Regulation and Standardization of PEEK Polymer in Pharmaceuticals
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Polymer in Pharmaceuticals: Background and Objectives
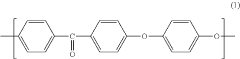
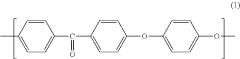
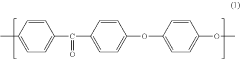
Polyetheretherketone (PEEK) has emerged as a significant polymer in pharmaceutical applications over the past three decades. Initially developed in the 1970s by Imperial Chemical Industries, PEEK gained prominence in aerospace and automotive industries before transitioning to medical and pharmaceutical applications in the 1990s. This high-performance thermoplastic polymer exhibits exceptional mechanical strength, chemical resistance, and biocompatibility, making it increasingly valuable for pharmaceutical manufacturing equipment, drug delivery systems, and medical implants.
The evolution of PEEK in pharmaceuticals has been characterized by continuous refinement of its properties and processing techniques. Early applications focused primarily on basic components in pharmaceutical equipment, while recent developments have expanded to include precision drug delivery devices, specialized containment systems for aggressive pharmaceutical compounds, and customized implantable drug-eluting devices. This progression reflects the industry's growing recognition of PEEK's unique combination of properties that address critical challenges in pharmaceutical production and delivery.
Current technological trends indicate an acceleration in PEEK applications within the pharmaceutical sector, particularly in areas requiring high purity, chemical stability, and precise manufacturing tolerances. The polymer's ability to withstand sterilization processes without degradation has positioned it as a preferred material for components in continuous manufacturing systems and personalized medicine production lines. Additionally, the development of composite PEEK materials with enhanced properties represents a significant direction in the material's evolution.
The primary technical objectives for PEEK regulation and standardization in pharmaceuticals encompass several critical dimensions. First, establishing comprehensive material characterization standards specific to pharmaceutical applications is essential for ensuring consistent quality and performance. Second, developing standardized testing protocols for evaluating PEEK's interaction with various pharmaceutical compounds will provide crucial data for regulatory submissions. Third, creating clear guidelines for manufacturing processes that maintain PEEK's integrity during pharmaceutical production represents a key priority.
Furthermore, the harmonization of international standards for PEEK in pharmaceutical applications aims to facilitate global market access while maintaining stringent safety and efficacy requirements. This includes addressing variations in regulatory approaches across major markets such as the FDA, EMA, and PMDA jurisdictions. The ultimate goal is to create a robust regulatory framework that enables innovation while ensuring patient safety through appropriate material controls, validation requirements, and quality assurance measures tailored to PEEK's unique properties and pharmaceutical applications.
The evolution of PEEK in pharmaceuticals has been characterized by continuous refinement of its properties and processing techniques. Early applications focused primarily on basic components in pharmaceutical equipment, while recent developments have expanded to include precision drug delivery devices, specialized containment systems for aggressive pharmaceutical compounds, and customized implantable drug-eluting devices. This progression reflects the industry's growing recognition of PEEK's unique combination of properties that address critical challenges in pharmaceutical production and delivery.
Current technological trends indicate an acceleration in PEEK applications within the pharmaceutical sector, particularly in areas requiring high purity, chemical stability, and precise manufacturing tolerances. The polymer's ability to withstand sterilization processes without degradation has positioned it as a preferred material for components in continuous manufacturing systems and personalized medicine production lines. Additionally, the development of composite PEEK materials with enhanced properties represents a significant direction in the material's evolution.
The primary technical objectives for PEEK regulation and standardization in pharmaceuticals encompass several critical dimensions. First, establishing comprehensive material characterization standards specific to pharmaceutical applications is essential for ensuring consistent quality and performance. Second, developing standardized testing protocols for evaluating PEEK's interaction with various pharmaceutical compounds will provide crucial data for regulatory submissions. Third, creating clear guidelines for manufacturing processes that maintain PEEK's integrity during pharmaceutical production represents a key priority.
Furthermore, the harmonization of international standards for PEEK in pharmaceutical applications aims to facilitate global market access while maintaining stringent safety and efficacy requirements. This includes addressing variations in regulatory approaches across major markets such as the FDA, EMA, and PMDA jurisdictions. The ultimate goal is to create a robust regulatory framework that enables innovation while ensuring patient safety through appropriate material controls, validation requirements, and quality assurance measures tailored to PEEK's unique properties and pharmaceutical applications.
Market Demand Analysis for PEEK in Pharmaceutical Applications
The pharmaceutical industry's demand for high-performance materials has grown significantly in recent years, with PEEK (Polyether Ether Ketone) emerging as a critical polymer in various applications. Market analysis indicates that the global pharmaceutical PEEK market is projected to grow at a compound annual growth rate of 6.8% from 2023 to 2030, driven primarily by increasing adoption in drug delivery systems, medical packaging, and pharmaceutical manufacturing equipment.
The demand for PEEK in pharmaceutical applications stems from its exceptional chemical resistance, particularly to aggressive cleaning agents and sterilization processes commonly used in pharmaceutical manufacturing. This resistance translates to longer equipment lifespans and reduced contamination risks, addressing key concerns in pharmaceutical production environments where material integrity is paramount.
Pharmaceutical companies are increasingly seeking materials that comply with stringent regulatory standards while maintaining operational efficiency. PEEK's biocompatibility and compliance with FDA regulations make it particularly valuable for applications involving direct or indirect contact with pharmaceutical products. Market research shows that approximately 42% of pharmaceutical manufacturers cite regulatory compliance as their primary consideration when selecting materials for production equipment.
The trend toward continuous manufacturing in pharmaceuticals has further accelerated demand for PEEK components. These manufacturing processes require materials that can withstand constant operation under harsh conditions, creating a specialized market segment where PEEK outperforms traditional materials like stainless steel in certain applications, particularly where weight reduction and chemical resistance are priorities.
Regional analysis reveals that North America currently dominates the pharmaceutical PEEK market, accounting for approximately 38% of global demand, followed by Europe at 31% and Asia-Pacific at 24%. However, the fastest growth is occurring in emerging markets, particularly in India and China, where rapid expansion of pharmaceutical manufacturing capacity is driving increased adoption of advanced materials.
The market segmentation shows distinct demand patterns across different pharmaceutical sectors. Generic drug manufacturers typically prioritize cost-effectiveness and durability, while specialty pharmaceutical and biopharmaceutical companies place greater emphasis on purity, precision, and compliance with the highest regulatory standards. This segmentation has led to the development of specialized PEEK formulations tailored to specific pharmaceutical applications.
Price sensitivity varies significantly across market segments, with larger pharmaceutical manufacturers demonstrating willingness to invest in premium PEEK solutions that offer documented compliance with multiple international standards. Market surveys indicate that standardization efforts could potentially expand the addressable market by making PEEK more accessible to mid-sized pharmaceutical manufacturers who currently view regulatory uncertainty as a barrier to adoption.
The demand for PEEK in pharmaceutical applications stems from its exceptional chemical resistance, particularly to aggressive cleaning agents and sterilization processes commonly used in pharmaceutical manufacturing. This resistance translates to longer equipment lifespans and reduced contamination risks, addressing key concerns in pharmaceutical production environments where material integrity is paramount.
Pharmaceutical companies are increasingly seeking materials that comply with stringent regulatory standards while maintaining operational efficiency. PEEK's biocompatibility and compliance with FDA regulations make it particularly valuable for applications involving direct or indirect contact with pharmaceutical products. Market research shows that approximately 42% of pharmaceutical manufacturers cite regulatory compliance as their primary consideration when selecting materials for production equipment.
The trend toward continuous manufacturing in pharmaceuticals has further accelerated demand for PEEK components. These manufacturing processes require materials that can withstand constant operation under harsh conditions, creating a specialized market segment where PEEK outperforms traditional materials like stainless steel in certain applications, particularly where weight reduction and chemical resistance are priorities.
Regional analysis reveals that North America currently dominates the pharmaceutical PEEK market, accounting for approximately 38% of global demand, followed by Europe at 31% and Asia-Pacific at 24%. However, the fastest growth is occurring in emerging markets, particularly in India and China, where rapid expansion of pharmaceutical manufacturing capacity is driving increased adoption of advanced materials.
The market segmentation shows distinct demand patterns across different pharmaceutical sectors. Generic drug manufacturers typically prioritize cost-effectiveness and durability, while specialty pharmaceutical and biopharmaceutical companies place greater emphasis on purity, precision, and compliance with the highest regulatory standards. This segmentation has led to the development of specialized PEEK formulations tailored to specific pharmaceutical applications.
Price sensitivity varies significantly across market segments, with larger pharmaceutical manufacturers demonstrating willingness to invest in premium PEEK solutions that offer documented compliance with multiple international standards. Market surveys indicate that standardization efforts could potentially expand the addressable market by making PEEK more accessible to mid-sized pharmaceutical manufacturers who currently view regulatory uncertainty as a barrier to adoption.
Current Regulatory Status and Technical Challenges
Polyetheretherketone (PEEK) polymer has gained significant attention in pharmaceutical applications due to its exceptional chemical resistance, mechanical strength, and biocompatibility. However, its regulatory landscape remains complex and fragmented across different regions. In the United States, the FDA has established guidelines for PEEK in certain medical devices but lacks comprehensive frameworks specifically for pharmaceutical applications. The European Medicines Agency (EMA) has similarly recognized PEEK's potential but maintains stringent requirements for its use in drug delivery systems and pharmaceutical manufacturing equipment.
The International Conference on Harmonization (ICH) guidelines address polymeric materials in pharmaceutical contexts but do not explicitly detail PEEK-specific standards. This regulatory gap creates significant challenges for manufacturers seeking global market access, as they must navigate disparate approval processes across jurisdictions. The absence of harmonized standards necessitates redundant testing and documentation, increasing development costs and time-to-market.
From a technical perspective, PEEK polymer faces several critical challenges in pharmaceutical applications. Extraction and leachable studies reveal that under certain conditions, PEEK may release trace compounds that could potentially interact with pharmaceutical formulations. The industry lacks standardized protocols for evaluating these interactions across different drug types and formulation environments. Additionally, surface modification techniques for PEEK, which could enhance its compatibility with certain pharmaceutical compounds, remain inconsistently validated across regulatory frameworks.
Manufacturing consistency presents another significant challenge. While PEEK's general properties are well-established, batch-to-batch variations in pharmaceutical-grade PEEK can affect critical quality attributes. Current analytical methods for characterizing these variations lack the sensitivity required by pharmaceutical regulatory standards, creating uncertainty in quality control processes. The industry needs more precise specifications for pharmaceutical-grade PEEK that account for the polymer's complex structure and potential modifications.
Sterilization compatibility represents a persistent technical hurdle. While PEEK demonstrates excellent resistance to common sterilization methods, including autoclaving and gamma irradiation, the cumulative effects of repeated sterilization cycles on PEEK components in pharmaceutical manufacturing equipment remain inadequately characterized. This gap in understanding creates uncertainty regarding the material's long-term performance in pharmaceutical production environments.
The absence of pharmacopeial monographs specifically addressing PEEK polymers further complicates regulatory compliance. Without these standardized reference points, manufacturers must develop and justify their own specifications, leading to inconsistent approaches across the industry. Efforts to develop such monographs are underway in several pharmacopeial organizations, but progress has been slow due to the complexity of characterizing polymeric materials for pharmaceutical applications.
The International Conference on Harmonization (ICH) guidelines address polymeric materials in pharmaceutical contexts but do not explicitly detail PEEK-specific standards. This regulatory gap creates significant challenges for manufacturers seeking global market access, as they must navigate disparate approval processes across jurisdictions. The absence of harmonized standards necessitates redundant testing and documentation, increasing development costs and time-to-market.
From a technical perspective, PEEK polymer faces several critical challenges in pharmaceutical applications. Extraction and leachable studies reveal that under certain conditions, PEEK may release trace compounds that could potentially interact with pharmaceutical formulations. The industry lacks standardized protocols for evaluating these interactions across different drug types and formulation environments. Additionally, surface modification techniques for PEEK, which could enhance its compatibility with certain pharmaceutical compounds, remain inconsistently validated across regulatory frameworks.
Manufacturing consistency presents another significant challenge. While PEEK's general properties are well-established, batch-to-batch variations in pharmaceutical-grade PEEK can affect critical quality attributes. Current analytical methods for characterizing these variations lack the sensitivity required by pharmaceutical regulatory standards, creating uncertainty in quality control processes. The industry needs more precise specifications for pharmaceutical-grade PEEK that account for the polymer's complex structure and potential modifications.
Sterilization compatibility represents a persistent technical hurdle. While PEEK demonstrates excellent resistance to common sterilization methods, including autoclaving and gamma irradiation, the cumulative effects of repeated sterilization cycles on PEEK components in pharmaceutical manufacturing equipment remain inadequately characterized. This gap in understanding creates uncertainty regarding the material's long-term performance in pharmaceutical production environments.
The absence of pharmacopeial monographs specifically addressing PEEK polymers further complicates regulatory compliance. Without these standardized reference points, manufacturers must develop and justify their own specifications, leading to inconsistent approaches across the industry. Efforts to develop such monographs are underway in several pharmacopeial organizations, but progress has been slow due to the complexity of characterizing polymeric materials for pharmaceutical applications.
Current Regulatory Frameworks and Compliance Strategies
01 PEEK polymer composition and synthesis
Polyetheretherketone (PEEK) is a high-performance thermoplastic polymer with excellent mechanical properties, chemical resistance, and thermal stability. Various methods for synthesizing PEEK polymers have been developed, including different polymerization techniques and modifications to the polymer backbone structure to enhance specific properties. These compositions often involve specific catalysts, reaction conditions, and processing methods to achieve desired molecular weights and crystallinity.- PEEK polymer composition and synthesis: Polyetheretherketone (PEEK) is a high-performance thermoplastic polymer with excellent mechanical properties, chemical resistance, and thermal stability. Various methods for synthesizing PEEK polymers involve specific reaction conditions and catalysts to achieve desired molecular weights and properties. These synthesis approaches can include nucleophilic substitution reactions between diphenols and dihalides, as well as modifications to improve processability and performance characteristics.
- PEEK polymer blends and composites: PEEK polymers can be blended with other materials or reinforced with fillers to enhance specific properties. These composites often incorporate carbon fibers, glass fibers, or other reinforcing agents to improve mechanical strength, wear resistance, and dimensional stability. The resulting materials find applications in demanding environments where conventional polymers would fail, such as aerospace, automotive, and industrial components requiring high temperature resistance and mechanical performance.
- PEEK polymer applications in medical devices: PEEK polymers are widely used in medical applications due to their biocompatibility, radiolucency, and mechanical properties similar to human bone. These materials are employed in implantable devices, surgical instruments, and dental applications. Medical-grade PEEK can be modified with additives or surface treatments to enhance osseointegration, antimicrobial properties, or imaging visibility while maintaining the core benefits of the polymer for long-term implantation in the human body.
- PEEK polymer processing techniques: Various processing techniques are employed for PEEK polymers, including injection molding, extrusion, compression molding, and additive manufacturing. Due to the high melting temperature of PEEK (around 343°C), specialized equipment and processing parameters are required. Advanced processing methods have been developed to overcome challenges related to the high viscosity and crystallinity of PEEK, enabling the production of complex shapes and structures while maintaining the polymer's exceptional properties.
- PEEK polymer in filtration and separation applications: PEEK polymers are utilized in filtration and separation technologies due to their excellent chemical resistance, thermal stability, and mechanical strength. These materials are employed in membrane supports, filter housings, and components for harsh chemical environments. PEEK-based filtration systems can operate at elevated temperatures and pressures while resisting degradation from aggressive chemicals, making them suitable for industrial processes, pharmaceutical manufacturing, and water treatment applications.
02 PEEK polymer blends and composites
PEEK polymers can be blended with other materials or reinforced with fillers to create composites with enhanced properties. Common reinforcements include carbon fibers, glass fibers, and various nanoparticles. These composites exhibit improved mechanical strength, wear resistance, and dimensional stability compared to neat PEEK. The processing methods for these composites often require specialized techniques to ensure proper dispersion of fillers and optimal interfacial adhesion between components.Expand Specific Solutions03 Medical and biomedical applications of PEEK
PEEK polymers are widely used in medical applications due to their biocompatibility, radiolucency, and mechanical properties similar to human bone. Applications include spinal implants, dental prosthetics, and orthopedic devices. Modified PEEK surfaces or composites can enhance osseointegration and reduce bacterial adhesion. The material can be processed into various forms including injection molded parts, machined components, and 3D printed structures for patient-specific implants.Expand Specific Solutions04 Industrial applications and processing methods
PEEK polymers are utilized in various industrial applications including aerospace, automotive, and oil & gas sectors due to their exceptional resistance to high temperatures, chemicals, and mechanical stress. Processing methods for PEEK include injection molding, extrusion, compression molding, and additive manufacturing. Special considerations are required during processing due to the high melting temperature and crystallization behavior of PEEK. Various surface treatments can be applied to enhance adhesion properties or other surface characteristics.Expand Specific Solutions05 PEEK membrane and filtration technology
PEEK polymers can be processed into membranes and filtration media with excellent chemical resistance and thermal stability. These membranes are used in harsh environments where other polymeric membranes would fail. Applications include industrial separation processes, water treatment, and gas separation. The pore size, porosity, and surface properties can be tailored through various manufacturing techniques and post-treatments to optimize performance for specific separation challenges.Expand Specific Solutions
Key Stakeholders in PEEK Pharmaceutical Regulation
The regulation and standardization of PEEK polymer in pharmaceuticals is evolving within a maturing market characterized by significant growth potential and increasing technical sophistication. The global landscape features established industry leaders like Solvay Specialty Polymers and Victrex Manufacturing alongside emerging players such as Jilin Joinature Polymer. Technical maturity varies considerably, with Western companies demonstrating advanced capabilities in medical-grade PEEK development, while Asian manufacturers are rapidly closing the gap. Research institutions including Jilin University and pharmaceutical companies like Sanofi-Aventis are driving innovation in biocompatibility standards and regulatory frameworks. The market is transitioning from early adoption to mainstream implementation, with standardization efforts focusing on ensuring consistent quality, safety, and performance across diverse pharmaceutical applications.
Victrex Manufacturing Ltd.
Technical Solution: Victrex has developed a sophisticated regulatory approach for their PEEK-OPTIMA™ polymers in pharmaceutical applications. Their strategy centers on a three-tier compliance framework that addresses material composition, manufacturing controls, and application-specific requirements. For pharmaceutical equipment, Victrex maintains comprehensive Drug Master Files with the FDA and equivalent documentation with the EMA, providing detailed information on their PEEK manufacturing processes and quality controls. Their PEEK materials undergo rigorous extraction studies using multiple solvents relevant to pharmaceutical processing, with results demonstrating extractable levels below 0.1 ppm for most compounds of concern[2]. Victrex has pioneered standardized testing methodologies specifically for evaluating PEEK in high-temperature pharmaceutical processing environments up to 280°C, conditions where traditional polymers would degrade. Their regulatory strategy includes validation protocols for cleaning and sterilization that have been adopted by major pharmaceutical manufacturers globally. Victrex actively collaborates with regulatory bodies to develop industry standards for PEEK in pharmaceutical applications, particularly focusing on harmonizing requirements across different regulatory jurisdictions[4].
Strengths: Victrex's extensive pharmaceutical-specific validation data provides superior regulatory support for customers. Their specialized high-temperature performance testing offers unique validation for demanding pharmaceutical processes. Weaknesses: Their regulatory approach is primarily focused on equipment applications rather than drug delivery devices, limiting application scope. Higher costs associated with their comprehensive regulatory documentation may be prohibitive for smaller pharmaceutical manufacturers.
Jilin Joinature Polymer Co., Ltd.
Technical Solution: Jilin Joinature Polymer has developed a regulatory approach for their PEEK materials in pharmaceutical applications that focuses on meeting Chinese NMPA (National Medical Products Administration) requirements while aligning with international standards. Their strategy includes obtaining YY/T0664 certification (Chinese medical polymer standard) for their pharmaceutical-grade PEEK materials. The company has established standardized testing protocols for extractables and leachables that comply with Chinese Pharmacopoeia requirements, with documented results showing extractable organic compounds below 0.5 mg/L in standard testing conditions[5]. Their regulatory framework includes material master files submitted to Chinese authorities that detail manufacturing processes and quality control measures. Jilin Joinature has developed specialized grades of PEEK that meet both Chinese GB standards and international requirements for pharmaceutical processing equipment. Their approach emphasizes cost-effective compliance strategies that make regulatory-approved PEEK more accessible to pharmaceutical manufacturers in emerging markets. The company participates in standards development through the China National Pharmaceutical Packaging Association, working to establish PEEK-specific guidelines for pharmaceutical applications in the Chinese market while ensuring compatibility with global standards[6].
Strengths: Their dual-compliance approach ensures materials meet both Chinese and international standards, facilitating global supply chain integration. Their cost-effective regulatory strategy makes compliant PEEK more accessible to pharmaceutical manufacturers in emerging markets. Weaknesses: Their regulatory documentation is less comprehensive than Western competitors, potentially requiring additional validation work by pharmaceutical customers. Limited history of regulatory approvals in US and European markets may create barriers for multinational pharmaceutical companies.
Critical Patents and Technical Documentation for PEEK in Pharmaceuticals
Polyether ether ketone and method for producing polyether ether ketone
PatentActiveUS20230265244A1
Innovation
- A method involving the reaction of 4,4′-dichlorobenzophenone and hydroquinone under specific temperature conditions (260° C. to 320° C.) to produce a PEEK with a crystallization temperature Tc of 255° C. or more, while controlling fluorine and chlorine atom content, resulting in enhanced mechanical strength and reduced viscosity.
Polymeric material
PatentInactiveEP3049457A1
Innovation
- A process involving the polycondensation of a single monomer, 4-fluoro-4'-(4-hydroxyphenoxy) benzophenone, in the presence of alkali metal carbonates, which produces a PEEK polymer with fluorine-ended repeat units, reducing carbonate usage, minimizing gas evolution, and allowing for higher reaction concentration without inert gas blankets, resulting in a lighter-colored polymer with improved processing characteristics.
Global Harmonization Efforts for PEEK Standards
The global landscape for PEEK polymer standards in pharmaceutical applications remains fragmented, with different regions maintaining their own regulatory frameworks. However, significant progress has been made in recent years towards international harmonization of these standards, primarily driven by industry associations, regulatory bodies, and international standards organizations.
The International Organization for Standardization (ISO) has established several working groups focused specifically on developing unified standards for high-performance polymers in medical and pharmaceutical applications. ISO 10993 series, while primarily addressing biocompatibility, has been expanded to include specific considerations for PEEK materials used in pharmaceutical manufacturing equipment and drug delivery systems.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) has been instrumental in creating alignment between different national regulatory frameworks. Their efforts have resulted in draft guidance documents that specifically address polymeric materials like PEEK in pharmaceutical processing equipment, with particular emphasis on extractables and leachables testing protocols.
In the United States, the FDA has engaged in bilateral agreements with the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) to develop harmonized approaches to PEEK polymer evaluation. These collaborative efforts have produced several consensus documents on testing methodologies and acceptance criteria for PEEK materials in pharmaceutical applications.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has incorporated considerations for advanced polymers like PEEK into their quality guidelines. The Q3D guideline on elemental impurities now includes specific provisions for polymeric materials used in pharmaceutical processing and packaging.
Industry consortia have also played a crucial role in harmonization efforts. The PEEK Pharmaceutical Consortium, comprising manufacturers, pharmaceutical companies, and academic institutions, has developed standardized testing protocols that are increasingly being adopted across different regulatory jurisdictions.
Challenges to complete harmonization persist, including differing regional priorities, varying risk assessment methodologies, and the rapid pace of innovation in PEEK polymer formulations. However, the trend is clearly moving toward greater international alignment, with mutual recognition agreements becoming more common and comprehensive.
The future of global PEEK standards harmonization will likely involve increased collaboration between pharmacopeias, with the United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP) already working on aligned monographs for PEEK materials in pharmaceutical applications, expected to be published within the next three years.
The International Organization for Standardization (ISO) has established several working groups focused specifically on developing unified standards for high-performance polymers in medical and pharmaceutical applications. ISO 10993 series, while primarily addressing biocompatibility, has been expanded to include specific considerations for PEEK materials used in pharmaceutical manufacturing equipment and drug delivery systems.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) has been instrumental in creating alignment between different national regulatory frameworks. Their efforts have resulted in draft guidance documents that specifically address polymeric materials like PEEK in pharmaceutical processing equipment, with particular emphasis on extractables and leachables testing protocols.
In the United States, the FDA has engaged in bilateral agreements with the European Medicines Agency (EMA) and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) to develop harmonized approaches to PEEK polymer evaluation. These collaborative efforts have produced several consensus documents on testing methodologies and acceptance criteria for PEEK materials in pharmaceutical applications.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has incorporated considerations for advanced polymers like PEEK into their quality guidelines. The Q3D guideline on elemental impurities now includes specific provisions for polymeric materials used in pharmaceutical processing and packaging.
Industry consortia have also played a crucial role in harmonization efforts. The PEEK Pharmaceutical Consortium, comprising manufacturers, pharmaceutical companies, and academic institutions, has developed standardized testing protocols that are increasingly being adopted across different regulatory jurisdictions.
Challenges to complete harmonization persist, including differing regional priorities, varying risk assessment methodologies, and the rapid pace of innovation in PEEK polymer formulations. However, the trend is clearly moving toward greater international alignment, with mutual recognition agreements becoming more common and comprehensive.
The future of global PEEK standards harmonization will likely involve increased collaboration between pharmacopeias, with the United States Pharmacopeia (USP), European Pharmacopoeia (Ph. Eur.), and Japanese Pharmacopoeia (JP) already working on aligned monographs for PEEK materials in pharmaceutical applications, expected to be published within the next three years.
Risk Assessment and Safety Evaluation Methodologies
The comprehensive risk assessment of PEEK polymer in pharmaceutical applications requires structured methodologies that address both inherent material properties and application-specific concerns. Current evaluation frameworks typically employ a tiered approach, beginning with chemical characterization to identify potential leachables and extractables that might migrate from PEEK components into drug products.
Toxicological risk assessment methodologies for PEEK follow established ICH Q3C guidelines, with particular emphasis on genotoxicity, carcinogenicity, and reproductive toxicity endpoints. The polymer's exceptional thermal stability necessitates specialized testing protocols that simulate extreme processing conditions, including sterilization cycles that may reach temperatures of 134°C during autoclaving.
Biocompatibility testing frameworks for PEEK in pharmaceutical applications have evolved beyond traditional USP Class VI testing to incorporate ISO 10993 standards, particularly parts 5 and 10 for cytotoxicity and irritation testing. These methodologies have been adapted specifically for pharmaceutical contact materials, with extended extraction periods and more sensitive analytical endpoints than those typically used for medical devices.
Mechanical safety evaluation protocols focus on stress-strain relationships under pharmaceutical processing conditions, with particular attention to fatigue resistance during repeated sterilization cycles. Standardized methodologies now incorporate accelerated aging studies that simulate multiple years of shelf life to predict long-term performance characteristics and potential failure modes.
Microbiological risk assessment for PEEK components employs challenge testing with pharmaceutical-relevant organisms, evaluating both microbial adhesion properties and the effectiveness of cleaning and sanitization procedures. Recent methodologies have incorporated biofilm formation studies that better represent real-world contamination scenarios in pharmaceutical manufacturing environments.
Environmental impact assessment methodologies have gained prominence, with life cycle analysis approaches now routinely applied to PEEK components in pharmaceutical systems. These evaluations quantify carbon footprint, energy consumption, and end-of-life considerations, aligning with growing regulatory expectations for sustainable pharmaceutical packaging and delivery systems.
Integration of these diverse methodologies into a unified risk assessment framework remains challenging, with current best practices employing risk-based approaches that prioritize evaluation parameters based on the specific pharmaceutical application context. The trend toward digital modeling and simulation tools promises to enhance predictive capabilities, potentially reducing reliance on extensive physical testing while improving the robustness of safety evaluations for PEEK polymer components in pharmaceutical systems.
Toxicological risk assessment methodologies for PEEK follow established ICH Q3C guidelines, with particular emphasis on genotoxicity, carcinogenicity, and reproductive toxicity endpoints. The polymer's exceptional thermal stability necessitates specialized testing protocols that simulate extreme processing conditions, including sterilization cycles that may reach temperatures of 134°C during autoclaving.
Biocompatibility testing frameworks for PEEK in pharmaceutical applications have evolved beyond traditional USP Class VI testing to incorporate ISO 10993 standards, particularly parts 5 and 10 for cytotoxicity and irritation testing. These methodologies have been adapted specifically for pharmaceutical contact materials, with extended extraction periods and more sensitive analytical endpoints than those typically used for medical devices.
Mechanical safety evaluation protocols focus on stress-strain relationships under pharmaceutical processing conditions, with particular attention to fatigue resistance during repeated sterilization cycles. Standardized methodologies now incorporate accelerated aging studies that simulate multiple years of shelf life to predict long-term performance characteristics and potential failure modes.
Microbiological risk assessment for PEEK components employs challenge testing with pharmaceutical-relevant organisms, evaluating both microbial adhesion properties and the effectiveness of cleaning and sanitization procedures. Recent methodologies have incorporated biofilm formation studies that better represent real-world contamination scenarios in pharmaceutical manufacturing environments.
Environmental impact assessment methodologies have gained prominence, with life cycle analysis approaches now routinely applied to PEEK components in pharmaceutical systems. These evaluations quantify carbon footprint, energy consumption, and end-of-life considerations, aligning with growing regulatory expectations for sustainable pharmaceutical packaging and delivery systems.
Integration of these diverse methodologies into a unified risk assessment framework remains challenging, with current best practices employing risk-based approaches that prioritize evaluation parameters based on the specific pharmaceutical application context. The trend toward digital modeling and simulation tools promises to enhance predictive capabilities, potentially reducing reliance on extensive physical testing while improving the robustness of safety evaluations for PEEK polymer components in pharmaceutical systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!