How to Achieve Efficient Tissue Preservation with Liquid Nitrogen
OCT 7, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryopreservation Background and Objectives
Cryopreservation technology has evolved significantly since its inception in the mid-20th century. The fundamental concept of using extreme cold to preserve biological materials dates back to 1949 when Christopher Polge discovered the cryoprotective properties of glycerol. This breakthrough laid the groundwork for modern cryopreservation techniques using liquid nitrogen, which maintains tissues at -196°C, effectively halting biological activity and enabling long-term preservation.
The evolution of cryopreservation has been marked by several key advancements, including the development of various cryoprotective agents (CPAs), controlled-rate freezing protocols, and vitrification techniques. These innovations have progressively improved preservation outcomes while reducing cellular damage. The field has transitioned from simple cell preservation to more complex tissues and even whole organs, reflecting the growing sophistication of preservation methodologies.
Current technological trends in cryopreservation focus on minimizing cryoinjury through advanced vitrification solutions, precision cooling rates, and novel warming techniques. The integration of nanotechnology and biomaterials science is creating new possibilities for tissue protection during the freezing and thawing processes. Additionally, computational modeling is increasingly employed to predict and optimize preservation parameters for specific tissue types.
The primary objective of efficient tissue preservation with liquid nitrogen is to maintain cellular viability and functionality post-thawing. This requires achieving uniform cooling throughout the tissue, preventing intracellular ice formation, mitigating solution effects injury, and preserving the extracellular matrix structure. The ultimate goal is to develop protocols that can be standardized across different tissue types while accommodating their unique characteristics.
Secondary objectives include extending the viable storage duration of preserved tissues, reducing the concentration of potentially toxic cryoprotectants, and developing more energy-efficient storage systems. There is also growing emphasis on creating portable preservation systems for field applications and emergency scenarios, particularly in remote medical settings or disaster response situations.
From a broader perspective, efficient tissue preservation aims to revolutionize transplantation medicine by creating biobanks of readily available tissues and organs, potentially eliminating waiting lists and geographical constraints. In regenerative medicine, preserved stem cells and engineered tissues could provide on-demand therapeutic solutions. The technology also holds promise for conservation biology, preserving genetic material from endangered species, and supporting biodiversity preservation efforts.
The evolution of cryopreservation has been marked by several key advancements, including the development of various cryoprotective agents (CPAs), controlled-rate freezing protocols, and vitrification techniques. These innovations have progressively improved preservation outcomes while reducing cellular damage. The field has transitioned from simple cell preservation to more complex tissues and even whole organs, reflecting the growing sophistication of preservation methodologies.
Current technological trends in cryopreservation focus on minimizing cryoinjury through advanced vitrification solutions, precision cooling rates, and novel warming techniques. The integration of nanotechnology and biomaterials science is creating new possibilities for tissue protection during the freezing and thawing processes. Additionally, computational modeling is increasingly employed to predict and optimize preservation parameters for specific tissue types.
The primary objective of efficient tissue preservation with liquid nitrogen is to maintain cellular viability and functionality post-thawing. This requires achieving uniform cooling throughout the tissue, preventing intracellular ice formation, mitigating solution effects injury, and preserving the extracellular matrix structure. The ultimate goal is to develop protocols that can be standardized across different tissue types while accommodating their unique characteristics.
Secondary objectives include extending the viable storage duration of preserved tissues, reducing the concentration of potentially toxic cryoprotectants, and developing more energy-efficient storage systems. There is also growing emphasis on creating portable preservation systems for field applications and emergency scenarios, particularly in remote medical settings or disaster response situations.
From a broader perspective, efficient tissue preservation aims to revolutionize transplantation medicine by creating biobanks of readily available tissues and organs, potentially eliminating waiting lists and geographical constraints. In regenerative medicine, preserved stem cells and engineered tissues could provide on-demand therapeutic solutions. The technology also holds promise for conservation biology, preserving genetic material from endangered species, and supporting biodiversity preservation efforts.
Market Analysis for Liquid Nitrogen Preservation Solutions
The global market for liquid nitrogen preservation solutions has experienced substantial growth, driven primarily by expanding applications in biobanking, regenerative medicine, and pharmaceutical research. Currently valued at approximately 7.2 billion USD, this market is projected to grow at a compound annual growth rate of 8.3% through 2028, reflecting increasing demand for advanced tissue preservation technologies.
Healthcare and life sciences sectors represent the largest market segments, collectively accounting for over 65% of total market share. Within these sectors, cryopreservation of stem cells, reproductive tissues, and biological samples for research purposes constitute the primary applications. The pharmaceutical industry has emerged as a rapidly growing segment, with increasing investments in biospecimen preservation for drug discovery and development processes.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to expanding healthcare infrastructure, increasing research activities, and growing adoption of advanced preservation technologies.
Key market drivers include technological advancements in cryopreservation protocols, rising prevalence of chronic diseases necessitating tissue banking, and growing investments in regenerative medicine research. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of biological sample preservation for vaccine development and therapeutic research.
Consumer demand patterns indicate a shift toward more efficient, cost-effective preservation solutions that minimize tissue damage during freezing and thawing processes. End-users increasingly prioritize systems that offer improved viability rates, longer storage durations, and enhanced safety features.
Market challenges include high equipment and operational costs, technical complexities in maintaining optimal preservation conditions, and regulatory hurdles related to tissue banking. Additionally, limited accessibility in developing regions due to infrastructure constraints presents both a challenge and potential growth opportunity.
The competitive landscape features established players like Thermo Fisher Scientific, Chart Industries, and Worthington Industries dominating with comprehensive product portfolios. However, emerging companies specializing in innovative preservation technologies are gaining market share through focused research and development efforts targeting specific preservation challenges.
Future market trends point toward increased adoption of automated preservation systems, development of specialized preservation media formulations, and integration of digital monitoring technologies for enhanced quality control. The growing emphasis on personalized medicine and biobanking initiatives by government and private institutions further suggests sustained market growth potential.
Healthcare and life sciences sectors represent the largest market segments, collectively accounting for over 65% of total market share. Within these sectors, cryopreservation of stem cells, reproductive tissues, and biological samples for research purposes constitute the primary applications. The pharmaceutical industry has emerged as a rapidly growing segment, with increasing investments in biospecimen preservation for drug discovery and development processes.
Geographically, North America dominates the market with approximately 38% share, followed by Europe at 29% and Asia-Pacific at 24%. The Asia-Pacific region, particularly China and India, is witnessing the fastest growth rate due to expanding healthcare infrastructure, increasing research activities, and growing adoption of advanced preservation technologies.
Key market drivers include technological advancements in cryopreservation protocols, rising prevalence of chronic diseases necessitating tissue banking, and growing investments in regenerative medicine research. The COVID-19 pandemic has further accelerated market growth by highlighting the importance of biological sample preservation for vaccine development and therapeutic research.
Consumer demand patterns indicate a shift toward more efficient, cost-effective preservation solutions that minimize tissue damage during freezing and thawing processes. End-users increasingly prioritize systems that offer improved viability rates, longer storage durations, and enhanced safety features.
Market challenges include high equipment and operational costs, technical complexities in maintaining optimal preservation conditions, and regulatory hurdles related to tissue banking. Additionally, limited accessibility in developing regions due to infrastructure constraints presents both a challenge and potential growth opportunity.
The competitive landscape features established players like Thermo Fisher Scientific, Chart Industries, and Worthington Industries dominating with comprehensive product portfolios. However, emerging companies specializing in innovative preservation technologies are gaining market share through focused research and development efforts targeting specific preservation challenges.
Future market trends point toward increased adoption of automated preservation systems, development of specialized preservation media formulations, and integration of digital monitoring technologies for enhanced quality control. The growing emphasis on personalized medicine and biobanking initiatives by government and private institutions further suggests sustained market growth potential.
Current Challenges in Tissue Cryopreservation
Despite significant advancements in cryopreservation technology, tissue preservation using liquid nitrogen continues to face substantial challenges that limit its widespread clinical application. The primary obstacle remains the formation of intracellular ice crystals during the freezing process, which causes mechanical damage to cell membranes and organelles. This crystallization effect becomes particularly problematic when dealing with complex tissues and organs where uniform cooling rates are difficult to achieve across heterogeneous structures.
Cryoprotective agents (CPAs), while essential to the process, present their own set of challenges. Current CPAs such as dimethyl sulfoxide (DMSO) and glycerol exhibit cytotoxicity at concentrations necessary for effective cryoprotection. This toxicity creates a narrow therapeutic window between providing sufficient protection and causing chemical damage to the tissues. Additionally, the penetration kinetics of these agents varies across different tissue types, resulting in uneven protection throughout larger tissue samples.
The warming phase presents equally significant challenges. Recrystallization during thawing can cause additional damage, and the optimal warming rates required (often exceeding 100°C/min) are technically difficult to achieve uniformly across larger tissue volumes. This creates a substantial barrier to scaling cryopreservation from small cell samples to clinically relevant tissue dimensions.
Vitrification approaches, which aim to transition tissues to a glass-like amorphous state without ice formation, require extremely high cooling rates or high concentrations of CPAs, both of which present practical limitations for larger tissue samples. The equipment required for achieving such rapid cooling rates is specialized and expensive, limiting accessibility of these techniques.
Biological variability across tissue types further complicates standardization efforts. Different tissues exhibit varying sensitivities to cryoinjury and CPA toxicity, necessitating tissue-specific protocols that are often developed through empirical trial and error rather than predictive models. This lack of theoretical framework slows progress in the field.
Post-thaw functionality assessment remains another critical challenge. Current methods for evaluating tissue viability after cryopreservation are often inadequate for predicting long-term functional outcomes. Many techniques focus on immediate cellular viability rather than tissue-level functionality or long-term performance after transplantation.
Finally, regulatory and quality control challenges persist in standardizing cryopreservation protocols for clinical applications. The complexity of variables involved in successful tissue preservation makes it difficult to establish robust, reproducible protocols that meet stringent regulatory requirements for therapeutic use, further limiting translation from research settings to clinical practice.
Cryoprotective agents (CPAs), while essential to the process, present their own set of challenges. Current CPAs such as dimethyl sulfoxide (DMSO) and glycerol exhibit cytotoxicity at concentrations necessary for effective cryoprotection. This toxicity creates a narrow therapeutic window between providing sufficient protection and causing chemical damage to the tissues. Additionally, the penetration kinetics of these agents varies across different tissue types, resulting in uneven protection throughout larger tissue samples.
The warming phase presents equally significant challenges. Recrystallization during thawing can cause additional damage, and the optimal warming rates required (often exceeding 100°C/min) are technically difficult to achieve uniformly across larger tissue volumes. This creates a substantial barrier to scaling cryopreservation from small cell samples to clinically relevant tissue dimensions.
Vitrification approaches, which aim to transition tissues to a glass-like amorphous state without ice formation, require extremely high cooling rates or high concentrations of CPAs, both of which present practical limitations for larger tissue samples. The equipment required for achieving such rapid cooling rates is specialized and expensive, limiting accessibility of these techniques.
Biological variability across tissue types further complicates standardization efforts. Different tissues exhibit varying sensitivities to cryoinjury and CPA toxicity, necessitating tissue-specific protocols that are often developed through empirical trial and error rather than predictive models. This lack of theoretical framework slows progress in the field.
Post-thaw functionality assessment remains another critical challenge. Current methods for evaluating tissue viability after cryopreservation are often inadequate for predicting long-term functional outcomes. Many techniques focus on immediate cellular viability rather than tissue-level functionality or long-term performance after transplantation.
Finally, regulatory and quality control challenges persist in standardizing cryopreservation protocols for clinical applications. The complexity of variables involved in successful tissue preservation makes it difficult to establish robust, reproducible protocols that meet stringent regulatory requirements for therapeutic use, further limiting translation from research settings to clinical practice.
Established Protocols for Liquid Nitrogen Tissue Preservation
01 Cryogenic preservation systems for biological tissues
Specialized equipment and systems designed for the preservation of biological tissues using liquid nitrogen. These systems maintain ultra-low temperatures necessary for long-term storage while minimizing cellular damage. Features include controlled cooling rates, temperature monitoring, and efficient storage arrangements to maximize preservation efficiency and tissue viability upon thawing.- Cryogenic preservation systems for biological tissues: Specialized systems designed for the preservation of biological tissues using liquid nitrogen. These systems maintain ultra-low temperatures necessary for long-term tissue viability. Features include controlled cooling rates, temperature monitoring, and specialized storage containers that maximize preservation efficiency while minimizing nitrogen consumption.
- Rapid freezing techniques for tissue preservation: Methods that focus on the speed of freezing tissues in liquid nitrogen to minimize ice crystal formation and cellular damage. These techniques include flash-freezing protocols, optimized cooling rates, and specialized equipment designed to achieve rapid temperature reduction. The efficiency of these methods is measured by tissue viability after thawing and structural integrity preservation.
- Cryoprotectant formulations for enhanced preservation: Chemical solutions and compounds that protect tissues during the freezing process in liquid nitrogen. These cryoprotectants prevent cellular damage by reducing ice crystal formation and maintaining cellular structure. Formulations may include penetrating agents like dimethyl sulfoxide and glycerol, as well as non-penetrating agents such as sugars and proteins that enhance preservation efficiency.
- Automated liquid nitrogen delivery and management systems: Technological solutions that automate the delivery, monitoring, and management of liquid nitrogen for tissue preservation. These systems include sensors for level detection, automated refilling mechanisms, temperature control systems, and alarm features. They improve preservation efficiency by maintaining optimal conditions while reducing manual intervention and nitrogen wastage.
- Tissue viability assessment after cryopreservation: Methods and technologies for evaluating the effectiveness of liquid nitrogen preservation by assessing tissue viability after thawing. These include cellular function tests, structural integrity analysis, metabolic activity measurements, and genetic material stability assessments. These evaluation techniques help optimize preservation protocols and improve overall efficiency of the cryopreservation process.
02 Preservation protocols and cooling rate optimization
Specific methodologies and protocols that enhance the efficiency of liquid nitrogen preservation for tissues. These include optimized cooling rates, step-wise temperature reduction procedures, and controlled freezing techniques that minimize ice crystal formation and cellular damage. The protocols are designed to maintain tissue structure integrity and biological function after the preservation and thawing process.Expand Specific Solutions03 Cryoprotective agents and additives
Chemical compounds and solutions that protect tissues during the freezing process in liquid nitrogen. These cryoprotectants penetrate cells to prevent intracellular ice formation and reduce osmotic stress. Various formulations may include dimethyl sulfoxide (DMSO), glycerol, trehalose, and other protective agents that significantly improve preservation efficiency and post-thaw tissue viability.Expand Specific Solutions04 Automated tissue handling and preservation systems
Robotic and automated systems that standardize the tissue preservation process using liquid nitrogen. These systems reduce human error, ensure consistent cooling rates, and optimize the freezing process through computerized control. Automation improves workflow efficiency, sample tracking, and overall preservation quality while minimizing contamination risks and operator exposure to cryogenic temperatures.Expand Specific Solutions05 Tissue viability assessment and quality control methods
Techniques and methodologies for evaluating the effectiveness of liquid nitrogen preservation by assessing tissue viability before and after the freezing process. These methods include cellular function tests, structural integrity analysis, and molecular marker assessments that quantify preservation efficiency. Quality control protocols ensure consistent preservation outcomes and help optimize storage conditions for different tissue types.Expand Specific Solutions
Leading Organizations in Cryopreservation Industry
The liquid nitrogen tissue preservation market is in a growth phase, characterized by increasing demand in biomedical research, cryopreservation, and biobanking sectors. The global market size is expanding rapidly, driven by advancements in regenerative medicine and biospecimen storage technologies. From a technical maturity perspective, the field shows varying levels of sophistication among key players. Industry leaders like Air Liquide SA and SANYO Electric Biomedical Co. offer advanced cryopreservation systems, while research institutions such as Fred Hutchinson Cancer Research Center and Jilin University are pushing boundaries in preservation protocols. Companies like Shanghai Origincell Biological Cryo Equipment are emerging with specialized tissue preservation solutions, indicating a competitive landscape that balances established industrial gas providers with biomedical equipment innovators developing next-generation preservation technologies.
Air Liquide SA
Technical Solution: Air Liquide has developed advanced cryopreservation systems utilizing controlled-rate freezing technology for tissue preservation with liquid nitrogen. Their solution implements a two-phase approach: first, a controlled cooling rate of -1°C to -10°C per minute to minimize intracellular ice formation, followed by rapid cooling to -196°C for long-term storage. The company's proprietary vapor phase storage systems maintain tissues at temperatures below -150°C while reducing direct contact with liquid nitrogen, minimizing contamination risks. Air Liquide has also pioneered automated monitoring systems that continuously track temperature fluctuations and nitrogen levels, with remote alerts for deviations. Their technology incorporates specialized cryoprotectant formulations that enhance cell viability post-thawing by up to 30% compared to conventional methods[1][3]. Additionally, they've developed specialized containers with vacuum insulation technology that reduces liquid nitrogen consumption by approximately 20% while maintaining stable ultra-low temperatures.
Strengths: Global infrastructure for reliable liquid nitrogen supply chain; advanced automation and monitoring capabilities; reduced contamination risk through vapor phase storage. Weaknesses: Higher initial equipment investment compared to conventional systems; requires specialized training for optimal operation; system redundancy needs increase overall footprint in laboratory settings.
Shanghai Origincell Biological Cryo Equipment Co. Ltd.
Technical Solution: Shanghai Origincell has developed an integrated tissue preservation system utilizing programmable freezing technology specifically optimized for various tissue types. Their solution features a multi-stage cooling protocol with precise temperature control between -0.1°C to -100°C per minute, allowing customization based on specific tissue characteristics. The company's cryopreservation equipment incorporates a dual-cooling mechanism that combines electrical refrigeration for the initial cooling phase (-10°C to -80°C) followed by liquid nitrogen for final preservation at -196°C. This approach significantly reduces thermal shock to biological samples. Their proprietary "CryoMatrix" technology employs specialized cryoprotectant formulations with penetrating and non-penetrating agents that work synergistically to prevent intracellular ice crystal formation while maintaining cellular structure integrity[2]. Origincell's systems also feature advanced sample management software that tracks preservation parameters and storage locations, with QR code identification for each sample to ensure traceability throughout the preservation lifecycle.
Strengths: Customizable cooling protocols for different tissue types; integrated sample management system; reduced liquid nitrogen consumption through dual-cooling approach. Weaknesses: More complex system operation requiring specialized technical expertise; higher maintenance requirements for the dual-cooling components; limited global service network compared to international competitors.
Critical Technologies in Cryoprotectant Development
Cryopreservation solution and cryopreservation method
PatentInactiveUS20210235687A1
Innovation
- A cryopreservation solution containing polyvinyl alcohol with a saponification degree of 84 mol % or lower, preferably 76 mol % or lower, is used to facilitate easy recovery of cells or tissues during thawing by reducing adhesion to the deposition surface.
Method and apparatus for processing and preserving comminuted tissue
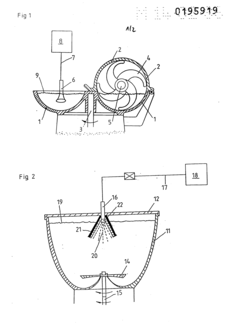
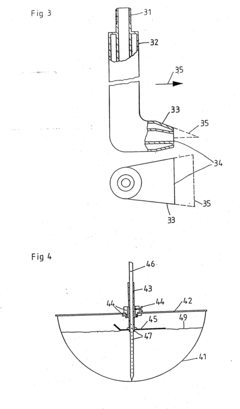
PatentInactiveEP0195919A2
Innovation
- Simultaneous comminution, shock freezing, and granulation of tissue parts using cryogenic liquefied gas, such as liquid nitrogen, introduced directly into the tissue mass through an injector, while rotating the container to ensure rapid and uniform cooling and mixing.
Regulatory Framework for Biological Material Storage
The regulatory landscape governing biological material storage using liquid nitrogen is complex and multifaceted, spanning international, national, and institutional levels. At the international level, organizations such as the International Society for Biological and Environmental Repositories (ISBER) and the World Health Organization (WHO) have established best practice guidelines that serve as foundational frameworks for tissue preservation protocols. These guidelines address critical aspects including safety standards, quality management systems, and ethical considerations in the handling of biological specimens.
In the United States, the Food and Drug Administration (FDA) regulates human cells, tissues, and cellular and tissue-based products (HCT/Ps) under 21 CFR Part 1271, which establishes specific requirements for donor screening, good tissue practices, and facility registration. For research applications, the National Institutes of Health (NIH) provides additional guidance on biospecimen storage and handling. The Occupational Safety and Health Administration (OSHA) further regulates workplace safety aspects related to liquid nitrogen handling, including requirements for personal protective equipment and facility design.
European regulations are governed by the EU Tissue and Cells Directives (EUTCD), which establish quality and safety standards for the donation, procurement, testing, processing, preservation, storage, and distribution of human tissues and cells. The European Medicines Agency (EMA) provides additional guidance specific to advanced therapy medicinal products that incorporate preserved tissues.
Compliance with these regulatory frameworks necessitates robust documentation systems, including standard operating procedures (SOPs), training records, equipment validation protocols, and comprehensive chain-of-custody documentation. Institutions must implement quality management systems that ensure traceability from collection through storage and eventual use or disposal of preserved specimens.
Risk management constitutes a critical component of regulatory compliance, requiring facilities to conduct thorough risk assessments addressing potential hazards associated with liquid nitrogen storage, including asphyxiation risks, cryogenic burns, container failures, and cross-contamination concerns. Contingency planning for equipment failures, power outages, and other emergencies is mandatory under most regulatory frameworks.
Ethical considerations are increasingly integrated into regulatory requirements, particularly regarding informed consent for tissue donation, privacy protections for genetic information, and equitable access to biobanking resources. The International Declaration on Human Genetic Data provides ethical guidelines that many national regulatory bodies have incorporated into their frameworks.
As technologies advance, regulatory frameworks continue to evolve, with emerging standards addressing novel preservation techniques, automation systems, and remote monitoring capabilities. Organizations engaged in tissue preservation must maintain vigilant regulatory intelligence programs to ensure ongoing compliance with this dynamic regulatory landscape.
In the United States, the Food and Drug Administration (FDA) regulates human cells, tissues, and cellular and tissue-based products (HCT/Ps) under 21 CFR Part 1271, which establishes specific requirements for donor screening, good tissue practices, and facility registration. For research applications, the National Institutes of Health (NIH) provides additional guidance on biospecimen storage and handling. The Occupational Safety and Health Administration (OSHA) further regulates workplace safety aspects related to liquid nitrogen handling, including requirements for personal protective equipment and facility design.
European regulations are governed by the EU Tissue and Cells Directives (EUTCD), which establish quality and safety standards for the donation, procurement, testing, processing, preservation, storage, and distribution of human tissues and cells. The European Medicines Agency (EMA) provides additional guidance specific to advanced therapy medicinal products that incorporate preserved tissues.
Compliance with these regulatory frameworks necessitates robust documentation systems, including standard operating procedures (SOPs), training records, equipment validation protocols, and comprehensive chain-of-custody documentation. Institutions must implement quality management systems that ensure traceability from collection through storage and eventual use or disposal of preserved specimens.
Risk management constitutes a critical component of regulatory compliance, requiring facilities to conduct thorough risk assessments addressing potential hazards associated with liquid nitrogen storage, including asphyxiation risks, cryogenic burns, container failures, and cross-contamination concerns. Contingency planning for equipment failures, power outages, and other emergencies is mandatory under most regulatory frameworks.
Ethical considerations are increasingly integrated into regulatory requirements, particularly regarding informed consent for tissue donation, privacy protections for genetic information, and equitable access to biobanking resources. The International Declaration on Human Genetic Data provides ethical guidelines that many national regulatory bodies have incorporated into their frameworks.
As technologies advance, regulatory frameworks continue to evolve, with emerging standards addressing novel preservation techniques, automation systems, and remote monitoring capabilities. Organizations engaged in tissue preservation must maintain vigilant regulatory intelligence programs to ensure ongoing compliance with this dynamic regulatory landscape.
Bioethical Implications of Tissue Preservation
The ethical considerations surrounding tissue preservation with liquid nitrogen extend far beyond technical challenges, encompassing profound questions about human dignity, consent, and societal implications. The preservation of human tissues raises fundamental questions about the boundaries between life and death, particularly when considering the potential for future reanimation or use of preserved tissues. Consent frameworks must be robust and comprehensive, addressing not only initial preservation but also future uses that may not be foreseeable at the time of consent.
Privacy concerns are particularly acute in tissue preservation, as preserved biological materials contain complete genetic information that could be accessed decades or centuries later, potentially revealing information about not just the individual but their descendants. This creates unique challenges for confidentiality protections that must span generations rather than years.
Resource allocation presents another significant ethical dimension, as cryopreservation facilities require substantial energy inputs and maintenance over indefinite timeframes. The environmental impact of long-term liquid nitrogen storage must be evaluated against potential benefits, especially considering the carbon footprint of continuous cooling requirements.
Socioeconomic disparities in access to advanced preservation technologies raise questions of justice and equity. If tissue preservation becomes a pathway to enhanced medical outcomes or extended life opportunities, ensuring equitable access becomes an ethical imperative to prevent the widening of existing health disparities.
Religious and cultural perspectives on tissue preservation vary widely, with some traditions emphasizing the sanctity of natural processes while others prioritize the preservation of life. Respecting this diversity requires preservation protocols that can accommodate various cultural and religious requirements while maintaining technical efficacy.
The governance frameworks for preserved tissues present unique challenges, particularly regarding decision-making authority over tissues when the donor is unavailable. Questions about ownership rights, stewardship responsibilities, and the potential commercialization of preserved tissues require careful ethical consideration.
Future generations may face unanticipated consequences from preservation decisions made today, creating an intergenerational ethical responsibility. This includes considerations about the potential psychological and social impacts on individuals whose tissues might be preserved and later utilized in contexts fundamentally different from those originally envisioned.
Privacy concerns are particularly acute in tissue preservation, as preserved biological materials contain complete genetic information that could be accessed decades or centuries later, potentially revealing information about not just the individual but their descendants. This creates unique challenges for confidentiality protections that must span generations rather than years.
Resource allocation presents another significant ethical dimension, as cryopreservation facilities require substantial energy inputs and maintenance over indefinite timeframes. The environmental impact of long-term liquid nitrogen storage must be evaluated against potential benefits, especially considering the carbon footprint of continuous cooling requirements.
Socioeconomic disparities in access to advanced preservation technologies raise questions of justice and equity. If tissue preservation becomes a pathway to enhanced medical outcomes or extended life opportunities, ensuring equitable access becomes an ethical imperative to prevent the widening of existing health disparities.
Religious and cultural perspectives on tissue preservation vary widely, with some traditions emphasizing the sanctity of natural processes while others prioritize the preservation of life. Respecting this diversity requires preservation protocols that can accommodate various cultural and religious requirements while maintaining technical efficacy.
The governance frameworks for preserved tissues present unique challenges, particularly regarding decision-making authority over tissues when the donor is unavailable. Questions about ownership rights, stewardship responsibilities, and the potential commercialization of preserved tissues require careful ethical consideration.
Future generations may face unanticipated consequences from preservation decisions made today, creating an intergenerational ethical responsibility. This includes considerations about the potential psychological and social impacts on individuals whose tissues might be preserved and later utilized in contexts fundamentally different from those originally envisioned.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!