecessary animal testing through chemical characterization and toxicological risk assessment. For PEEK implants with long-term patient contact, comprehensive testing typically includes cytotoxicity, sensitization, irritation, acute systemic toxicity, sub-chronic toxicity, and genotoxicity evaluations.
European regulatory bodies enforce similar requirements through the Medical Device Regulation (MDR), which mandates thorough biocompatibility documentation for CE marking. The MDR places particular emphasis on the biological evaluation plan and report as essential components of technical documentation.
Testing protocols for PEEK materials generally begin with in vitro cytotoxicity assays using methods such as MEM elution or direct contact with L929 fibroblast cells. These tests evaluate whether PEEK extracts cause cell death or morphological changes. Sensitization testing typically employs guinea pig maximization tests or local lymph node assays to assess allergenic potential, while irritation testing uses intracutaneous reactivity tests in animal models.
For long-term implantable PEEK devices, additional protocols include implantation studies ranging from 12 weeks to over 26 weeks, depending on the intended duration of use. These studies evaluate local tissue responses, potential degradation, and long-term compatibility. Genotoxicity testing through Ames tests, chromosomal aberration assays, and micronucleus tests is also required to assess mutagenic potential.
A significant challenge in PEEK biocompatibility testing is addressing the variability in material formulations. Different grades of PEEK may contain varying levels of additives, processing aids, or reinforcing materials like carbon fiber, each requiring separate evaluation. Additionally, manufacturing processes such as injection molding or machining can introduce surface characteristics that influence biocompatibility outcomes, necessitating testing of finished devices rather than raw materials alone.
The FDA has adopted these ISO standards while adding specific guidance through its "Use of International Standard ISO 10993-1" document. This guidance emphasizes a risk-based approach to biocompatibility testing, allowing manufacturers to potentially reduce unnecessary animal testing through chemical characterization and toxicological risk assessment. For PEEK implants with long-term patient contact, comprehensive testing typically includes cytotoxicity, sensitization, irritation, acute systemic toxicity, sub-chronic toxicity, and genotoxicity evaluations.
European regulatory bodies enforce similar requirements through the Medical Device Regulation (MDR), which mandates thorough biocompatibility documentation for CE marking. The MDR places particular emphasis on the biological evaluation plan and report as essential components of technical documentation.
Testing protocols for PEEK materials generally begin with in vitro cytotoxicity assays using methods such as MEM elution or direct contact with L929 fibroblast cells. These tests evaluate whether PEEK extracts cause cell death or morphological changes. Sensitization testing typically employs guinea pig maximization tests or local lymph node assays to assess allergenic potential, while irritation testing uses intracutaneous reactivity tests in animal models.
For long-term implantable PEEK devices, additional protocols include implantation studies ranging from 12 weeks to over 26 weeks, depending on the intended duration of use. These studies evaluate local tissue responses, potential degradation, and long-term compatibility. Genotoxicity testing through Ames tests, chromosomal aberration assays, and micronucleus tests is also required to assess mutagenic potential.
A significant challenge in PEEK biocompatibility testing is addressing the variability in material formulations. Different grades of PEEK may contain varying levels of additives, processing aids, or reinforcing materials like carbon fiber, each requiring separate evaluation. Additionally, manufacturing processes such as injection molding or machining can introduce surface characteristics that influence biocompatibility outcomes, necessitating testing of finished devices rather than raw materials alone.
International Regulatory Harmonization Opportunities
The global landscape of medical device regulations presents significant challenges for PEEK polymer applications, with manufacturers facing a complex web of country-specific requirements. Harmonization efforts represent a critical opportunity to streamline approval processes and accelerate market access for PEEK-based medical innovations.
The International Medical Device Regulators Forum (IMDRF) has emerged as a pivotal platform for advancing regulatory convergence. Their initiatives to standardize technical documentation requirements through the Table of Contents (ToC) format offer particular promise for PEEK polymer applications, potentially reducing redundant testing and documentation across jurisdictions. This framework could significantly decrease the regulatory burden for manufacturers seeking multi-market approvals for PEEK-based implants and devices.
Regional harmonization blocks are also making notable progress. The European Medical Device Regulation (MDR) has established a unified approach across EU member states, while the ASEAN Medical Device Directive aims to create regulatory coherence across Southeast Asian markets. These regional frameworks provide templates for broader international alignment on PEEK polymer evaluation standards.
Mutual Recognition Agreements (MRAs) between major regulatory authorities represent another promising avenue. The FDA-EU MRA for quality management system inspections, though currently limited in scope, could potentially expand to include aspects of material evaluation relevant to PEEK applications. Similar agreements between other major markets could substantially reduce duplicative conformity assessments.
International standards organizations, particularly ISO and ASTM, are developing globally recognized testing protocols specifically addressing polymer biocompatibility and mechanical performance. The adoption of ISO 10993-1:2018 for biological evaluation of medical devices provides a harmonized approach to PEEK biocompatibility assessment that is increasingly recognized across jurisdictions.
Emerging opportunities exist in the development of international consensus on PEEK-specific testing requirements. Currently, variations in testing protocols for leachables, degradation products, and long-term performance create significant market barriers. A coordinated effort between industry stakeholders, standards organizations, and regulatory bodies could establish globally accepted evaluation criteria specifically for PEEK polymers in medical applications.
Digital submission platforms and harmonized electronic documentation standards represent another frontier for regulatory efficiency. The implementation of systems like the European Database on Medical Devices (EUDAMED) points toward future possibilities for streamlined international submissions, potentially reducing the administrative burden for PEEK device manufacturers targeting multiple markets.
The International Medical Device Regulators Forum (IMDRF) has emerged as a pivotal platform for advancing regulatory convergence. Their initiatives to standardize technical documentation requirements through the Table of Contents (ToC) format offer particular promise for PEEK polymer applications, potentially reducing redundant testing and documentation across jurisdictions. This framework could significantly decrease the regulatory burden for manufacturers seeking multi-market approvals for PEEK-based implants and devices.
Regional harmonization blocks are also making notable progress. The European Medical Device Regulation (MDR) has established a unified approach across EU member states, while the ASEAN Medical Device Directive aims to create regulatory coherence across Southeast Asian markets. These regional frameworks provide templates for broader international alignment on PEEK polymer evaluation standards.
Mutual Recognition Agreements (MRAs) between major regulatory authorities represent another promising avenue. The FDA-EU MRA for quality management system inspections, though currently limited in scope, could potentially expand to include aspects of material evaluation relevant to PEEK applications. Similar agreements between other major markets could substantially reduce duplicative conformity assessments.
International standards organizations, particularly ISO and ASTM, are developing globally recognized testing protocols specifically addressing polymer biocompatibility and mechanical performance. The adoption of ISO 10993-1:2018 for biological evaluation of medical devices provides a harmonized approach to PEEK biocompatibility assessment that is increasingly recognized across jurisdictions.
Emerging opportunities exist in the development of international consensus on PEEK-specific testing requirements. Currently, variations in testing protocols for leachables, degradation products, and long-term performance create significant market barriers. A coordinated effort between industry stakeholders, standards organizations, and regulatory bodies could establish globally accepted evaluation criteria specifically for PEEK polymers in medical applications.
Digital submission platforms and harmonized electronic documentation standards represent another frontier for regulatory efficiency. The implementation of systems like the European Database on Medical Devices (EUDAMED) points toward future possibilities for streamlined international submissions, potentially reducing the administrative burden for PEEK device manufacturers targeting multiple markets.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!