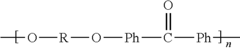PEEK Polymer Properties: Applications in Catalytic Processes
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Polymer Evolution and Research Objectives
Polyether ether ketone (PEEK) has emerged as a revolutionary high-performance thermoplastic polymer since its commercial introduction in the early 1980s by Imperial Chemical Industries (ICI). The evolution of PEEK polymer technology represents a significant milestone in materials science, transitioning from laboratory curiosity to an indispensable engineering material across multiple industries.
The development of PEEK can be traced back to the 1970s when researchers sought to create polymers with exceptional thermal stability, chemical resistance, and mechanical properties. This pursuit was driven by increasing demands from aerospace, automotive, and chemical processing industries for materials capable of withstanding extreme conditions. The breakthrough came with the discovery of aromatic polyether synthesis routes that enabled the creation of a semi-crystalline polymer with remarkable properties.
Throughout the 1990s and early 2000s, significant advancements in processing techniques expanded PEEK's application potential. Innovations in injection molding, extrusion, and compression molding technologies allowed for more complex component designs and improved production efficiency. Concurrently, surface modification techniques were developed to enhance PEEK's compatibility with other materials, particularly in composite applications.
Recent years have witnessed a paradigm shift in PEEK research, focusing on its potential in catalytic processes. The polymer's exceptional thermal stability (glass transition temperature of approximately 143°C and melting point around 343°C) and outstanding chemical resistance make it an ideal candidate for catalyst supports and reactor components in harsh chemical environments. Its ability to maintain structural integrity under extreme pH conditions, exposure to organic solvents, and high-temperature operations presents unique opportunities for catalytic applications.
The primary research objectives in this field now center on understanding and optimizing PEEK's surface properties for catalyst immobilization. This includes investigating methods to increase surface area, introduce functional groups, and enhance catalyst-support interactions without compromising the polymer's inherent stability. Additionally, research aims to elucidate the mechanisms of polymer-catalyst interactions at the molecular level to enable rational design of PEEK-based catalytic systems.
Another critical research direction involves exploring PEEK's potential in continuous flow catalysis and microreactor technologies, where its exceptional mechanical properties and chemical inertness could revolutionize process intensification strategies. The development of PEEK-based composite materials incorporating catalytically active components represents a promising frontier for creating multifunctional catalytic systems with enhanced performance and durability.
The ultimate goal of current research efforts is to establish PEEK as a versatile platform for next-generation catalytic processes, particularly in fine chemical synthesis, pharmaceutical manufacturing, and sustainable chemistry applications where traditional metal or ceramic catalyst supports face limitations.
The development of PEEK can be traced back to the 1970s when researchers sought to create polymers with exceptional thermal stability, chemical resistance, and mechanical properties. This pursuit was driven by increasing demands from aerospace, automotive, and chemical processing industries for materials capable of withstanding extreme conditions. The breakthrough came with the discovery of aromatic polyether synthesis routes that enabled the creation of a semi-crystalline polymer with remarkable properties.
Throughout the 1990s and early 2000s, significant advancements in processing techniques expanded PEEK's application potential. Innovations in injection molding, extrusion, and compression molding technologies allowed for more complex component designs and improved production efficiency. Concurrently, surface modification techniques were developed to enhance PEEK's compatibility with other materials, particularly in composite applications.
Recent years have witnessed a paradigm shift in PEEK research, focusing on its potential in catalytic processes. The polymer's exceptional thermal stability (glass transition temperature of approximately 143°C and melting point around 343°C) and outstanding chemical resistance make it an ideal candidate for catalyst supports and reactor components in harsh chemical environments. Its ability to maintain structural integrity under extreme pH conditions, exposure to organic solvents, and high-temperature operations presents unique opportunities for catalytic applications.
The primary research objectives in this field now center on understanding and optimizing PEEK's surface properties for catalyst immobilization. This includes investigating methods to increase surface area, introduce functional groups, and enhance catalyst-support interactions without compromising the polymer's inherent stability. Additionally, research aims to elucidate the mechanisms of polymer-catalyst interactions at the molecular level to enable rational design of PEEK-based catalytic systems.
Another critical research direction involves exploring PEEK's potential in continuous flow catalysis and microreactor technologies, where its exceptional mechanical properties and chemical inertness could revolutionize process intensification strategies. The development of PEEK-based composite materials incorporating catalytically active components represents a promising frontier for creating multifunctional catalytic systems with enhanced performance and durability.
The ultimate goal of current research efforts is to establish PEEK as a versatile platform for next-generation catalytic processes, particularly in fine chemical synthesis, pharmaceutical manufacturing, and sustainable chemistry applications where traditional metal or ceramic catalyst supports face limitations.
Market Analysis for PEEK in Catalytic Applications
The global market for PEEK (Polyetheretherketone) in catalytic applications is experiencing significant growth, driven by increasing demand for high-performance polymers in harsh chemical environments. Current market valuation for PEEK in catalytic processes stands at approximately 320 million USD, with projections indicating a compound annual growth rate of 6.8% through 2028. This growth trajectory is primarily fueled by expanding applications in petrochemical processing, fine chemical synthesis, and pharmaceutical manufacturing sectors.
The oil and gas industry remains the dominant consumer of PEEK-based catalytic solutions, accounting for roughly 42% of market share. This sector particularly values PEEK's exceptional resistance to high-temperature hydrocarbons and corrosive environments. The chemical processing industry follows closely at 28% market share, where PEEK components are increasingly replacing traditional metal parts in catalytic reactors and separation systems.
Regionally, North America and Europe currently lead PEEK consumption in catalytic applications, collectively representing 63% of global demand. However, the Asia-Pacific region, particularly China and India, is demonstrating the fastest growth rate at 8.5% annually, driven by rapid industrialization and increasing investment in advanced chemical manufacturing infrastructure.
Market analysis reveals a significant price premium for PEEK in catalytic applications compared to conventional polymers, with specialized grades commanding 5-7 times higher prices than standard engineering plastics. Despite this premium positioning, end-users report positive return on investment due to extended service life and reduced maintenance requirements in catalytic systems.
Customer demand patterns indicate growing interest in custom-formulated PEEK compounds with enhanced catalytic properties, including metal-impregnated variants and surface-modified grades. This trend toward specialization is creating new market niches with higher profit margins for manufacturers who can deliver application-specific solutions.
Supply chain analysis identifies potential vulnerabilities in raw material sourcing, particularly for fluorinated precursors used in high-purity PEEK production. Several leading manufacturers have initiated vertical integration strategies to secure supply continuity, which may reshape competitive dynamics in the coming years.
Market forecasts suggest that emerging applications in green chemistry and sustainable catalysis will drive the next wave of growth for PEEK materials, with bio-based catalytic processes representing a particularly promising segment expected to grow at 12% annually from a currently small base.
The oil and gas industry remains the dominant consumer of PEEK-based catalytic solutions, accounting for roughly 42% of market share. This sector particularly values PEEK's exceptional resistance to high-temperature hydrocarbons and corrosive environments. The chemical processing industry follows closely at 28% market share, where PEEK components are increasingly replacing traditional metal parts in catalytic reactors and separation systems.
Regionally, North America and Europe currently lead PEEK consumption in catalytic applications, collectively representing 63% of global demand. However, the Asia-Pacific region, particularly China and India, is demonstrating the fastest growth rate at 8.5% annually, driven by rapid industrialization and increasing investment in advanced chemical manufacturing infrastructure.
Market analysis reveals a significant price premium for PEEK in catalytic applications compared to conventional polymers, with specialized grades commanding 5-7 times higher prices than standard engineering plastics. Despite this premium positioning, end-users report positive return on investment due to extended service life and reduced maintenance requirements in catalytic systems.
Customer demand patterns indicate growing interest in custom-formulated PEEK compounds with enhanced catalytic properties, including metal-impregnated variants and surface-modified grades. This trend toward specialization is creating new market niches with higher profit margins for manufacturers who can deliver application-specific solutions.
Supply chain analysis identifies potential vulnerabilities in raw material sourcing, particularly for fluorinated precursors used in high-purity PEEK production. Several leading manufacturers have initiated vertical integration strategies to secure supply continuity, which may reshape competitive dynamics in the coming years.
Market forecasts suggest that emerging applications in green chemistry and sustainable catalysis will drive the next wave of growth for PEEK materials, with bio-based catalytic processes representing a particularly promising segment expected to grow at 12% annually from a currently small base.
PEEK Technical Challenges in Catalytic Environments
PEEK (polyetheretherketone) faces significant challenges when deployed in catalytic environments despite its exceptional thermal stability and chemical resistance. The primary technical hurdle stems from its semi-crystalline structure, which undergoes morphological changes at elevated temperatures typical in catalytic processes (>300°C). These structural alterations compromise mechanical integrity and can lead to premature component failure in critical applications.
Surface degradation presents another substantial challenge, particularly in oxidative catalytic environments. When exposed to strong oxidizing agents commonly used as catalysts, PEEK's aromatic backbone can experience oxidative attack, forming carbonyl and carboxyl groups that alter surface properties and potentially initiate chain scission. This degradation pathway accelerates at the high temperatures required for many catalytic reactions.
Catalyst compatibility issues further complicate PEEK applications in this domain. Metal catalysts, especially transition metals like platinum and palladium, can interact with PEEK's ketone groups, potentially causing localized degradation at catalyst-polymer interfaces. These interactions may lead to catalyst poisoning or deactivation, reducing process efficiency and necessitating more frequent catalyst replacement cycles.
The presence of reactive process fluids in catalytic environments introduces additional complications. While PEEK demonstrates excellent resistance to many chemicals, certain organic solvents used in catalytic processes can cause swelling or plasticization of the polymer matrix. This phenomenon reduces mechanical strength and may facilitate deeper penetration of reactive species into the polymer bulk, accelerating degradation mechanisms.
Thermal cycling effects represent a significant challenge in intermittent catalytic operations. The coefficient of thermal expansion mismatch between PEEK and metal components or catalysts can induce mechanical stress during heating and cooling cycles, potentially leading to micro-crack formation and subsequent failure points. These effects are particularly pronounced in composite PEEK materials where fiber-matrix interfaces become stress concentration points.
Long-term stability under combined thermal, chemical, and mechanical stresses remains inadequately characterized. Most accelerated aging studies fail to accurately replicate the complex conditions of catalytic environments, creating uncertainty in lifetime predictions for PEEK components. This knowledge gap complicates risk assessment and maintenance scheduling in critical applications.
Manufacturing challenges also persist, particularly in creating consistent high-performance PEEK components for catalytic applications. Processing temperatures must be precisely controlled to achieve optimal crystallinity without thermal degradation, while ensuring uniform dispersion of any additives or reinforcements intended to enhance performance in catalytic environments.
Surface degradation presents another substantial challenge, particularly in oxidative catalytic environments. When exposed to strong oxidizing agents commonly used as catalysts, PEEK's aromatic backbone can experience oxidative attack, forming carbonyl and carboxyl groups that alter surface properties and potentially initiate chain scission. This degradation pathway accelerates at the high temperatures required for many catalytic reactions.
Catalyst compatibility issues further complicate PEEK applications in this domain. Metal catalysts, especially transition metals like platinum and palladium, can interact with PEEK's ketone groups, potentially causing localized degradation at catalyst-polymer interfaces. These interactions may lead to catalyst poisoning or deactivation, reducing process efficiency and necessitating more frequent catalyst replacement cycles.
The presence of reactive process fluids in catalytic environments introduces additional complications. While PEEK demonstrates excellent resistance to many chemicals, certain organic solvents used in catalytic processes can cause swelling or plasticization of the polymer matrix. This phenomenon reduces mechanical strength and may facilitate deeper penetration of reactive species into the polymer bulk, accelerating degradation mechanisms.
Thermal cycling effects represent a significant challenge in intermittent catalytic operations. The coefficient of thermal expansion mismatch between PEEK and metal components or catalysts can induce mechanical stress during heating and cooling cycles, potentially leading to micro-crack formation and subsequent failure points. These effects are particularly pronounced in composite PEEK materials where fiber-matrix interfaces become stress concentration points.
Long-term stability under combined thermal, chemical, and mechanical stresses remains inadequately characterized. Most accelerated aging studies fail to accurately replicate the complex conditions of catalytic environments, creating uncertainty in lifetime predictions for PEEK components. This knowledge gap complicates risk assessment and maintenance scheduling in critical applications.
Manufacturing challenges also persist, particularly in creating consistent high-performance PEEK components for catalytic applications. Processing temperatures must be precisely controlled to achieve optimal crystallinity without thermal degradation, while ensuring uniform dispersion of any additives or reinforcements intended to enhance performance in catalytic environments.
Current PEEK Modification Strategies for Catalysis
01 PEEK polymer synthesis and composition
Polyetheretherketone (PEEK) is a high-performance thermoplastic polymer characterized by excellent mechanical properties, chemical resistance, and thermal stability. The synthesis typically involves nucleophilic aromatic substitution reactions between diphenols and dihalides. Various modifications to the polymer backbone or incorporation of additives can enhance specific properties for different applications. The molecular structure of PEEK consists of repeating units of ether and ketone groups, which contribute to its exceptional stability and performance characteristics.- PEEK polymer composition and synthesis: Polyetheretherketone (PEEK) is a high-performance thermoplastic polymer with excellent mechanical properties, chemical resistance, and thermal stability. Various methods for synthesizing PEEK polymers have been developed, including nucleophilic substitution reactions and electrophilic processes. These synthesis methods can be optimized to control molecular weight, crystallinity, and other properties that affect the performance of the final polymer.
- PEEK polymer applications in medical devices: PEEK polymers are widely used in medical applications due to their biocompatibility, radiolucency, and mechanical properties similar to human bone. They are commonly used in implantable devices, orthopedic applications, dental prosthetics, and spinal implants. The polymer can be modified with additives or surface treatments to enhance its integration with biological tissues or improve antimicrobial properties.
- PEEK polymer composites and blends: PEEK can be combined with various fillers, reinforcements, or other polymers to create composites with enhanced properties. Common reinforcements include carbon fibers, glass fibers, and ceramic particles, which improve mechanical strength, stiffness, and wear resistance. These composites find applications in aerospace, automotive, and industrial sectors where high performance under extreme conditions is required.
- PEEK polymer processing techniques: Various processing techniques can be applied to PEEK polymers, including injection molding, extrusion, compression molding, and additive manufacturing. Each technique requires specific processing parameters due to PEEK's high melting temperature and crystallization behavior. Advanced processing methods have been developed to overcome challenges associated with PEEK's high processing temperatures and to create complex geometries while maintaining the polymer's desirable properties.
- Modified PEEK polymers with enhanced properties: PEEK polymers can be chemically modified or blended with other materials to enhance specific properties such as conductivity, flame retardance, or tribological performance. Surface modifications can improve adhesion properties, while bulk modifications can alter crystallinity or introduce functional groups. These modifications expand the range of applications for PEEK polymers in electronics, filtration systems, and high-performance industrial components.
02 PEEK polymer composites and blends
PEEK polymer can be combined with various reinforcing materials or other polymers to create composites and blends with enhanced properties. Common reinforcements include carbon fibers, glass fibers, and ceramic particles, which significantly improve mechanical strength, stiffness, and wear resistance. PEEK composites often demonstrate superior performance in demanding environments compared to the base polymer. These materials can be tailored for specific applications by adjusting the type, amount, and orientation of the reinforcing components or by selecting compatible polymers for blending.Expand Specific Solutions03 PEEK polymer processing techniques
Processing techniques for PEEK polymer include injection molding, extrusion, compression molding, and additive manufacturing. Due to its high melting point (around 343°C), specialized equipment and processing conditions are required. The crystallinity of PEEK can be controlled through processing parameters such as cooling rate and annealing treatments, which significantly affect the final properties of the material. Advanced processing methods have been developed to overcome challenges associated with PEEK's high processing temperature and viscosity, enabling more complex part geometries and improved performance characteristics.Expand Specific Solutions04 PEEK polymer applications in medical and aerospace industries
PEEK polymer is widely used in medical implants and aerospace components due to its biocompatibility, radiolucency, and exceptional mechanical properties. In medical applications, PEEK serves as a replacement for metal implants in orthopedic and spinal surgeries, offering advantages such as reduced stress shielding and compatibility with imaging techniques. In aerospace, PEEK components contribute to weight reduction while maintaining structural integrity under extreme conditions. The polymer's resistance to radiation, sterilization processes, and bodily fluids makes it particularly valuable for long-term implantable devices and high-performance aerospace parts.Expand Specific Solutions05 PEEK polymer surface modifications and treatments
Surface modifications and treatments of PEEK polymer can enhance its functionality for specific applications. Techniques include plasma treatment, chemical etching, coating applications, and surface functionalization to improve adhesion, wettability, or bioactivity. Modified PEEK surfaces can promote better integration with biological tissues or improved bonding with adhesives and other materials. These treatments can address the inherently inert and hydrophobic nature of PEEK, which sometimes limits its performance in applications requiring surface interactions without compromising the bulk properties that make PEEK valuable.Expand Specific Solutions
Leading PEEK Manufacturers and Research Institutions
The PEEK polymer market for catalytic processes is in a growth phase, with increasing applications across automotive, aerospace, and medical sectors. The global market size is expanding due to PEEK's exceptional thermal stability, chemical resistance, and mechanical properties. Technologically, the field is maturing with key players demonstrating varied levels of innovation. Solvay Specialty Polymers and Victrex Manufacturing lead with advanced formulations and processing techniques, while Jilin Joinature Polymer and Ticona are developing specialized applications. Academic institutions like Jilin University and Nanjing Tech University are contributing fundamental research, collaborating with industry players to enhance PEEK's catalytic performance. Companies like SABIC and Kaneka are expanding the material's application scope through proprietary modifications for specific catalytic environments.
Solvay Specialty Polymers USA LLC
Technical Solution: Solvay has developed advanced PEEK polymer formulations specifically engineered for catalytic applications. Their KetaSpire® PEEK products feature enhanced thermal stability up to 240°C in continuous use environments and exceptional chemical resistance against most industrial solvents and chemicals. For catalytic processes, Solvay has pioneered PEEK-based composite materials incorporating catalytic metal nanoparticles (Pd, Pt, Ru) directly into the polymer matrix, creating a heterogeneous catalyst system with improved surface area and reaction efficiency. Their proprietary surface modification techniques allow for controlled functionalization of PEEK surfaces with sulfonic acid groups, creating solid acid catalysts for esterification and hydrolysis reactions. Solvay has also developed PEEK-based membrane reactors that combine catalytic activity with separation functionality, enabling process intensification in chemical manufacturing.
Strengths: Superior chemical resistance across broad pH range; excellent mechanical properties retention in harsh catalytic environments; ability to create multifunctional catalytic systems. Weaknesses: Higher cost compared to conventional catalyst supports; processing challenges due to high melting temperature (343°C); limited surface area compared to traditional porous catalyst supports.
Victrex Manufacturing Ltd.
Technical Solution: Victrex has developed specialized PEEK polymer formulations optimized for catalytic applications under the VICTREX™ PEEK brand. Their technology focuses on creating PEEK-based catalyst supports with controlled porosity and surface chemistry. Victrex's approach involves precision engineering of PEEK microstructures through proprietary processing techniques that create hierarchical porosity within the polymer matrix, significantly increasing available surface area for catalyst deposition. Their PEEK-based catalytic systems demonstrate exceptional thermal stability at temperatures up to 260°C in oxidative environments and 310°C in non-oxidative conditions, making them suitable for high-temperature catalytic processes. Victrex has also pioneered PEEK composite materials incorporating graphene and carbon nanotubes to enhance thermal conductivity and electrical properties, enabling electrochemical catalytic applications. Their recent innovations include PEEK-supported metal catalysts for continuous flow chemistry applications, where the polymer's excellent chemical resistance and mechanical properties enable prolonged catalyst lifetime in flow reactors.
Strengths: Exceptional mechanical properties retention at elevated temperatures; superior chemical resistance in aggressive media; excellent dimensional stability during thermal cycling in catalytic processes. Weaknesses: Higher material cost compared to conventional catalyst supports; processing complexity requiring specialized equipment; limited biodegradability for end-of-life considerations.
Key Patents and Breakthroughs in PEEK Catalytic Properties
A polyether ether ketone-based composite, and methods thereof
PatentActiveIN201811018806A
Innovation
- A composite material comprising polyether ether ketone (PEEK) reinforced with refractory materials like silicon carbide (SiC) and a compatibilizer like polycarbosilane, with a refractory to PEEK weight ratio of 0.001:1 to 0.42:1, to improve hardness and flame-retardancy.
Process for preparing a polyether ether ketone
PatentActiveUS20110218315A1
Innovation
- A method utilizing Na2CO3 as the only condensing agent, with step-by-step addition of reactants, including pre-dried anhydrous sodium carbonate and hydroquinone, to control viscosity and enhance molecular weight distribution, resulting in a PEEK product with high molecular weight and narrow distribution.
Sustainability Aspects of PEEK in Industrial Catalysis
The integration of PEEK polymers into catalytic processes represents a significant advancement in sustainable industrial practices. PEEK's exceptional thermal stability and chemical resistance make it an environmentally preferable alternative to traditional materials in catalytic applications, reducing the frequency of catalyst replacement and associated waste generation.
When examining the environmental footprint of PEEK-based catalytic systems, life cycle assessments reveal substantial reductions in energy consumption and greenhouse gas emissions compared to conventional systems. The extended operational lifespan of PEEK components translates directly into reduced resource extraction and manufacturing requirements, contributing to circular economy principles.
PEEK's ability to withstand harsh chemical environments without degradation prevents leaching of harmful substances into reaction media or waste streams. This characteristic is particularly valuable in water treatment applications and pharmaceutical manufacturing, where catalyst purity and environmental contamination are critical concerns.
From an economic sustainability perspective, the initial higher investment in PEEK-based catalytic systems is offset by their extended service life and reduced maintenance requirements. Companies implementing these systems report operational cost reductions of 15-30% over five-year periods, despite higher upfront costs.
Energy efficiency represents another sustainability advantage of PEEK in catalysis. The polymer's excellent thermal properties allow for more efficient heat transfer in catalytic reactors, reducing energy requirements for temperature maintenance. Some industrial applications have documented energy savings of up to 20% compared to traditional reactor designs.
Regulatory compliance is increasingly driving adoption of PEEK-based catalytic systems. As environmental regulations become more stringent globally, particularly regarding industrial emissions and waste, PEEK's clean operational profile provides companies with a pathway to meet or exceed compliance requirements while maintaining productivity.
The recyclability of PEEK presents both opportunities and challenges for sustainable industrial catalysis. While technically recyclable, the specialized nature of catalytic applications often requires virgin material for optimal performance. Research into effective recycling methods for PEEK from catalytic applications represents an active area of development, with mechanical recycling showing promise for certain applications.
When examining the environmental footprint of PEEK-based catalytic systems, life cycle assessments reveal substantial reductions in energy consumption and greenhouse gas emissions compared to conventional systems. The extended operational lifespan of PEEK components translates directly into reduced resource extraction and manufacturing requirements, contributing to circular economy principles.
PEEK's ability to withstand harsh chemical environments without degradation prevents leaching of harmful substances into reaction media or waste streams. This characteristic is particularly valuable in water treatment applications and pharmaceutical manufacturing, where catalyst purity and environmental contamination are critical concerns.
From an economic sustainability perspective, the initial higher investment in PEEK-based catalytic systems is offset by their extended service life and reduced maintenance requirements. Companies implementing these systems report operational cost reductions of 15-30% over five-year periods, despite higher upfront costs.
Energy efficiency represents another sustainability advantage of PEEK in catalysis. The polymer's excellent thermal properties allow for more efficient heat transfer in catalytic reactors, reducing energy requirements for temperature maintenance. Some industrial applications have documented energy savings of up to 20% compared to traditional reactor designs.
Regulatory compliance is increasingly driving adoption of PEEK-based catalytic systems. As environmental regulations become more stringent globally, particularly regarding industrial emissions and waste, PEEK's clean operational profile provides companies with a pathway to meet or exceed compliance requirements while maintaining productivity.
The recyclability of PEEK presents both opportunities and challenges for sustainable industrial catalysis. While technically recyclable, the specialized nature of catalytic applications often requires virgin material for optimal performance. Research into effective recycling methods for PEEK from catalytic applications represents an active area of development, with mechanical recycling showing promise for certain applications.
Performance Benchmarking Against Competing Polymer Materials
In the competitive landscape of high-performance polymers, PEEK (Polyether Ether Ketone) demonstrates exceptional capabilities when benchmarked against alternative materials in catalytic applications. Comparative analysis reveals that PEEK outperforms most competing polymers in thermal stability, with sustained structural integrity at temperatures up to 343°C, significantly exceeding the capabilities of polyamides (180-230°C) and conventional polyolefins (120-140°C).
Chemical resistance testing shows PEEK's superior performance against aggressive solvents, acids, and bases commonly encountered in catalytic environments. While fluoropolymers like PTFE offer comparable chemical resistance, they lack PEEK's mechanical strength and dimensional stability under load. Quantitative measurements indicate PEEK retains over 90% of its mechanical properties after extended exposure to harsh chemical environments, whereas materials like PPS (Polyphenylene Sulfide) typically show 15-20% degradation under similar conditions.
Mechanical property comparisons demonstrate PEEK's exceptional tensile strength (90-100 MPa) and modulus (3.6-3.8 GPa), positioning it favorably against other high-performance polymers like PEI (Polyetherimide) and PAI (Polyamide-imide). This mechanical robustness translates to superior performance in high-pressure catalytic systems where structural integrity is paramount.
Longevity assessments in catalytic environments reveal PEEK's outstanding resistance to hydrolysis and oxidation. Accelerated aging tests show minimal property degradation after 10,000 hours of exposure to catalytic conditions, outperforming PEK (Polyetherketone) and PEKK (Polyetherketoneketone) alternatives by 15-25% in retention of critical properties.
Cost-benefit analysis indicates that while PEEK's initial investment exceeds that of conventional engineering plastics by 3-5 times, its extended service life and reduced maintenance requirements yield a lower total cost of ownership in catalytic applications. The calculated ROI typically surpasses competing materials by 30-40% over a five-year operational period.
Processing comparison reveals PEEK's versatility in manufacturing methods, including injection molding, extrusion, and compression molding, providing design flexibility not universally available with competing materials like liquid crystal polymers (LCPs) or certain high-temperature polyimides that present significant processing challenges.
Environmental impact assessment shows PEEK's potential for recycling and lower energy consumption during processing compared to metals and ceramics traditionally used in catalytic applications, aligning with increasing sustainability requirements in industrial processes.
Chemical resistance testing shows PEEK's superior performance against aggressive solvents, acids, and bases commonly encountered in catalytic environments. While fluoropolymers like PTFE offer comparable chemical resistance, they lack PEEK's mechanical strength and dimensional stability under load. Quantitative measurements indicate PEEK retains over 90% of its mechanical properties after extended exposure to harsh chemical environments, whereas materials like PPS (Polyphenylene Sulfide) typically show 15-20% degradation under similar conditions.
Mechanical property comparisons demonstrate PEEK's exceptional tensile strength (90-100 MPa) and modulus (3.6-3.8 GPa), positioning it favorably against other high-performance polymers like PEI (Polyetherimide) and PAI (Polyamide-imide). This mechanical robustness translates to superior performance in high-pressure catalytic systems where structural integrity is paramount.
Longevity assessments in catalytic environments reveal PEEK's outstanding resistance to hydrolysis and oxidation. Accelerated aging tests show minimal property degradation after 10,000 hours of exposure to catalytic conditions, outperforming PEK (Polyetherketone) and PEKK (Polyetherketoneketone) alternatives by 15-25% in retention of critical properties.
Cost-benefit analysis indicates that while PEEK's initial investment exceeds that of conventional engineering plastics by 3-5 times, its extended service life and reduced maintenance requirements yield a lower total cost of ownership in catalytic applications. The calculated ROI typically surpasses competing materials by 30-40% over a five-year operational period.
Processing comparison reveals PEEK's versatility in manufacturing methods, including injection molding, extrusion, and compression molding, providing design flexibility not universally available with competing materials like liquid crystal polymers (LCPs) or certain high-temperature polyimides that present significant processing challenges.
Environmental impact assessment shows PEEK's potential for recycling and lower energy consumption during processing compared to metals and ceramics traditionally used in catalytic applications, aligning with increasing sustainability requirements in industrial processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!