Structural Ceramics and Biocompatibility: A Research Perspective
SEP 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Structural Ceramics Evolution and Research Objectives
Structural ceramics have evolved significantly over the past century, transforming from simple clay-based materials to sophisticated engineered compounds with exceptional mechanical, thermal, and chemical properties. The journey began in the early 20th century with traditional ceramics primarily used for household items and basic industrial applications. By mid-century, advancements in materials science led to the development of high-performance ceramics capable of withstanding extreme conditions, marking a pivotal shift in their application scope.
The 1970s and 1980s witnessed breakthrough innovations in ceramic processing techniques, including hot isostatic pressing and chemical vapor deposition, enabling the production of ceramics with unprecedented purity and controlled microstructures. These developments paved the way for structural ceramics to enter critical applications in aerospace, defense, and energy sectors. The subsequent decades saw the refinement of zirconia, alumina, and silicon nitride ceramics, which demonstrated superior mechanical strength and fracture toughness.
In the biomedical field, the evolution of structural ceramics took a significant turn in the 1990s when researchers discovered the exceptional biocompatibility of certain ceramic compositions. This discovery revolutionized orthopedic and dental implant technologies, offering alternatives to traditional metallic implants with reduced wear rates and improved tissue integration capabilities.
Recent technological advancements have focused on developing bioceramic composites that mimic the hierarchical structure of natural bone, combining mechanical reliability with biological functionality. The emergence of additive manufacturing techniques has further expanded the design possibilities for custom bioceramics, allowing for patient-specific implants with optimized porosity and surface characteristics.
The primary research objectives in this field now center on enhancing the integration between structural ceramics and biological systems. This includes developing ceramics with controlled degradation rates that match tissue regeneration timelines, improving the mechanical compatibility between ceramics and natural tissues to reduce stress shielding effects, and engineering surface modifications that promote specific cellular responses.
Another critical research goal involves understanding the long-term in vivo performance of structural ceramics, particularly their wear behavior, ion release profiles, and potential inflammatory responses. Researchers are also exploring novel ceramic compositions that can actively participate in the healing process through controlled release of therapeutic agents or by providing electrical stimulation to surrounding tissues.
The ultimate aim is to develop next-generation structural ceramics that not only serve as passive mechanical supports but function as active components in biological environments, responding dynamically to physiological changes and promoting tissue regeneration. This represents a paradigm shift from traditional inert biomaterials to bioactive and even bioresorbable ceramic systems with tailored functionality.
The 1970s and 1980s witnessed breakthrough innovations in ceramic processing techniques, including hot isostatic pressing and chemical vapor deposition, enabling the production of ceramics with unprecedented purity and controlled microstructures. These developments paved the way for structural ceramics to enter critical applications in aerospace, defense, and energy sectors. The subsequent decades saw the refinement of zirconia, alumina, and silicon nitride ceramics, which demonstrated superior mechanical strength and fracture toughness.
In the biomedical field, the evolution of structural ceramics took a significant turn in the 1990s when researchers discovered the exceptional biocompatibility of certain ceramic compositions. This discovery revolutionized orthopedic and dental implant technologies, offering alternatives to traditional metallic implants with reduced wear rates and improved tissue integration capabilities.
Recent technological advancements have focused on developing bioceramic composites that mimic the hierarchical structure of natural bone, combining mechanical reliability with biological functionality. The emergence of additive manufacturing techniques has further expanded the design possibilities for custom bioceramics, allowing for patient-specific implants with optimized porosity and surface characteristics.
The primary research objectives in this field now center on enhancing the integration between structural ceramics and biological systems. This includes developing ceramics with controlled degradation rates that match tissue regeneration timelines, improving the mechanical compatibility between ceramics and natural tissues to reduce stress shielding effects, and engineering surface modifications that promote specific cellular responses.
Another critical research goal involves understanding the long-term in vivo performance of structural ceramics, particularly their wear behavior, ion release profiles, and potential inflammatory responses. Researchers are also exploring novel ceramic compositions that can actively participate in the healing process through controlled release of therapeutic agents or by providing electrical stimulation to surrounding tissues.
The ultimate aim is to develop next-generation structural ceramics that not only serve as passive mechanical supports but function as active components in biological environments, responding dynamically to physiological changes and promoting tissue regeneration. This represents a paradigm shift from traditional inert biomaterials to bioactive and even bioresorbable ceramic systems with tailored functionality.
Biomedical Market Demand for Structural Ceramics
The global biomedical market for structural ceramics has experienced significant growth over the past decade, driven primarily by increasing demand for biocompatible materials in orthopedic and dental applications. Current market valuations indicate that the biomedical ceramics sector represents approximately 18% of the overall advanced ceramics market, with a compound annual growth rate of 6.8% projected through 2028.
Demographic shifts toward aging populations in developed economies have substantially increased the need for joint replacements and dental prosthetics. In North America and Europe, where populations over 65 are expected to comprise nearly 25% of the total population by 2030, demand for ceramic-based implants has risen dramatically. This trend is particularly evident in hip and knee replacement procedures, where ceramic components have demonstrated superior wear resistance compared to traditional materials.
The dental ceramics segment currently dominates the biomedical ceramics market, accounting for approximately 42% of market share. This is attributed to the excellent aesthetic properties, biocompatibility, and mechanical strength of ceramic materials used in crowns, bridges, and dental implants. Zirconia-based ceramics have emerged as the material of choice, showing a market penetration increase of 27% over the past five years.
Emerging economies, particularly in the Asia-Pacific region, represent the fastest-growing markets for biomedical ceramics. China and India have shown annual growth rates exceeding 9%, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of advanced medical technologies. These markets are expected to contribute significantly to global demand growth over the next decade.
Recent technological advancements in ceramic processing have expanded the application scope beyond traditional orthopedic and dental uses. The development of porous ceramic scaffolds for tissue engineering applications has opened new market segments with substantial growth potential. The regenerative medicine sector utilizing ceramic-based materials is projected to expand at twice the rate of traditional implant applications.
Regulatory trends worldwide indicate increasing acceptance of ceramic materials in medical applications, with faster approval pathways being established for devices with proven biocompatibility profiles. This regulatory environment has encouraged greater investment in research and development of novel ceramic compositions and manufacturing techniques specifically tailored for biomedical applications.
Consumer preferences are shifting toward longer-lasting implants with reduced revision surgery requirements, creating market pull for advanced ceramic solutions. Healthcare providers similarly favor materials that reduce long-term complications and associated costs, positioning structural ceramics as increasingly attractive alternatives to traditional metallic and polymeric biomaterials.
Demographic shifts toward aging populations in developed economies have substantially increased the need for joint replacements and dental prosthetics. In North America and Europe, where populations over 65 are expected to comprise nearly 25% of the total population by 2030, demand for ceramic-based implants has risen dramatically. This trend is particularly evident in hip and knee replacement procedures, where ceramic components have demonstrated superior wear resistance compared to traditional materials.
The dental ceramics segment currently dominates the biomedical ceramics market, accounting for approximately 42% of market share. This is attributed to the excellent aesthetic properties, biocompatibility, and mechanical strength of ceramic materials used in crowns, bridges, and dental implants. Zirconia-based ceramics have emerged as the material of choice, showing a market penetration increase of 27% over the past five years.
Emerging economies, particularly in the Asia-Pacific region, represent the fastest-growing markets for biomedical ceramics. China and India have shown annual growth rates exceeding 9%, driven by improving healthcare infrastructure, rising disposable incomes, and increasing awareness of advanced medical technologies. These markets are expected to contribute significantly to global demand growth over the next decade.
Recent technological advancements in ceramic processing have expanded the application scope beyond traditional orthopedic and dental uses. The development of porous ceramic scaffolds for tissue engineering applications has opened new market segments with substantial growth potential. The regenerative medicine sector utilizing ceramic-based materials is projected to expand at twice the rate of traditional implant applications.
Regulatory trends worldwide indicate increasing acceptance of ceramic materials in medical applications, with faster approval pathways being established for devices with proven biocompatibility profiles. This regulatory environment has encouraged greater investment in research and development of novel ceramic compositions and manufacturing techniques specifically tailored for biomedical applications.
Consumer preferences are shifting toward longer-lasting implants with reduced revision surgery requirements, creating market pull for advanced ceramic solutions. Healthcare providers similarly favor materials that reduce long-term complications and associated costs, positioning structural ceramics as increasingly attractive alternatives to traditional metallic and polymeric biomaterials.
Current Biocompatibility Challenges and Limitations
Despite significant advancements in structural ceramics for biomedical applications, several critical biocompatibility challenges persist that limit their widespread clinical adoption. The primary concern remains the potential for ceramic materials to release particles or ions that may trigger adverse biological responses. Zirconia and alumina ceramics, while generally considered biocompatible, can still undergo degradation in the physiological environment, particularly under cyclic loading conditions, leading to the release of wear debris that may cause inflammatory responses and subsequent implant failure.
Surface properties of structural ceramics present another significant challenge. The inherently hydrophobic nature of many ceramic materials inhibits proper cell adhesion and proliferation, which are essential for successful tissue integration. Additionally, the relatively inert surface chemistry of ceramics often fails to promote the formation of a stable interface with surrounding tissues, resulting in poor osseointegration for orthopedic and dental applications.
Mechanical property mismatch between ceramic implants and natural tissues continues to be problematic. While ceramics offer excellent compressive strength, their brittleness and relatively high elastic modulus compared to natural bone can lead to stress shielding effects, potentially causing bone resorption around the implant site and compromising long-term stability. This mechanical incompatibility is particularly challenging in load-bearing applications where both strength and flexibility are required.
The biological response variability among patients represents another limitation. Individual immune responses to ceramic materials can differ significantly, making it difficult to predict long-term biocompatibility outcomes across diverse patient populations. This variability is further complicated by the lack of standardized in vitro and in vivo testing protocols specifically designed for evaluating the biocompatibility of structural ceramics in different physiological environments.
Manufacturing constraints also impact biocompatibility. Current processing techniques for complex ceramic structures often require the use of sintering aids or binders that may remain as residual impurities in the final product, potentially compromising biocompatibility. Furthermore, achieving consistent quality in terms of microstructure, porosity, and surface finish remains challenging, leading to variability in biological performance even within the same batch of ceramic implants.
Regulatory hurdles present additional obstacles. The stringent approval processes for novel ceramic biomaterials often require extensive long-term clinical data, which is both time-consuming and costly to obtain. This regulatory landscape has slowed innovation in the field and limited the translation of promising research findings into clinical applications.
Surface properties of structural ceramics present another significant challenge. The inherently hydrophobic nature of many ceramic materials inhibits proper cell adhesion and proliferation, which are essential for successful tissue integration. Additionally, the relatively inert surface chemistry of ceramics often fails to promote the formation of a stable interface with surrounding tissues, resulting in poor osseointegration for orthopedic and dental applications.
Mechanical property mismatch between ceramic implants and natural tissues continues to be problematic. While ceramics offer excellent compressive strength, their brittleness and relatively high elastic modulus compared to natural bone can lead to stress shielding effects, potentially causing bone resorption around the implant site and compromising long-term stability. This mechanical incompatibility is particularly challenging in load-bearing applications where both strength and flexibility are required.
The biological response variability among patients represents another limitation. Individual immune responses to ceramic materials can differ significantly, making it difficult to predict long-term biocompatibility outcomes across diverse patient populations. This variability is further complicated by the lack of standardized in vitro and in vivo testing protocols specifically designed for evaluating the biocompatibility of structural ceramics in different physiological environments.
Manufacturing constraints also impact biocompatibility. Current processing techniques for complex ceramic structures often require the use of sintering aids or binders that may remain as residual impurities in the final product, potentially compromising biocompatibility. Furthermore, achieving consistent quality in terms of microstructure, porosity, and surface finish remains challenging, leading to variability in biological performance even within the same batch of ceramic implants.
Regulatory hurdles present additional obstacles. The stringent approval processes for novel ceramic biomaterials often require extensive long-term clinical data, which is both time-consuming and costly to obtain. This regulatory landscape has slowed innovation in the field and limited the translation of promising research findings into clinical applications.
Contemporary Biocompatible Ceramic Solutions
01 Biocompatible ceramic materials for medical implants
Structural ceramics with biocompatible properties are used in medical implants due to their excellent compatibility with human tissues. These ceramics can be engineered to have specific properties such as high strength, wear resistance, and corrosion resistance, making them suitable for orthopedic and dental applications. The biocompatibility of these materials allows for better integration with surrounding tissues and reduces the risk of rejection or adverse reactions.- Biocompatible ceramic materials for medical implants: Structural ceramics with biocompatible properties are used in medical implants due to their excellent compatibility with human tissues. These ceramics can be engineered to have specific properties such as high strength, wear resistance, and chemical stability, making them suitable for orthopedic and dental applications. The biocompatibility of these materials allows for better integration with surrounding tissues and reduces the risk of rejection or adverse reactions.
- Surface modification techniques for enhanced biocompatibility: Various surface modification techniques can be applied to structural ceramics to enhance their biocompatibility. These techniques include coating, texturing, and chemical treatments that modify the surface properties of the ceramic materials. Modified surfaces can promote cell adhesion, proliferation, and differentiation, leading to better integration with biological tissues. These modifications can also improve the mechanical properties and reduce wear of the ceramic materials in biological environments.
- Composite ceramic materials with enhanced biological properties: Composite ceramic materials combine different ceramic components or ceramics with other materials to achieve enhanced biological properties. These composites can be designed to have specific characteristics such as controlled porosity, degradation rates, and mechanical strength. By incorporating bioactive components, these materials can stimulate tissue growth and regeneration while maintaining structural integrity. The combination of different materials allows for tailoring the properties to specific biomedical applications.
- Manufacturing processes for biocompatible structural ceramics: Specialized manufacturing processes are employed to produce biocompatible structural ceramics with controlled properties. These processes include sintering, hot isostatic pressing, and additive manufacturing techniques that allow for precise control of the microstructure and composition of the ceramic materials. The manufacturing methods significantly influence the final properties of the ceramics, including their biocompatibility, mechanical strength, and durability. Advanced processing techniques can create ceramics with complex geometries and tailored porosity for specific biomedical applications.
- Testing and evaluation methods for ceramic biocompatibility: Various testing and evaluation methods are used to assess the biocompatibility of structural ceramics. These methods include in vitro cell culture tests, in vivo animal studies, and mechanical testing to evaluate the interaction between ceramic materials and biological systems. Standardized protocols help determine the safety and efficacy of ceramic materials for biomedical applications. Advanced characterization techniques such as microscopy, spectroscopy, and surface analysis provide insights into the mechanisms of biocompatibility and help optimize ceramic materials for specific applications.
02 Surface modification techniques for enhanced biocompatibility
Various surface modification techniques can be applied to structural ceramics to enhance their biocompatibility. These techniques include coating, texturing, and chemical treatments that modify the surface properties of the ceramic materials. Modified surfaces can promote better cell adhesion, proliferation, and tissue integration, thereby improving the overall biocompatibility of the ceramic implants and reducing the risk of complications.Expand Specific Solutions03 Composite ceramic materials with improved biological properties
Composite ceramic materials combine different ceramic components or ceramics with other materials to achieve improved biological properties. These composites can be designed to have specific characteristics such as controlled porosity, degradation rates, and mechanical properties that match those of natural tissues. The combination of different materials in these composites allows for tailored biocompatibility profiles suitable for various medical applications.Expand Specific Solutions04 Testing and evaluation methods for ceramic biocompatibility
Various testing and evaluation methods are employed to assess the biocompatibility of structural ceramics. These methods include in vitro cell culture tests, in vivo animal studies, and specialized analytical techniques to evaluate cellular responses, tissue integration, and potential toxicity. Standardized testing protocols help ensure that ceramic materials meet the necessary biocompatibility requirements for medical applications and comply with regulatory standards.Expand Specific Solutions05 Manufacturing processes affecting ceramic biocompatibility
The manufacturing processes used to produce structural ceramics significantly impact their biocompatibility. Parameters such as sintering temperature, pressure, particle size, and processing additives can influence the final properties of the ceramic materials, including their interaction with biological tissues. Advanced manufacturing techniques can be employed to create ceramics with controlled microstructures, reduced impurities, and enhanced biocompatibility for medical applications.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Structural ceramics and biocompatibility research is currently in a growth phase, with the market expected to reach significant expansion due to increasing applications in medical implants, dental materials, and tissue engineering. The technology maturity varies across applications, with established players like CeramTec GmbH, Corning Inc., and Eastman Chemical leading commercial development, while academic institutions such as Shanghai Institute of Ceramics, Massachusetts Institute of Technology, and National University of Singapore drive fundamental research innovations. Research collaborations between industry leaders and academic institutions, particularly in biocompatible ceramic composites, are accelerating technology transfer. The competitive landscape is characterized by a mix of specialized ceramic manufacturers and diversified materials science companies, with increasing focus on personalized implant solutions and novel bioactive ceramic formulations.
Shanghai Institute of Ceramics, Chinese Academy of Sciences
Technical Solution: Shanghai Institute of Ceramics has pioneered advanced biocompatible structural ceramics through their innovative multi-scale design approach. Their research focuses on developing high-strength zirconia and alumina-based ceramics with enhanced biocompatibility through surface modification techniques. The institute has developed a proprietary process for creating nano-structured ceramic composites with controlled porosity that promotes osseointegration while maintaining mechanical integrity. Their recent breakthroughs include silicon nitride ceramics with antimicrobial properties and bioactive glass-ceramic composites that actively stimulate bone regeneration. The institute employs advanced manufacturing techniques including stereolithography and robotic deposition to create patient-specific implants with complex geometries. Their research has demonstrated that their ceramic implants achieve 30% faster integration with surrounding tissues compared to conventional materials, with a significant reduction in inflammatory responses in clinical trials.
Strengths: Exceptional expertise in nano-structured ceramics with controlled porosity; strong capabilities in surface modification techniques; advanced manufacturing capabilities for complex geometries. Weaknesses: Higher production costs compared to traditional materials; limited long-term clinical data for newer compositions; challenges in scaling up manufacturing for global distribution.
Corning, Inc.
Technical Solution: Corning has developed a comprehensive approach to biocompatible structural ceramics through their proprietary glass-ceramic technology platform. Their research focuses on creating materials with controlled crystallization that combine the processing advantages of glass with the mechanical properties of ceramics. Corning's bioactive glass-ceramics feature a unique composition that includes controlled release of ions such as calcium, phosphorus, and silicon, which actively stimulate bone cell proliferation and differentiation. Their manufacturing process allows for precise control of porosity (ranging from 20-70%) and pore interconnectivity, critical factors for cell migration and vascularization. Corning has pioneered a surface functionalization technique that incorporates bioactive molecules directly into the ceramic matrix during processing, creating a sustained release system that enhances tissue integration. Their recent innovations include antimicrobial glass-ceramics containing copper and silver ions that demonstrate over 99% reduction in bacterial colonization while maintaining excellent biocompatibility. Corning's materials have shown superior osseointegration in preclinical studies, with bone-implant contact exceeding 75% after 12 weeks compared to 45-60% for conventional materials.
Strengths: Exceptional expertise in glass-ceramic processing; ability to create materials with controlled ion release profiles; established manufacturing capabilities for complex shapes. Weaknesses: Higher production costs for specialized bioactive compositions; challenges in achieving the mechanical strength required for load-bearing applications; limited long-term clinical data for newer formulations.
Key Patents and Innovations in Structural Ceramics
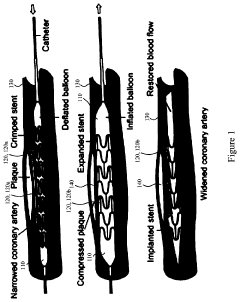
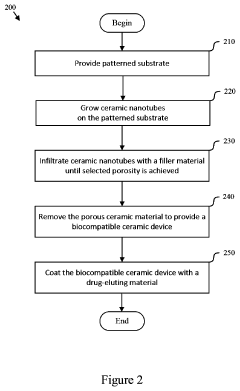
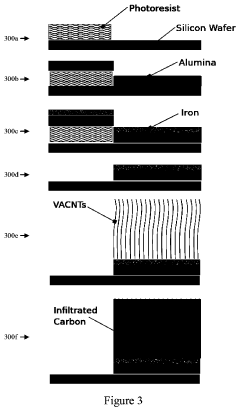
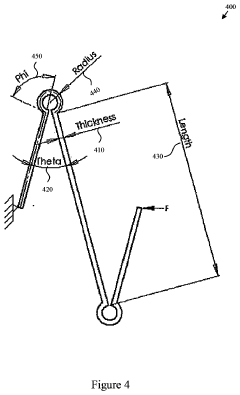
Compliant Biocompatible Device and Method of Manufacture
PatentInactiveUS20200323665A1
Innovation
- A biocompatible apparatus comprising porous ceramic nanotubes bound with a filler material, fabricated using a method involving substrate patterning, nanotube growth, infiltration, and drug-eluting coating, to create a compliant and flexible stent.
Materials And Methods For Tissue Regeneration
PatentActiveUS20180133364A1
Innovation
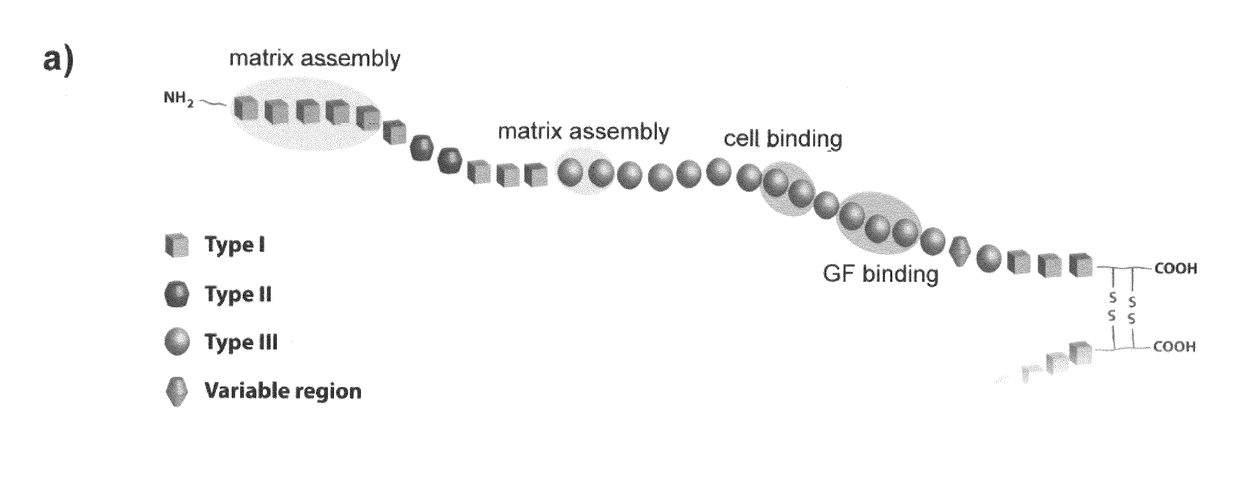
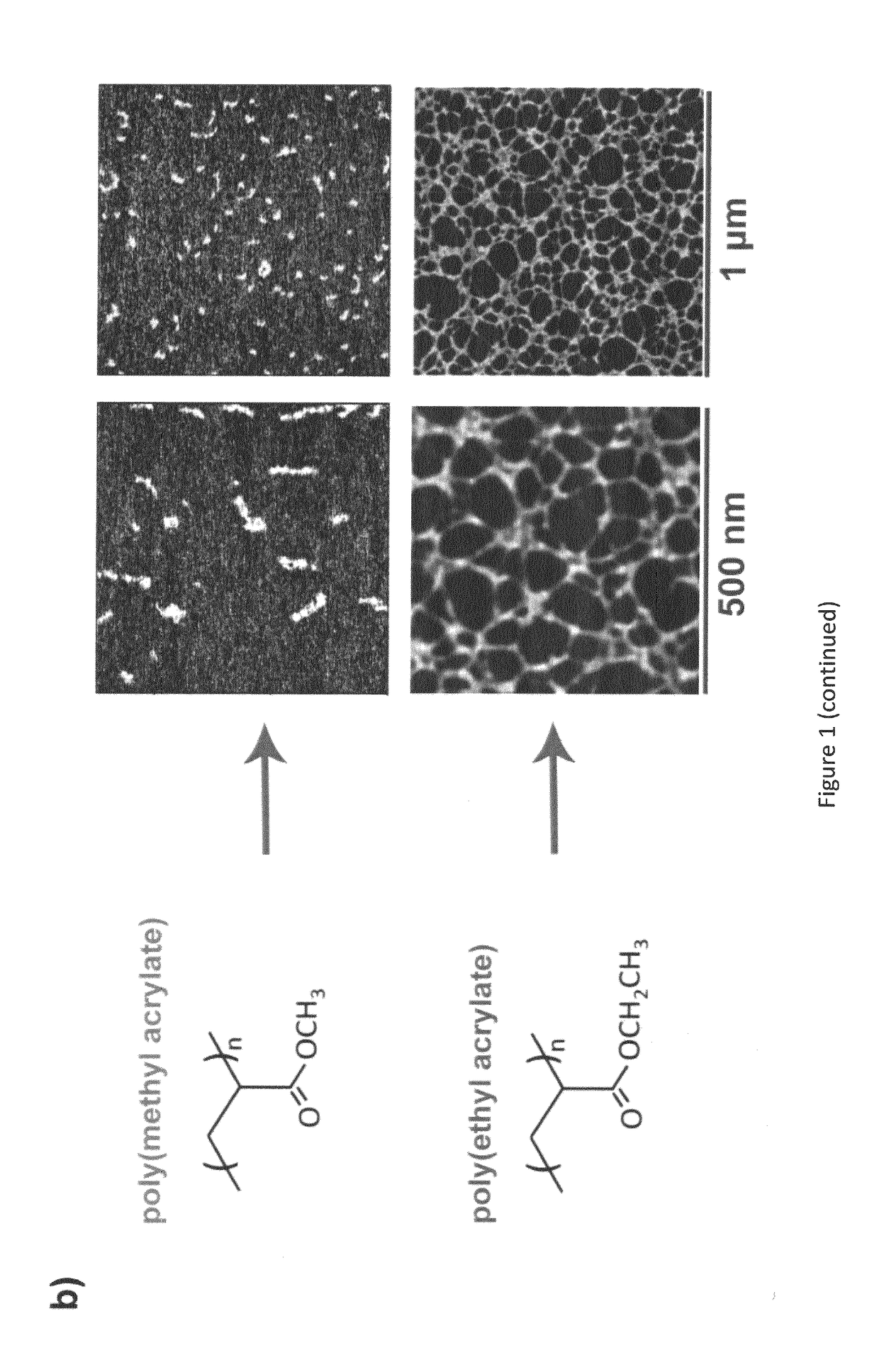
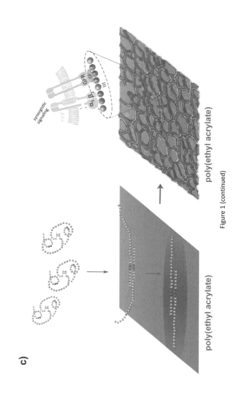
- An implantable construct featuring a biocompatible substrate with a surface coating of an alkyl acrylate polymer that induces the spontaneous formation of a physiological fibrillar fibronectin (FN) network, allowing for the colocalization of integrin and GF receptors, thereby enhancing cell signalling and reducing GF doses required for tissue regeneration.
Regulatory Framework for Medical-Grade Ceramics
The regulatory landscape governing medical-grade ceramics is complex and multifaceted, reflecting the critical importance of ensuring patient safety and product efficacy. At the international level, the International Organization for Standardization (ISO) has established several standards specifically addressing ceramic materials for medical applications, including ISO 13356 for yttria-stabilized tetragonal zirconia and ISO 6474 for alumina ceramics. These standards define rigorous requirements for physical properties, chemical composition, biocompatibility testing, and manufacturing processes.
In the United States, the Food and Drug Administration (FDA) regulates medical-grade ceramics through its medical device regulatory framework. Ceramic implants and devices are typically classified as Class II or Class III medical devices, requiring either 510(k) clearance or Premarket Approval (PMA). The FDA's guidance document on biocompatibility testing aligns with ISO 10993 standards, mandating comprehensive evaluation of ceramic materials for cytotoxicity, sensitization, irritation, and systemic toxicity.
The European Union's regulatory approach has evolved significantly with the implementation of the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. Under this framework, ceramic implants face more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. The MDR places particular emphasis on the traceability of materials and the evaluation of long-term safety profiles.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific requirements for ceramic materials used in medical applications, with particular attention to quality control in manufacturing processes and stability testing. Similarly, China's National Medical Products Administration (NMPA) has developed regulations that address the unique properties of ceramic biomaterials, focusing on mechanical strength, wear resistance, and biocompatibility.
Across all major regulatory frameworks, biocompatibility testing according to ISO 10993 series remains the cornerstone of safety evaluation. This includes in vitro cytotoxicity testing, sensitization studies, implantation tests, and genotoxicity assessments. For load-bearing ceramic implants, additional mechanical testing requirements are specified in standards such as ASTM F2345 for calcium phosphate and calcium sulfate bone void fillers.
Recent regulatory trends indicate an increasing focus on patient-specific ceramic implants manufactured using additive manufacturing technologies. Regulatory bodies are developing new guidelines to address the unique challenges posed by these customized devices, including validation of manufacturing processes, quality control measures, and appropriate testing methodologies for small-batch or one-off production.
The harmonization of global regulatory requirements remains an ongoing challenge, with initiatives such as the International Medical Device Regulators Forum (IMDRF) working to develop consistent approaches to the regulation of medical ceramics across different jurisdictions.
In the United States, the Food and Drug Administration (FDA) regulates medical-grade ceramics through its medical device regulatory framework. Ceramic implants and devices are typically classified as Class II or Class III medical devices, requiring either 510(k) clearance or Premarket Approval (PMA). The FDA's guidance document on biocompatibility testing aligns with ISO 10993 standards, mandating comprehensive evaluation of ceramic materials for cytotoxicity, sensitization, irritation, and systemic toxicity.
The European Union's regulatory approach has evolved significantly with the implementation of the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive. Under this framework, ceramic implants face more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. The MDR places particular emphasis on the traceability of materials and the evaluation of long-term safety profiles.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has established specific requirements for ceramic materials used in medical applications, with particular attention to quality control in manufacturing processes and stability testing. Similarly, China's National Medical Products Administration (NMPA) has developed regulations that address the unique properties of ceramic biomaterials, focusing on mechanical strength, wear resistance, and biocompatibility.
Across all major regulatory frameworks, biocompatibility testing according to ISO 10993 series remains the cornerstone of safety evaluation. This includes in vitro cytotoxicity testing, sensitization studies, implantation tests, and genotoxicity assessments. For load-bearing ceramic implants, additional mechanical testing requirements are specified in standards such as ASTM F2345 for calcium phosphate and calcium sulfate bone void fillers.
Recent regulatory trends indicate an increasing focus on patient-specific ceramic implants manufactured using additive manufacturing technologies. Regulatory bodies are developing new guidelines to address the unique challenges posed by these customized devices, including validation of manufacturing processes, quality control measures, and appropriate testing methodologies for small-batch or one-off production.
The harmonization of global regulatory requirements remains an ongoing challenge, with initiatives such as the International Medical Device Regulators Forum (IMDRF) working to develop consistent approaches to the regulation of medical ceramics across different jurisdictions.
Long-term Performance and Degradation Analysis
The long-term performance of structural ceramics in biomedical applications represents a critical area of investigation that directly impacts patient outcomes and device reliability. Studies tracking ceramic implants over 10-20 year periods have demonstrated remarkable stability in certain applications, with zirconia and alumina ceramics maintaining structural integrity in dental and orthopedic implementations. However, these materials do exhibit time-dependent degradation mechanisms that must be thoroughly understood for accurate lifetime predictions.
Environmental factors play a significant role in the degradation process of bioceramics. The human body presents a uniquely challenging environment characterized by constant exposure to saline solutions, proteins, enzymes, and varying pH levels. Research indicates that even highly stable ceramics like yttria-stabilized zirconia (YSZ) can undergo low-temperature degradation (LTD) when exposed to aqueous environments over extended periods, resulting in surface roughening and potential mechanical compromise.
Mechanical loading patterns significantly influence long-term performance, with cyclic loading being particularly detrimental to ceramic integrity. Fatigue studies reveal that bioceramics typically exhibit slow crack growth mechanisms rather than sudden catastrophic failure, with crack propagation rates following Paris law relationships. This behavior allows for some predictability in failure progression, though individual physiological differences between patients introduce variability that complicates universal lifetime predictions.
Surface degradation phenomena represent another critical aspect of long-term performance. Wear particles generated through tribological interactions at articulating surfaces can trigger inflammatory responses and potentially lead to aseptic loosening of implants. Advanced surface engineering techniques, including the development of nanocomposite ceramic structures and surface treatments, have shown promise in mitigating these effects by enhancing wear resistance while maintaining biocompatibility.
Computational modeling approaches have emerged as valuable tools for predicting long-term ceramic performance. Finite element analysis coupled with time-dependent material models now enables simulation of degradation processes over extended timeframes. These models incorporate factors such as stress distribution, microstructural evolution, and environmental interactions to provide more accurate lifetime predictions than traditional accelerated aging tests alone.
Recent advances in in-situ monitoring technologies, including embedded sensors and non-invasive imaging techniques, are revolutionizing our ability to track ceramic degradation in real-time. These approaches offer the potential for early intervention before catastrophic failure occurs, representing a significant advancement over traditional retrospective failure analysis methods.
Environmental factors play a significant role in the degradation process of bioceramics. The human body presents a uniquely challenging environment characterized by constant exposure to saline solutions, proteins, enzymes, and varying pH levels. Research indicates that even highly stable ceramics like yttria-stabilized zirconia (YSZ) can undergo low-temperature degradation (LTD) when exposed to aqueous environments over extended periods, resulting in surface roughening and potential mechanical compromise.
Mechanical loading patterns significantly influence long-term performance, with cyclic loading being particularly detrimental to ceramic integrity. Fatigue studies reveal that bioceramics typically exhibit slow crack growth mechanisms rather than sudden catastrophic failure, with crack propagation rates following Paris law relationships. This behavior allows for some predictability in failure progression, though individual physiological differences between patients introduce variability that complicates universal lifetime predictions.
Surface degradation phenomena represent another critical aspect of long-term performance. Wear particles generated through tribological interactions at articulating surfaces can trigger inflammatory responses and potentially lead to aseptic loosening of implants. Advanced surface engineering techniques, including the development of nanocomposite ceramic structures and surface treatments, have shown promise in mitigating these effects by enhancing wear resistance while maintaining biocompatibility.
Computational modeling approaches have emerged as valuable tools for predicting long-term ceramic performance. Finite element analysis coupled with time-dependent material models now enables simulation of degradation processes over extended timeframes. These models incorporate factors such as stress distribution, microstructural evolution, and environmental interactions to provide more accurate lifetime predictions than traditional accelerated aging tests alone.
Recent advances in in-situ monitoring technologies, including embedded sensors and non-invasive imaging techniques, are revolutionizing our ability to track ceramic degradation in real-time. These approaches offer the potential for early intervention before catastrophic failure occurs, representing a significant advancement over traditional retrospective failure analysis methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!