How Structural Ceramics Contribute to Medical Implant Success
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Structural Ceramics in Medical Implants: Background and Objectives
Structural ceramics have emerged as revolutionary materials in the field of medical implants, marking a significant departure from traditional metallic implants that dominated the industry for decades. The evolution of these advanced ceramics began in the 1970s with the introduction of alumina (Al₂O₃) for hip replacements, followed by the development of zirconia (ZrO₂) in the 1980s. This technological progression has continued with the recent advent of silicon nitride, aluminum nitride, and various ceramic composites specifically engineered for biomedical applications.
The fundamental appeal of structural ceramics in medical implants stems from their exceptional combination of mechanical properties, biocompatibility, and chemical stability. Unlike metallic implants that may release ions through corrosion processes, high-quality ceramics remain inert within the physiological environment, significantly reducing adverse tissue reactions and implant rejection risks. This characteristic has positioned ceramics as materials of choice for long-term implantable devices.
Current technological trends in structural ceramics for medical implants focus on enhancing mechanical reliability through innovations in processing techniques, microstructural engineering, and surface modifications. The field is witnessing a shift toward nano-structured ceramics that mimic the hierarchical organization of natural bone, potentially improving osseointegration and long-term stability. Additionally, researchers are exploring functionally graded ceramic materials that can provide spatially varied properties to match the complex mechanical requirements of different anatomical locations.
The primary technical objectives in this domain include developing ceramics with improved fracture toughness without compromising biocompatibility, creating surfaces that actively promote tissue integration while preventing bacterial colonization, and establishing manufacturing processes that allow for patient-specific customization through advanced fabrication technologies such as additive manufacturing. These objectives address the critical challenges of implant longevity and functionality.
Global research efforts are increasingly concentrated on multifunctional ceramic systems that not only provide structural support but also deliver therapeutic agents, incorporate diagnostic capabilities, or respond to physiological changes. This represents a paradigm shift from passive implants to active, smart materials that can adapt to the dynamic biological environment and potentially communicate with external monitoring systems.
The ultimate goal of structural ceramic development for medical implants is to create materials that seamlessly integrate with the human body, providing lifelong functionality without degradation or adverse effects. This requires interdisciplinary collaboration between materials scientists, biomedical engineers, clinicians, and regulatory experts to translate promising laboratory developments into clinically viable and commercially successful implant technologies.
The fundamental appeal of structural ceramics in medical implants stems from their exceptional combination of mechanical properties, biocompatibility, and chemical stability. Unlike metallic implants that may release ions through corrosion processes, high-quality ceramics remain inert within the physiological environment, significantly reducing adverse tissue reactions and implant rejection risks. This characteristic has positioned ceramics as materials of choice for long-term implantable devices.
Current technological trends in structural ceramics for medical implants focus on enhancing mechanical reliability through innovations in processing techniques, microstructural engineering, and surface modifications. The field is witnessing a shift toward nano-structured ceramics that mimic the hierarchical organization of natural bone, potentially improving osseointegration and long-term stability. Additionally, researchers are exploring functionally graded ceramic materials that can provide spatially varied properties to match the complex mechanical requirements of different anatomical locations.
The primary technical objectives in this domain include developing ceramics with improved fracture toughness without compromising biocompatibility, creating surfaces that actively promote tissue integration while preventing bacterial colonization, and establishing manufacturing processes that allow for patient-specific customization through advanced fabrication technologies such as additive manufacturing. These objectives address the critical challenges of implant longevity and functionality.
Global research efforts are increasingly concentrated on multifunctional ceramic systems that not only provide structural support but also deliver therapeutic agents, incorporate diagnostic capabilities, or respond to physiological changes. This represents a paradigm shift from passive implants to active, smart materials that can adapt to the dynamic biological environment and potentially communicate with external monitoring systems.
The ultimate goal of structural ceramic development for medical implants is to create materials that seamlessly integrate with the human body, providing lifelong functionality without degradation or adverse effects. This requires interdisciplinary collaboration between materials scientists, biomedical engineers, clinicians, and regulatory experts to translate promising laboratory developments into clinically viable and commercially successful implant technologies.
Market Analysis of Ceramic-Based Medical Implants
The global market for ceramic-based medical implants has experienced substantial growth over the past decade, reaching approximately $19.3 billion in 2022 and projected to expand at a compound annual growth rate (CAGR) of 6.8% through 2028. This growth is primarily driven by increasing prevalence of musculoskeletal disorders, rising geriatric population, and technological advancements in ceramic materials science.
Dental implants represent the largest segment within the ceramic medical implants market, accounting for roughly 38% of the total market share. Hip and knee replacements follow closely, collectively representing about 35% of the market. Emerging applications in spinal implants and small joint replacements are showing promising growth trajectories, with CAGRs exceeding 8% in some regional markets.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region is witnessing the fastest growth rate due to improving healthcare infrastructure, increasing disposable income, and growing awareness about advanced medical treatments in countries like China, India, and South Korea.
The competitive landscape features both established medical device manufacturers and specialized ceramic implant producers. Key market players include Zimmer Biomet, Stryker Corporation, DePuy Synthes (Johnson & Johnson), CeramTec, and Kyocera Medical, collectively holding over 65% of the global market share. These companies are increasingly focusing on R&D investments to develop advanced ceramic composites with enhanced biocompatibility and mechanical properties.
Consumer demand patterns indicate a growing preference for longer-lasting implants with reduced revision surgery requirements. This trend has accelerated the adoption of ceramic materials over traditional metal implants in various applications. Market research indicates that patients are increasingly willing to pay premium prices for ceramic implants due to their superior biocompatibility and reduced risk of allergic reactions.
Reimbursement policies significantly influence market dynamics, with variations across different regions affecting adoption rates. Countries with favorable reimbursement structures for ceramic implants show higher market penetration rates. Additionally, regulatory approvals play a crucial role in market access, with stringent evaluation processes for novel ceramic materials potentially delaying product launches but ultimately ensuring safety and efficacy.
Future market growth is expected to be driven by innovations in ceramic manufacturing technologies, including 3D printing of custom ceramic implants and development of bioresorbable ceramic composites. These advancements are anticipated to expand the application scope of ceramic implants beyond the current mainstream uses.
Dental implants represent the largest segment within the ceramic medical implants market, accounting for roughly 38% of the total market share. Hip and knee replacements follow closely, collectively representing about 35% of the market. Emerging applications in spinal implants and small joint replacements are showing promising growth trajectories, with CAGRs exceeding 8% in some regional markets.
Geographically, North America dominates the market with approximately 42% share, followed by Europe at 31% and Asia-Pacific at 21%. However, the Asia-Pacific region is witnessing the fastest growth rate due to improving healthcare infrastructure, increasing disposable income, and growing awareness about advanced medical treatments in countries like China, India, and South Korea.
The competitive landscape features both established medical device manufacturers and specialized ceramic implant producers. Key market players include Zimmer Biomet, Stryker Corporation, DePuy Synthes (Johnson & Johnson), CeramTec, and Kyocera Medical, collectively holding over 65% of the global market share. These companies are increasingly focusing on R&D investments to develop advanced ceramic composites with enhanced biocompatibility and mechanical properties.
Consumer demand patterns indicate a growing preference for longer-lasting implants with reduced revision surgery requirements. This trend has accelerated the adoption of ceramic materials over traditional metal implants in various applications. Market research indicates that patients are increasingly willing to pay premium prices for ceramic implants due to their superior biocompatibility and reduced risk of allergic reactions.
Reimbursement policies significantly influence market dynamics, with variations across different regions affecting adoption rates. Countries with favorable reimbursement structures for ceramic implants show higher market penetration rates. Additionally, regulatory approvals play a crucial role in market access, with stringent evaluation processes for novel ceramic materials potentially delaying product launches but ultimately ensuring safety and efficacy.
Future market growth is expected to be driven by innovations in ceramic manufacturing technologies, including 3D printing of custom ceramic implants and development of bioresorbable ceramic composites. These advancements are anticipated to expand the application scope of ceramic implants beyond the current mainstream uses.
Current Challenges in Structural Ceramics for Implantology
Despite significant advancements in structural ceramics for medical implants, several critical challenges continue to impede their widespread adoption in implantology. The primary obstacle remains the inherent brittleness of ceramic materials, which limits their application in load-bearing implants where sudden failure could be catastrophic. This fundamental material property creates a significant engineering dilemma: while ceramics offer excellent biocompatibility and wear resistance, their fracture toughness remains substantially lower than metallic alternatives.
Manufacturing precision presents another substantial challenge. The production of complex ceramic implant geometries with consistent quality requires sophisticated processing techniques. Current sintering methods often introduce microscopic defects and residual stresses that can serve as fracture initiation sites. Additionally, achieving the necessary dimensional accuracy for implant components, particularly those requiring precise interfacing with biological tissues, remains technically demanding and cost-intensive.
Surface engineering of ceramic implants constitutes a multifaceted challenge. While bioactive ceramic surfaces can promote osseointegration, controlling the exact surface properties to optimize cell attachment while minimizing bacterial adhesion requires nanoscale precision that current manufacturing processes struggle to deliver consistently. The long-term stability of these engineered surfaces in the dynamic biological environment also remains inadequately characterized.
The biological response variability to ceramic implants presents significant clinical challenges. Patient-specific factors can influence osseointegration rates and long-term implant stability. Current ceramic formulations lack adaptability to different patient physiologies, creating unpredictable outcomes in diverse patient populations. This variability complicates clinical decision-making and risk assessment.
Cost factors continue to limit broader implementation of ceramic implants. The complex manufacturing processes, specialized equipment, and stringent quality control measures significantly increase production costs compared to traditional implant materials. These economic barriers particularly affect adoption in cost-sensitive healthcare markets and developing regions.
Regulatory hurdles compound these technical challenges. The approval pathway for novel ceramic implant materials involves extensive testing regimes and clinical trials that can span many years. The regulatory framework struggles to keep pace with rapid material innovations, creating bottlenecks in bringing new ceramic implant technologies to market.
Finally, there exists a significant knowledge gap in long-term performance data. While short-term clinical outcomes for ceramic implants are promising, comprehensive longitudinal studies spanning decades remain scarce. This uncertainty regarding long-term stability, wear characteristics, and potential degradation mechanisms in vivo creates hesitation among clinicians when selecting ceramic implants for younger patients with longer life expectancies.
Manufacturing precision presents another substantial challenge. The production of complex ceramic implant geometries with consistent quality requires sophisticated processing techniques. Current sintering methods often introduce microscopic defects and residual stresses that can serve as fracture initiation sites. Additionally, achieving the necessary dimensional accuracy for implant components, particularly those requiring precise interfacing with biological tissues, remains technically demanding and cost-intensive.
Surface engineering of ceramic implants constitutes a multifaceted challenge. While bioactive ceramic surfaces can promote osseointegration, controlling the exact surface properties to optimize cell attachment while minimizing bacterial adhesion requires nanoscale precision that current manufacturing processes struggle to deliver consistently. The long-term stability of these engineered surfaces in the dynamic biological environment also remains inadequately characterized.
The biological response variability to ceramic implants presents significant clinical challenges. Patient-specific factors can influence osseointegration rates and long-term implant stability. Current ceramic formulations lack adaptability to different patient physiologies, creating unpredictable outcomes in diverse patient populations. This variability complicates clinical decision-making and risk assessment.
Cost factors continue to limit broader implementation of ceramic implants. The complex manufacturing processes, specialized equipment, and stringent quality control measures significantly increase production costs compared to traditional implant materials. These economic barriers particularly affect adoption in cost-sensitive healthcare markets and developing regions.
Regulatory hurdles compound these technical challenges. The approval pathway for novel ceramic implant materials involves extensive testing regimes and clinical trials that can span many years. The regulatory framework struggles to keep pace with rapid material innovations, creating bottlenecks in bringing new ceramic implant technologies to market.
Finally, there exists a significant knowledge gap in long-term performance data. While short-term clinical outcomes for ceramic implants are promising, comprehensive longitudinal studies spanning decades remain scarce. This uncertainty regarding long-term stability, wear characteristics, and potential degradation mechanisms in vivo creates hesitation among clinicians when selecting ceramic implants for younger patients with longer life expectancies.
Contemporary Ceramic Implant Solutions and Methodologies
01 Advanced manufacturing techniques for structural ceramics
Various manufacturing techniques have been developed to enhance the properties and performance of structural ceramics. These include specialized sintering processes, precision molding methods, and advanced forming techniques that improve the mechanical strength and durability of ceramic components. These manufacturing innovations help overcome traditional limitations of ceramic materials, resulting in products with superior structural integrity and reliability for demanding applications.- Advanced manufacturing techniques for structural ceramics: Various manufacturing techniques have been developed to enhance the properties and performance of structural ceramics. These include specialized sintering processes, hot pressing, and innovative molding methods that improve density, strength, and reliability. These advanced manufacturing approaches help overcome traditional limitations of ceramic materials, resulting in components with superior mechanical properties and thermal resistance for demanding applications.
- Composite ceramic materials with enhanced properties: Composite ceramic materials combine different ceramic components or ceramics with other materials to achieve enhanced properties. These composites often feature improved fracture toughness, thermal shock resistance, and mechanical strength compared to monolithic ceramics. By incorporating reinforcing phases such as fibers, whiskers, or particles, these materials can overcome the inherent brittleness of traditional ceramics while maintaining their high-temperature capabilities and chemical stability.
- Silicon-based structural ceramics: Silicon-based ceramics, including silicon carbide, silicon nitride, and sialon materials, represent a significant category of structural ceramics with exceptional high-temperature properties. These materials offer excellent thermal shock resistance, low thermal expansion, and good mechanical strength at elevated temperatures. Their unique combination of properties makes them suitable for applications in harsh environments, such as gas turbine components, cutting tools, and high-temperature bearings.
- Ceramic coatings and surface treatments: Specialized ceramic coatings and surface treatments can significantly enhance the performance of structural ceramic components. These treatments improve wear resistance, reduce friction, increase chemical stability, and enhance thermal barrier properties. Various deposition techniques, including plasma spraying, chemical vapor deposition, and sol-gel methods, are employed to apply these coatings, extending the service life and expanding the application range of ceramic components in demanding environments.
- Novel applications of structural ceramics: Structural ceramics have found success in numerous innovative applications across various industries. These include advanced aerospace components, cutting-edge medical implants, high-efficiency energy systems, and next-generation electronics. The unique combination of properties offered by structural ceramics, such as high hardness, wear resistance, biocompatibility, and electrical insulation, makes them ideal for these specialized applications where traditional materials cannot meet performance requirements.
02 Composite ceramic materials with enhanced properties
Composite ceramic materials combine different ceramic compounds or incorporate reinforcing elements to achieve enhanced mechanical and thermal properties. These composites often feature improved fracture toughness, strength, and resistance to thermal shock compared to conventional ceramics. By carefully engineering the composition and microstructure of these materials, manufacturers can create structural ceramics with tailored properties suitable for specific high-performance applications.Expand Specific Solutions03 High-temperature applications of structural ceramics
Structural ceramics demonstrate exceptional performance in high-temperature environments where traditional materials would fail. These ceramics maintain their mechanical properties, dimensional stability, and chemical resistance at elevated temperatures, making them ideal for applications in aerospace, energy generation, and industrial processing. Advanced formulations have been developed specifically to withstand extreme thermal conditions while providing reliable structural support.Expand Specific Solutions04 Novel ceramic coating technologies
Innovative coating technologies have been developed to enhance the surface properties of structural ceramics or to apply ceramic coatings to other materials. These coatings can provide wear resistance, thermal insulation, chemical protection, or improved tribological properties. The application methods include plasma spraying, chemical vapor deposition, and sol-gel techniques, allowing for precise control of coating thickness and properties to meet specific performance requirements.Expand Specific Solutions05 Structural ceramics for electronic and energy applications
Specialized structural ceramics have been developed for electronic components and energy systems, offering unique combinations of electrical, thermal, and mechanical properties. These materials serve as substrates, insulators, or functional components in electronic devices, fuel cells, batteries, and energy conversion systems. Their ability to withstand harsh operating conditions while providing specific electrical or thermal characteristics makes them essential for advancing technology in these fields.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Medical Ceramics
The structural ceramics market for medical implants is in a growth phase, characterized by increasing adoption due to superior biocompatibility and mechanical properties. The global market is expanding rapidly, driven by aging populations and rising demand for long-lasting implant solutions. Technologically, the field shows varying maturity levels across applications, with dental implants (led by Straumann, Nobel Biocare, and Z-Systems) being most advanced, while orthopedic applications (where CeramTec, Zimmer, and Warsaw Orthopedic dominate) continue to evolve. Research institutions like MIT and Sichuan University are advancing next-generation ceramic biomaterials, while companies like Smith + Nephew and VITA Zahnfabrik are commercializing innovations in load-bearing implants and aesthetic dental solutions, creating a competitive landscape balanced between established players and emerging specialists.
CeramTec GmbH
Technical Solution: CeramTec has pioneered advanced bioceramics, particularly their BIOLOX® family of alumina and zirconia-toughened alumina (ZTA) ceramics for orthopedic implants. Their technology focuses on high-purity aluminum oxide and zirconia composites that offer exceptional wear resistance and biocompatibility. The company's manufacturing process involves hot isostatic pressing (HIP) and precision machining to create components with superior mechanical properties. Their ceramic femoral heads and acetabular liners have demonstrated wear rates approximately 100 times lower than metal-on-polyethylene alternatives, with debris particles that cause significantly less inflammatory response. CeramTec's latest innovation includes BIOLOX®delta, a mixed ceramic matrix containing 82% alumina, 17% zirconia, and other oxide additives that create a unique microstructure with exceptional fracture resistance and longevity in hip replacement applications.
Strengths: Superior wear resistance (>20 years functional lifetime), excellent biocompatibility with minimal inflammatory response, and high mechanical strength (fracture toughness >8 MPa·m1/2). Weaknesses: Higher manufacturing costs compared to metal alternatives, limited design flexibility due to ceramic processing constraints, and potential for catastrophic failure though rare (<0.001% fracture rate).
Z-Systems AG
Technical Solution: Z-Systems specializes in zirconia dental implants, developing a proprietary ceramic implant system that offers metal-free alternatives for dental restoration. Their Z-Look3 and newer Z5 implant systems utilize yttria-stabilized tetragonal zirconia polycrystals (Y-TZP) with a unique surface treatment technology that enhances osseointegration. The company's patented manufacturing process includes precision CNC milling followed by specialized sintering protocols that achieve optimal density while maintaining dimensional accuracy. Their implants feature a specially designed macro and micro surface topography created through a combination of sandblasting and acid etching, which clinical studies have shown promotes bone-to-implant contact comparable to titanium standards (approximately 70-80% after 12 weeks). Z-Systems has also developed a proprietary two-piece ceramic implant system that overcomes traditional limitations of one-piece designs, allowing for more versatile prosthetic options while maintaining the biocompatibility advantages of ceramic materials.
Strengths: Superior esthetics with no grayish discoloration of surrounding tissue, excellent soft tissue response with reduced plaque accumulation, and complete biocompatibility for metal-sensitive patients. Weaknesses: Lower fracture toughness compared to titanium alternatives (requiring specific design considerations), more technique-sensitive placement protocol, and higher cost than conventional metal implants.
Critical Patents and Innovations in Biomedical Ceramic Technology
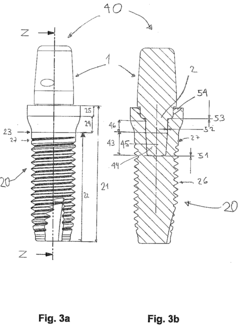
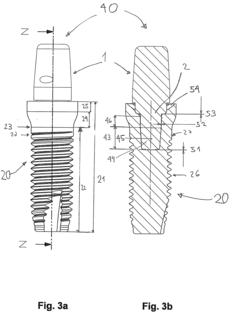
Ceramic part having at least one ceramic foam for medical applications
PatentWO2019038145A1
Innovation
- Ceramic parts made partially or completely of ceramic foam with a significant proportion of pores, allowing for the integration of fasteners like screws or nails without catastrophic failure, featuring a combination of porous and dense ceramic areas for enhanced mechanical stability and osseointegration.
Ceramic implant system
PatentInactiveEP2767254A2
Innovation
- A ceramic implant system featuring a clamping connection between the implant and abutment using precisely manufactured conical clamping surfaces with a small opening angle, minimizing tensile and ring stresses, and optionally supplemented with an adhesive connection to enhance stability.
Biocompatibility and Long-term Performance Assessment
Biocompatibility assessment of structural ceramics in medical implants involves rigorous evaluation protocols that exceed standard material testing. These ceramics, primarily aluminum oxide (alumina), zirconium dioxide (zirconia), and silicon nitride, undergo comprehensive in vitro cytotoxicity, genotoxicity, and sensitization tests. The evaluation process typically begins with cell culture studies to assess direct cellular interactions, followed by more complex tissue response analyses.
Long-term performance assessment requires both accelerated aging studies and real-time monitoring of implanted ceramics. Simulated body fluid (SBF) immersion tests provide valuable data on material degradation patterns under physiological conditions. Advanced techniques including micro-CT scanning and Raman spectroscopy enable non-destructive evaluation of structural integrity and surface chemistry changes over time. These methodologies have demonstrated that high-purity alumina and zirconia ceramics maintain exceptional stability, with degradation rates below 0.01% annually in optimal formulations.
Clinical follow-up studies spanning 15-20 years have established the superior biocompatibility profile of structural ceramics compared to metallic alternatives. Particularly noteworthy is the significantly reduced inflammatory response and absence of metal ion release that can trigger hypersensitivity reactions. The ceramic-tissue interface typically develops a stable fibrous encapsulation with minimal foreign body reaction, contributing to long-term implant stability.
Wear particle analysis represents a critical component of performance assessment, as ceramic particulates demonstrate fundamentally different biological interactions compared to metal or polymer debris. Studies indicate that ceramic wear particles, while potentially present in small quantities, exhibit lower inflammatory potential and reduced osteolytic activity. This characteristic significantly contributes to the extended functional lifespan of ceramic-based implant systems.
Recent advances in assessment methodologies include real-time biosensing technologies that can be integrated with implants to provide continuous monitoring of the local biochemical environment. These innovations allow for earlier detection of potential compatibility issues before clinical symptoms manifest. Additionally, machine learning algorithms applied to longitudinal implant performance data are enhancing predictive capabilities regarding ceramic implant longevity in specific patient populations.
Regulatory frameworks for ceramic implant assessment continue to evolve, with ISO 10993 standards providing the foundation for biocompatibility evaluation. However, specialized protocols for ceramic-specific degradation mechanisms and wear characteristics are increasingly being incorporated into testing regimens to better predict in vivo performance.
Long-term performance assessment requires both accelerated aging studies and real-time monitoring of implanted ceramics. Simulated body fluid (SBF) immersion tests provide valuable data on material degradation patterns under physiological conditions. Advanced techniques including micro-CT scanning and Raman spectroscopy enable non-destructive evaluation of structural integrity and surface chemistry changes over time. These methodologies have demonstrated that high-purity alumina and zirconia ceramics maintain exceptional stability, with degradation rates below 0.01% annually in optimal formulations.
Clinical follow-up studies spanning 15-20 years have established the superior biocompatibility profile of structural ceramics compared to metallic alternatives. Particularly noteworthy is the significantly reduced inflammatory response and absence of metal ion release that can trigger hypersensitivity reactions. The ceramic-tissue interface typically develops a stable fibrous encapsulation with minimal foreign body reaction, contributing to long-term implant stability.
Wear particle analysis represents a critical component of performance assessment, as ceramic particulates demonstrate fundamentally different biological interactions compared to metal or polymer debris. Studies indicate that ceramic wear particles, while potentially present in small quantities, exhibit lower inflammatory potential and reduced osteolytic activity. This characteristic significantly contributes to the extended functional lifespan of ceramic-based implant systems.
Recent advances in assessment methodologies include real-time biosensing technologies that can be integrated with implants to provide continuous monitoring of the local biochemical environment. These innovations allow for earlier detection of potential compatibility issues before clinical symptoms manifest. Additionally, machine learning algorithms applied to longitudinal implant performance data are enhancing predictive capabilities regarding ceramic implant longevity in specific patient populations.
Regulatory frameworks for ceramic implant assessment continue to evolve, with ISO 10993 standards providing the foundation for biocompatibility evaluation. However, specialized protocols for ceramic-specific degradation mechanisms and wear characteristics are increasingly being incorporated into testing regimens to better predict in vivo performance.
Regulatory Framework and Certification Processes for Ceramic Implants
The regulatory landscape for ceramic medical implants is complex and multifaceted, requiring manufacturers to navigate various approval pathways across different global jurisdictions. In the United States, the Food and Drug Administration (FDA) classifies most ceramic implants as Class II or Class III medical devices, necessitating either a 510(k) clearance or a more rigorous Premarket Approval (PMA) process. The determination largely depends on the implant's intended use, anatomical location, and risk profile.
European markets operate under the Medical Device Regulation (MDR), which replaced the Medical Device Directive (MDD) in 2021, significantly increasing requirements for clinical evidence and post-market surveillance. Ceramic implants typically fall under Class IIb or Class III categories, requiring Notified Body assessment and CE marking before market access. The MDR has introduced more stringent requirements for biocompatibility testing and technical documentation.
ISO 13356 specifically addresses requirements for surgical implants made of yttria-stabilized tetragonal zirconia (Y-TZP), while ISO 6474 covers alumina ceramic materials. These standards define essential properties including chemical composition, microstructure, mechanical strength, and aging resistance. Manufacturers must demonstrate compliance through extensive testing protocols that evaluate both short and long-term material stability.
Biocompatibility testing follows ISO 10993 series guidelines, with particular emphasis on cytotoxicity, sensitization, irritation, and systemic toxicity. For ceramic implants, additional testing for genotoxicity and carcinogenicity may be required depending on the duration of patient contact. The testing regimen must account for potential ceramic degradation products and their biological interactions.
Clinical evaluation requirements have become increasingly stringent, with regulatory bodies demanding robust clinical data demonstrating both safety and performance. For novel ceramic compositions or applications, randomized controlled trials may be necessary, while established ceramic materials with substantial clinical history may leverage existing data through literature-based clinical evaluations.
Post-market surveillance represents a critical component of the regulatory framework, with manufacturers required to implement comprehensive systems for adverse event reporting and trend analysis. The Medical Device Single Audit Program (MDSAP) allows for single regulatory audits to satisfy requirements across multiple jurisdictions, streamlining compliance for global manufacturers of ceramic implants.
Emerging regulatory considerations include unique device identification (UDI) requirements and increasing scrutiny of manufacturing processes, particularly for advanced ceramics with complex microstructures. Regulatory bodies are also developing frameworks for additive manufacturing of ceramic implants, addressing the unique validation challenges of 3D-printed ceramic components for medical applications.
European markets operate under the Medical Device Regulation (MDR), which replaced the Medical Device Directive (MDD) in 2021, significantly increasing requirements for clinical evidence and post-market surveillance. Ceramic implants typically fall under Class IIb or Class III categories, requiring Notified Body assessment and CE marking before market access. The MDR has introduced more stringent requirements for biocompatibility testing and technical documentation.
ISO 13356 specifically addresses requirements for surgical implants made of yttria-stabilized tetragonal zirconia (Y-TZP), while ISO 6474 covers alumina ceramic materials. These standards define essential properties including chemical composition, microstructure, mechanical strength, and aging resistance. Manufacturers must demonstrate compliance through extensive testing protocols that evaluate both short and long-term material stability.
Biocompatibility testing follows ISO 10993 series guidelines, with particular emphasis on cytotoxicity, sensitization, irritation, and systemic toxicity. For ceramic implants, additional testing for genotoxicity and carcinogenicity may be required depending on the duration of patient contact. The testing regimen must account for potential ceramic degradation products and their biological interactions.
Clinical evaluation requirements have become increasingly stringent, with regulatory bodies demanding robust clinical data demonstrating both safety and performance. For novel ceramic compositions or applications, randomized controlled trials may be necessary, while established ceramic materials with substantial clinical history may leverage existing data through literature-based clinical evaluations.
Post-market surveillance represents a critical component of the regulatory framework, with manufacturers required to implement comprehensive systems for adverse event reporting and trend analysis. The Medical Device Single Audit Program (MDSAP) allows for single regulatory audits to satisfy requirements across multiple jurisdictions, streamlining compliance for global manufacturers of ceramic implants.
Emerging regulatory considerations include unique device identification (UDI) requirements and increasing scrutiny of manufacturing processes, particularly for advanced ceramics with complex microstructures. Regulatory bodies are also developing frameworks for additive manufacturing of ceramic implants, addressing the unique validation challenges of 3D-printed ceramic components for medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!