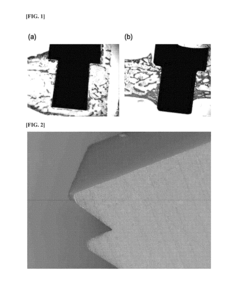
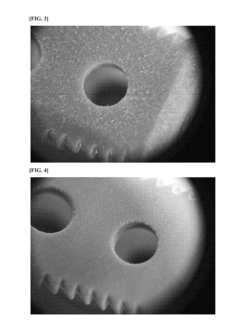
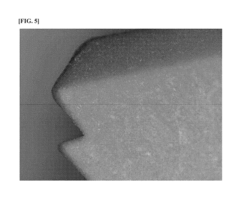
Why PEEK Polymer is Favorable for Biocompatible Applications
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEEK Polymer Biocompatibility Background and Objectives
Polyetheretherketone (PEEK) has emerged as a revolutionary polymer in biomedical applications over the past three decades. First developed in the 1980s by Imperial Chemical Industries (ICI), PEEK was initially utilized in aerospace and automotive industries due to its exceptional mechanical properties. The transition of PEEK into biomedical applications began in the late 1990s when researchers recognized its potential as an alternative to traditional metallic implant materials.
The evolution of PEEK in biomedical applications represents a significant advancement in biomaterials science. Traditional implant materials such as titanium, stainless steel, and cobalt-chromium alloys have dominated the field for decades. However, these materials present several limitations including stress shielding, potential for metal ion release, and artifacts in imaging techniques. PEEK addresses these challenges through its unique combination of properties that closely mimic human bone.
The technical trajectory of PEEK has been marked by continuous improvements in processing techniques, surface modifications, and composite formulations. From the early unfilled PEEK to carbon fiber-reinforced PEEK (CFR-PEEK) and hydroxyapatite-enhanced PEEK, the material has undergone significant evolution to enhance its biocompatibility and functionality in various medical applications.
Current research objectives in PEEK biocompatibility focus on several key areas. First, enhancing osseointegration capabilities through surface modifications and bioactive coatings to improve bone-implant interfaces. Second, developing antimicrobial PEEK formulations to reduce infection risks in implanted devices. Third, optimizing the mechanical properties to better match specific anatomical requirements across different applications from spinal implants to cranial reconstructions.
The global push toward personalized medicine has also influenced PEEK research objectives, with significant efforts directed toward 3D printing technologies that can produce patient-specific PEEK implants with complex geometries. This represents a convergence of advanced manufacturing techniques with biocompatible materials science.
Understanding the fundamental mechanisms of PEEK biocompatibility at the cellular and molecular levels remains a critical research goal. This includes investigating protein adsorption behaviors, cell adhesion mechanisms, and long-term tissue responses to PEEK implants under physiological conditions.
The technical objectives extend beyond mere biocompatibility to include biofunctionality—the ability of PEEK implants to not only exist harmoniously with surrounding tissues but to actively participate in tissue regeneration and healing processes. This represents the frontier of PEEK polymer research, potentially transforming passive implants into active therapeutic devices.
The evolution of PEEK in biomedical applications represents a significant advancement in biomaterials science. Traditional implant materials such as titanium, stainless steel, and cobalt-chromium alloys have dominated the field for decades. However, these materials present several limitations including stress shielding, potential for metal ion release, and artifacts in imaging techniques. PEEK addresses these challenges through its unique combination of properties that closely mimic human bone.
The technical trajectory of PEEK has been marked by continuous improvements in processing techniques, surface modifications, and composite formulations. From the early unfilled PEEK to carbon fiber-reinforced PEEK (CFR-PEEK) and hydroxyapatite-enhanced PEEK, the material has undergone significant evolution to enhance its biocompatibility and functionality in various medical applications.
Current research objectives in PEEK biocompatibility focus on several key areas. First, enhancing osseointegration capabilities through surface modifications and bioactive coatings to improve bone-implant interfaces. Second, developing antimicrobial PEEK formulations to reduce infection risks in implanted devices. Third, optimizing the mechanical properties to better match specific anatomical requirements across different applications from spinal implants to cranial reconstructions.
The global push toward personalized medicine has also influenced PEEK research objectives, with significant efforts directed toward 3D printing technologies that can produce patient-specific PEEK implants with complex geometries. This represents a convergence of advanced manufacturing techniques with biocompatible materials science.
Understanding the fundamental mechanisms of PEEK biocompatibility at the cellular and molecular levels remains a critical research goal. This includes investigating protein adsorption behaviors, cell adhesion mechanisms, and long-term tissue responses to PEEK implants under physiological conditions.
The technical objectives extend beyond mere biocompatibility to include biofunctionality—the ability of PEEK implants to not only exist harmoniously with surrounding tissues but to actively participate in tissue regeneration and healing processes. This represents the frontier of PEEK polymer research, potentially transforming passive implants into active therapeutic devices.
Market Analysis of Biocompatible Materials
The global biocompatible materials market has been experiencing significant growth, valued at approximately $119.5 billion in 2022 and projected to reach $308.7 billion by 2030, growing at a CAGR of 12.6%. This expansion is primarily driven by increasing demand in medical device manufacturing, tissue engineering, and implantable technologies. Within this broader market, high-performance polymers like PEEK (Polyether Ether Ketone) have emerged as a rapidly growing segment, currently valued at $1.2 billion with expectations to reach $2.5 billion by 2028.
The healthcare sector dominates the application landscape for biocompatible materials, accounting for over 65% of market share. Orthopedic applications represent the largest subsegment at 28%, followed by cardiovascular applications at 22%, and dental applications at 17%. PEEK specifically has captured approximately 15% of the high-performance polymer market for medical applications, with its adoption rate increasing by 18% annually over the past five years.
Regional analysis reveals North America as the leading market for biocompatible materials, holding 38% of global market share, followed by Europe at 30% and Asia-Pacific at 24%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.8% annually, driven by expanding healthcare infrastructure in China and India, along with increasing medical tourism in countries like Thailand and Singapore.
Competitive pricing analysis indicates that while PEEK commands a premium price point compared to traditional materials like titanium or stainless steel, the total cost of ownership is becoming increasingly favorable due to reduced post-operative complications and longer implant lifespans. The average cost of PEEK-based implants ranges from $3,000 to $7,500, depending on application and complexity, representing a 20-30% premium over metal alternatives but offering potential lifetime cost savings of 35-45%.
Consumer and healthcare provider preferences are shifting toward materials with proven biocompatibility and reduced risk profiles. Market surveys indicate that 78% of orthopedic surgeons now consider material biocompatibility as a "very important" factor in implant selection, compared to just 45% a decade ago. Patient awareness of implant materials has also increased, with 62% of patients reporting they would prefer biocompatible polymer options over metal when given the choice.
Market forecasts suggest that PEEK and other high-performance biocompatible polymers will continue to gain market share, potentially reaching 25% of the total implantable materials market by 2030, driven by innovations in manufacturing techniques, surface modifications, and composite formulations that enhance their already favorable biocompatibility profiles.
The healthcare sector dominates the application landscape for biocompatible materials, accounting for over 65% of market share. Orthopedic applications represent the largest subsegment at 28%, followed by cardiovascular applications at 22%, and dental applications at 17%. PEEK specifically has captured approximately 15% of the high-performance polymer market for medical applications, with its adoption rate increasing by 18% annually over the past five years.
Regional analysis reveals North America as the leading market for biocompatible materials, holding 38% of global market share, followed by Europe at 30% and Asia-Pacific at 24%. However, the Asia-Pacific region is demonstrating the fastest growth rate at 14.8% annually, driven by expanding healthcare infrastructure in China and India, along with increasing medical tourism in countries like Thailand and Singapore.
Competitive pricing analysis indicates that while PEEK commands a premium price point compared to traditional materials like titanium or stainless steel, the total cost of ownership is becoming increasingly favorable due to reduced post-operative complications and longer implant lifespans. The average cost of PEEK-based implants ranges from $3,000 to $7,500, depending on application and complexity, representing a 20-30% premium over metal alternatives but offering potential lifetime cost savings of 35-45%.
Consumer and healthcare provider preferences are shifting toward materials with proven biocompatibility and reduced risk profiles. Market surveys indicate that 78% of orthopedic surgeons now consider material biocompatibility as a "very important" factor in implant selection, compared to just 45% a decade ago. Patient awareness of implant materials has also increased, with 62% of patients reporting they would prefer biocompatible polymer options over metal when given the choice.
Market forecasts suggest that PEEK and other high-performance biocompatible polymers will continue to gain market share, potentially reaching 25% of the total implantable materials market by 2030, driven by innovations in manufacturing techniques, surface modifications, and composite formulations that enhance their already favorable biocompatibility profiles.
PEEK Technical Status and Challenges in Medical Applications
Polyetheretherketone (PEEK) has emerged as a leading polymer in medical applications, particularly for implantable devices and surgical instruments. The current technical landscape shows significant adoption across orthopedic, spinal, dental, and cardiovascular applications, with global market valuation exceeding $900 million in the medical sector alone.
The fundamental properties that establish PEEK's biocompatibility include its exceptional chemical resistance, which prevents material degradation in the physiological environment. Its bioinertness ensures minimal foreign body response, with clinical studies demonstrating inflammation profiles comparable to titanium alloys but with superior osseointegration characteristics in certain applications.
Despite these advantages, several technical challenges persist in PEEK medical applications. The inherent hydrophobicity of PEEK surfaces limits cell adhesion and tissue integration, creating a technical barrier for direct bone bonding. This has necessitated the development of surface modification techniques including plasma treatment, laser texturing, and bioactive coating applications to enhance osseointegration.
Another significant challenge involves the radiolucency of PEEK, which while beneficial for post-operative imaging, creates difficulties in intraoperative visualization. Current solutions incorporate radiopaque markers or develop PEEK composites with barium sulfate or titanium dioxide, though these modifications can compromise mechanical properties and introduce new biocompatibility concerns.
Manufacturing consistency represents a persistent technical hurdle, as processing parameters significantly impact the crystallinity and mechanical properties of PEEK implants. The high processing temperatures (370-400°C) required for PEEK fabrication limit manufacturing options and increase production costs compared to conventional polymers.
Antimicrobial resistance remains an unresolved challenge, with PEEK surfaces susceptible to bacterial colonization and biofilm formation. Research efforts focus on incorporating antimicrobial agents or developing surface treatments that inhibit bacterial adhesion without compromising biocompatibility or mechanical integrity.
Regulatory frameworks present additional complexities, with varying requirements across global markets for demonstrating long-term safety and efficacy. The FDA classification of PEEK devices typically requires extensive preclinical and clinical testing, creating significant barriers to market entry for novel PEEK applications.
Geographically, technical development in PEEK medical applications is concentrated in North America and Europe, with emerging research centers in Asia, particularly Japan and China. The technical landscape is characterized by a combination of proprietary technologies held by major medical device manufacturers and academic research focused on addressing the fundamental limitations of PEEK in biological environments.
The fundamental properties that establish PEEK's biocompatibility include its exceptional chemical resistance, which prevents material degradation in the physiological environment. Its bioinertness ensures minimal foreign body response, with clinical studies demonstrating inflammation profiles comparable to titanium alloys but with superior osseointegration characteristics in certain applications.
Despite these advantages, several technical challenges persist in PEEK medical applications. The inherent hydrophobicity of PEEK surfaces limits cell adhesion and tissue integration, creating a technical barrier for direct bone bonding. This has necessitated the development of surface modification techniques including plasma treatment, laser texturing, and bioactive coating applications to enhance osseointegration.
Another significant challenge involves the radiolucency of PEEK, which while beneficial for post-operative imaging, creates difficulties in intraoperative visualization. Current solutions incorporate radiopaque markers or develop PEEK composites with barium sulfate or titanium dioxide, though these modifications can compromise mechanical properties and introduce new biocompatibility concerns.
Manufacturing consistency represents a persistent technical hurdle, as processing parameters significantly impact the crystallinity and mechanical properties of PEEK implants. The high processing temperatures (370-400°C) required for PEEK fabrication limit manufacturing options and increase production costs compared to conventional polymers.
Antimicrobial resistance remains an unresolved challenge, with PEEK surfaces susceptible to bacterial colonization and biofilm formation. Research efforts focus on incorporating antimicrobial agents or developing surface treatments that inhibit bacterial adhesion without compromising biocompatibility or mechanical integrity.
Regulatory frameworks present additional complexities, with varying requirements across global markets for demonstrating long-term safety and efficacy. The FDA classification of PEEK devices typically requires extensive preclinical and clinical testing, creating significant barriers to market entry for novel PEEK applications.
Geographically, technical development in PEEK medical applications is concentrated in North America and Europe, with emerging research centers in Asia, particularly Japan and China. The technical landscape is characterized by a combination of proprietary technologies held by major medical device manufacturers and academic research focused on addressing the fundamental limitations of PEEK in biological environments.
Current PEEK Modification Approaches for Biomedical Use
01 Biocompatibility of PEEK for medical implants
PEEK (Polyetheretherketone) polymer demonstrates excellent biocompatibility for medical implant applications. Its chemical stability, mechanical properties similar to human bone, and resistance to degradation in the body make it suitable for long-term implantation. PEEK implants show minimal inflammatory response and good tissue integration, making them valuable alternatives to traditional metal implants in orthopedic and spinal applications.- Biocompatibility of PEEK for medical implants: PEEK (Polyetheretherketone) polymer demonstrates excellent biocompatibility properties making it suitable for various medical implant applications. Its chemical stability, mechanical strength similar to human bone, and resistance to degradation in biological environments contribute to its success as an implant material. PEEK implants show minimal inflammatory response and good tissue integration, making them ideal for long-term implantable devices such as spinal implants, orthopedic components, and dental prosthetics.
- Surface modifications to enhance PEEK biocompatibility: Various surface modification techniques are employed to enhance the biocompatibility of PEEK polymers. These include plasma treatment, coating with bioactive materials like hydroxyapatite, surface texturing, and chemical functionalization. These modifications improve cell adhesion, proliferation, and osseointegration properties of PEEK implants. Modified PEEK surfaces show improved wettability and can incorporate antimicrobial properties, reducing infection risks while maintaining the core mechanical advantages of the base polymer.
- PEEK composites with enhanced biological properties: PEEK composites incorporating bioactive materials such as carbon fibers, glass fibers, and ceramic particles demonstrate improved biocompatibility and mechanical properties. These composites can be engineered to match specific tissue properties while maintaining the biocompatibility of pure PEEK. The addition of bioactive fillers promotes bone ingrowth and tissue attachment, creating stronger interfaces between the implant and surrounding tissues. These composites find applications in load-bearing implants where both strength and biocompatibility are critical requirements.
- Biocompatibility testing methods for PEEK materials: Specialized testing protocols have been developed to evaluate the biocompatibility of PEEK polymers and their composites. These include in vitro cytotoxicity assays, cell adhesion and proliferation studies, protein adsorption tests, and in vivo implantation studies. Advanced imaging techniques are used to assess tissue integration and inflammatory responses. These testing methods ensure that PEEK materials meet regulatory requirements for medical applications and help in predicting their long-term performance in biological environments.
- PEEK applications in tissue engineering and regenerative medicine: PEEK polymers are increasingly used in tissue engineering scaffolds and regenerative medicine applications due to their biocompatibility and customizable properties. PEEK-based scaffolds can be manufactured with controlled porosity and surface chemistry to support cell growth and tissue regeneration. These materials can be processed using advanced manufacturing techniques like 3D printing to create patient-specific implants with optimized biological interfaces. The combination of PEEK with growth factors and stem cells shows promise for enhanced tissue regeneration applications.
02 Surface modifications to enhance PEEK biocompatibility
Various surface modification techniques can be applied to PEEK polymers to enhance their biocompatibility and functionality. These include plasma treatment, coating with bioactive materials, surface texturing, and chemical modifications. Such treatments can improve cell adhesion, osseointegration, and reduce bacterial colonization, thereby enhancing the overall performance of PEEK-based medical devices.Expand Specific Solutions03 PEEK composites with bioactive materials
PEEK can be combined with various bioactive materials to create composites with enhanced biocompatibility and functionality. Incorporation of materials such as hydroxyapatite, calcium phosphates, carbon fibers, or bioactive glass into the PEEK matrix can improve osseointegration and tissue response. These composites maintain the mechanical advantages of PEEK while adding bioactive properties that promote healing and tissue integration.Expand Specific Solutions04 Biocompatibility testing methods for PEEK materials
Various standardized and novel testing methods are employed to evaluate the biocompatibility of PEEK polymers and their composites. These include in vitro cell culture studies, protein adsorption tests, bacterial adhesion assays, and in vivo implantation studies. Advanced techniques such as microscopy, spectroscopy, and molecular biology methods are used to assess cell attachment, proliferation, inflammatory response, and tissue integration with PEEK materials.Expand Specific Solutions05 PEEK applications in biomedical devices
PEEK polymer is utilized in various biomedical applications due to its biocompatibility. These applications include spinal implants, orthopedic devices, dental prosthetics, cranial implants, and cardiovascular devices. The polymer's combination of mechanical strength, chemical resistance, sterilizability, and radiolucency makes it particularly valuable for these medical applications where long-term biocompatibility is essential.Expand Specific Solutions
Key Manufacturers and Research Institutions in PEEK Industry
The PEEK polymer biocompatible applications market is currently in a growth phase, characterized by increasing adoption across medical device sectors. The global market size is expanding rapidly, driven by PEEK's exceptional mechanical properties, chemical resistance, and biocompatibility. From a technological maturity perspective, industry leaders like Solvay Specialty Polymers and Victrex Manufacturing have established commercial-scale production capabilities, while companies such as DiFusion and Yunyi Medical Device are developing innovative PEEK composites with enhanced bioactive properties. Medical device manufacturers including Stryker, Howmedica Osteonics, and ConforMIS are increasingly incorporating PEEK into implantable devices. Academic institutions like Shanghai Jiao Tong University and The University of Sydney are advancing fundamental research, suggesting the technology continues to evolve despite its commercial readiness.
Solvay Specialty Polymers USA LLC
Technical Solution: Solvay has developed advanced PEEK (polyetheretherketone) formulations specifically engineered for biomedical applications. Their KetaSpire® PEEK products feature exceptional purity levels and are manufactured under stringent controlled conditions to meet medical-grade requirements. Solvay's PEEK polymers undergo specialized processing techniques to enhance biocompatibility while maintaining the inherent chemical resistance and mechanical properties of the base polymer. Their technology includes surface modification treatments that improve osseointegration when used in implantable devices. Solvay has also developed radiopaque PEEK compounds by incorporating barium sulfate or bismuth compounds, allowing for visibility under imaging techniques while preserving biocompatibility. Their PEEK formulations demonstrate excellent resistance to hydrolysis and sterilization processes, maintaining structural integrity even after multiple sterilization cycles[1][3].
Strengths: Industry-leading expertise in high-performance polymers with extensive regulatory compliance documentation. Their PEEK formulations offer superior mechanical properties and chemical resistance compared to competitors. Weaknesses: Higher cost compared to conventional medical polymers, and some formulations may require specialized processing equipment, limiting accessibility for smaller medical device manufacturers.
DiFusion, Inc.
Technical Solution: DiFusion has pioneered ZFUZE™, a proprietary PEEK-based composite technology specifically designed for orthopedic applications. This innovative approach modifies traditional PEEK polymer by incorporating zeolite particles, creating a surface nanotopography that significantly enhances osteoblast activity while maintaining PEEK's mechanical advantages. Their technology addresses one of PEEK's primary limitations in orthopedic applications - its bioinert nature that can lead to poor osseointegration. The zeolite-enhanced PEEK demonstrates improved hydrophilicity and surface energy, promoting better cell attachment and bone growth. Clinical studies have shown that DiFusion's modified PEEK exhibits osteogenic properties comparable to titanium while retaining PEEK's advantageous modulus of elasticity that more closely matches bone. The company has developed specialized manufacturing processes that ensure uniform distribution of zeolite particles throughout the polymer matrix, maintaining consistent bioactive properties across the entire implant surface[2][5].
Strengths: Their zeolite-enhanced PEEK technology bridges the gap between titanium's excellent osseointegration and PEEK's favorable mechanical properties, offering a truly differentiated product. Weaknesses: The specialized formulation may have higher production costs and more complex manufacturing requirements than standard PEEK, potentially limiting applications to higher-value medical devices.
Critical Patents and Research on PEEK Biocompatibility
Polyether ether ketone surface-modified with hydroxyapatite
PatentInactiveUS20180339082A1
Innovation
- Embedding hydroxyapatite (HA) particles discontinuously on the surface of PEEK using a blasting process with controlled particle size and pressure to form HA islands, enhancing osteoconductivity while maintaining PEEK's high tensile strength.
A polyether ether ketone-based composite, and methods thereof
PatentActiveIN201811018806A
Innovation
- A composite material comprising polyether ether ketone (PEEK) reinforced with refractory materials like silicon carbide (SiC) and a compatibilizer like polycarbosilane, with a refractory to PEEK weight ratio of 0.001:1 to 0.42:1, to improve hardness and flame-retardancy.
Regulatory Framework for Medical-Grade Polymers
The regulatory landscape for medical-grade polymers is complex and multifaceted, with PEEK (Polyetheretherketone) being subject to stringent oversight due to its increasing use in biomedical applications. In the United States, the Food and Drug Administration (FDA) regulates PEEK-based medical devices through various pathways, including 510(k) clearance for devices substantially equivalent to predicate devices, and Premarket Approval (PMA) for novel applications. The FDA specifically evaluates PEEK materials under ISO 10993 standards for biocompatibility, requiring comprehensive testing for cytotoxicity, sensitization, irritation, and systemic toxicity.
In Europe, PEEK medical devices must comply with the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive (MDD) with more rigorous requirements for clinical evaluation and post-market surveillance. The European regulatory framework emphasizes risk management throughout the product lifecycle and requires CE marking to indicate conformity with health, safety, and environmental protection standards.
The International Organization for Standardization (ISO) provides several standards directly applicable to PEEK in medical applications, including ISO 13485 for quality management systems and ISO 16061 for instrumentation used with non-active surgical implants. These standards establish baseline requirements for manufacturing processes, quality control, and performance characteristics of medical-grade PEEK.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have also established specific regulatory pathways for PEEK-based medical devices, often requiring local testing and certification despite international harmonization efforts. These regional variations in regulatory requirements create significant challenges for global manufacturers of PEEK medical devices.
Regulatory compliance for PEEK involves extensive documentation of material sourcing, processing methods, sterilization validation, and shelf-life stability. Manufacturers must demonstrate that their PEEK formulations maintain consistent properties throughout the manufacturing process and after sterilization, as changes in crystallinity or molecular weight can affect biocompatibility and mechanical performance.
Recent regulatory trends indicate increasing scrutiny of leachables and extractables from polymeric materials, with particular attention to potential endocrine-disrupting compounds. While PEEK generally performs favorably in these assessments due to its chemical stability, manufacturers must still conduct comprehensive chemical characterization studies to satisfy evolving regulatory requirements.
The regulatory framework also addresses end-of-life considerations, with growing emphasis on sustainable disposal or recycling options for medical polymers. PEEK's durability presents both advantages in terms of device longevity and challenges regarding environmental persistence, factors that regulatory bodies are beginning to incorporate into approval processes for new medical materials.
In Europe, PEEK medical devices must comply with the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive (MDD) with more rigorous requirements for clinical evaluation and post-market surveillance. The European regulatory framework emphasizes risk management throughout the product lifecycle and requires CE marking to indicate conformity with health, safety, and environmental protection standards.
The International Organization for Standardization (ISO) provides several standards directly applicable to PEEK in medical applications, including ISO 13485 for quality management systems and ISO 16061 for instrumentation used with non-active surgical implants. These standards establish baseline requirements for manufacturing processes, quality control, and performance characteristics of medical-grade PEEK.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have also established specific regulatory pathways for PEEK-based medical devices, often requiring local testing and certification despite international harmonization efforts. These regional variations in regulatory requirements create significant challenges for global manufacturers of PEEK medical devices.
Regulatory compliance for PEEK involves extensive documentation of material sourcing, processing methods, sterilization validation, and shelf-life stability. Manufacturers must demonstrate that their PEEK formulations maintain consistent properties throughout the manufacturing process and after sterilization, as changes in crystallinity or molecular weight can affect biocompatibility and mechanical performance.
Recent regulatory trends indicate increasing scrutiny of leachables and extractables from polymeric materials, with particular attention to potential endocrine-disrupting compounds. While PEEK generally performs favorably in these assessments due to its chemical stability, manufacturers must still conduct comprehensive chemical characterization studies to satisfy evolving regulatory requirements.
The regulatory framework also addresses end-of-life considerations, with growing emphasis on sustainable disposal or recycling options for medical polymers. PEEK's durability presents both advantages in terms of device longevity and challenges regarding environmental persistence, factors that regulatory bodies are beginning to incorporate into approval processes for new medical materials.
Sustainability Aspects of PEEK in Healthcare Applications
The sustainability profile of PEEK in healthcare applications represents a significant advantage in the growing movement toward environmentally responsible medical practices. PEEK demonstrates exceptional durability with an estimated lifespan of 25-30 years in medical implant applications, substantially reducing the need for revision surgeries and replacement devices. This longevity directly translates to decreased resource consumption and waste generation across the healthcare supply chain.
From a manufacturing perspective, PEEK processing requires less energy compared to metal alternatives like titanium or stainless steel. The polymer can be processed at temperatures around 400°C, whereas metals often require temperatures exceeding 1000°C. This energy efficiency extends throughout the entire production cycle, from raw material extraction to final product manufacturing, contributing to a reduced carbon footprint.
PEEK's recyclability presents another sustainability advantage. While medical-grade PEEK products face regulatory challenges regarding reuse, industrial PEEK components can be effectively recycled through mechanical grinding and reprocessing. Research indicates that recycled PEEK maintains approximately 85-90% of its original mechanical properties through multiple recycling cycles, demonstrating excellent material conservation potential.
The lightweight nature of PEEK compared to metal alternatives reduces transportation-related emissions throughout the supply chain. Studies suggest that PEEK components weigh approximately 60-70% less than equivalent metal parts, resulting in significant fuel savings and reduced carbon emissions during distribution.
End-of-life considerations for PEEK also demonstrate favorable environmental characteristics. Unlike some medical polymers that release harmful substances during incineration, PEEK produces minimal toxic byproducts when properly disposed of through controlled medical waste management systems. Additionally, its chemical stability prevents leaching of harmful compounds into groundwater when disposed of in landfills.
Healthcare facilities increasingly recognize PEEK's contribution to their sustainability goals through reduced energy consumption in sterilization processes. PEEK components can withstand multiple sterilization cycles using various methods, including autoclave, gamma radiation, and chemical treatments, without significant degradation. This versatility allows healthcare providers to select the most environmentally efficient sterilization method available at their facility.
As healthcare systems worldwide implement sustainability metrics into procurement decisions, PEEK's favorable environmental profile positions it advantageously against competing materials, aligning with the industry's movement toward more sustainable medical technologies and practices.
From a manufacturing perspective, PEEK processing requires less energy compared to metal alternatives like titanium or stainless steel. The polymer can be processed at temperatures around 400°C, whereas metals often require temperatures exceeding 1000°C. This energy efficiency extends throughout the entire production cycle, from raw material extraction to final product manufacturing, contributing to a reduced carbon footprint.
PEEK's recyclability presents another sustainability advantage. While medical-grade PEEK products face regulatory challenges regarding reuse, industrial PEEK components can be effectively recycled through mechanical grinding and reprocessing. Research indicates that recycled PEEK maintains approximately 85-90% of its original mechanical properties through multiple recycling cycles, demonstrating excellent material conservation potential.
The lightweight nature of PEEK compared to metal alternatives reduces transportation-related emissions throughout the supply chain. Studies suggest that PEEK components weigh approximately 60-70% less than equivalent metal parts, resulting in significant fuel savings and reduced carbon emissions during distribution.
End-of-life considerations for PEEK also demonstrate favorable environmental characteristics. Unlike some medical polymers that release harmful substances during incineration, PEEK produces minimal toxic byproducts when properly disposed of through controlled medical waste management systems. Additionally, its chemical stability prevents leaching of harmful compounds into groundwater when disposed of in landfills.
Healthcare facilities increasingly recognize PEEK's contribution to their sustainability goals through reduced energy consumption in sterilization processes. PEEK components can withstand multiple sterilization cycles using various methods, including autoclave, gamma radiation, and chemical treatments, without significant degradation. This versatility allows healthcare providers to select the most environmentally efficient sterilization method available at their facility.
As healthcare systems worldwide implement sustainability metrics into procurement decisions, PEEK's favorable environmental profile positions it advantageously against competing materials, aligning with the industry's movement toward more sustainable medical technologies and practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!