Liquid Nitrogen vs Solid CO2 in Laboratory Settings: A Comparison
OCT 7, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryogenic Technology Background and Objectives
Cryogenic technology has evolved significantly since the early 20th century, with major breakthroughs occurring in the 1940s during the development of rocket propulsion systems and superconductivity research. The field has expanded from primarily industrial applications to become an essential component in modern laboratory settings, particularly in biological sample preservation, material testing, and various scientific experiments requiring ultra-low temperatures.
The evolution of cryogenic technology has been characterized by continuous improvements in insulation materials, storage vessels, and handling techniques. Early systems suffered from significant evaporation losses and safety concerns, while contemporary solutions offer enhanced efficiency, reduced maintenance requirements, and improved safety profiles. This progression has made cryogenic cooling increasingly accessible to research institutions of varying sizes and budgets.
Liquid nitrogen (LN2) and solid carbon dioxide (dry ice) represent two distinct approaches to laboratory cooling, each with unique thermodynamic properties. Liquid nitrogen, with its boiling point of -196°C, provides significantly lower temperatures than solid CO2, which sublimates at -78.5°C. This temperature differential creates distinct application niches for each substance across scientific disciplines.
The primary objective of this technical assessment is to comprehensively evaluate the comparative advantages, limitations, and optimal application scenarios for liquid nitrogen versus solid CO2 in contemporary laboratory environments. This analysis will consider factors including cooling efficiency, temperature stability, handling safety, infrastructure requirements, operational costs, and environmental impact.
Current technological trends indicate growing interest in hybrid cooling systems that leverage the complementary properties of both substances, as well as innovations in micro-scale cryogenic applications for specialized laboratory procedures. Additionally, there is increasing focus on developing more energy-efficient cryogenic systems with reduced carbon footprints, particularly important as research facilities face growing pressure to minimize environmental impact.
The global cryogenic technology market is projected to expand at a CAGR of approximately 6.5% through 2028, driven by increasing demand in healthcare, biotechnology, and materials science research. This growth trajectory suggests continued innovation in both liquid nitrogen and solid CO2 delivery systems, with particular emphasis on miniaturization, automation, and integration with digital laboratory management systems.
This assessment aims to provide actionable insights for laboratory managers, research directors, and procurement specialists seeking to optimize their cryogenic infrastructure investments while balancing performance requirements, operational constraints, and sustainability objectives.
The evolution of cryogenic technology has been characterized by continuous improvements in insulation materials, storage vessels, and handling techniques. Early systems suffered from significant evaporation losses and safety concerns, while contemporary solutions offer enhanced efficiency, reduced maintenance requirements, and improved safety profiles. This progression has made cryogenic cooling increasingly accessible to research institutions of varying sizes and budgets.
Liquid nitrogen (LN2) and solid carbon dioxide (dry ice) represent two distinct approaches to laboratory cooling, each with unique thermodynamic properties. Liquid nitrogen, with its boiling point of -196°C, provides significantly lower temperatures than solid CO2, which sublimates at -78.5°C. This temperature differential creates distinct application niches for each substance across scientific disciplines.
The primary objective of this technical assessment is to comprehensively evaluate the comparative advantages, limitations, and optimal application scenarios for liquid nitrogen versus solid CO2 in contemporary laboratory environments. This analysis will consider factors including cooling efficiency, temperature stability, handling safety, infrastructure requirements, operational costs, and environmental impact.
Current technological trends indicate growing interest in hybrid cooling systems that leverage the complementary properties of both substances, as well as innovations in micro-scale cryogenic applications for specialized laboratory procedures. Additionally, there is increasing focus on developing more energy-efficient cryogenic systems with reduced carbon footprints, particularly important as research facilities face growing pressure to minimize environmental impact.
The global cryogenic technology market is projected to expand at a CAGR of approximately 6.5% through 2028, driven by increasing demand in healthcare, biotechnology, and materials science research. This growth trajectory suggests continued innovation in both liquid nitrogen and solid CO2 delivery systems, with particular emphasis on miniaturization, automation, and integration with digital laboratory management systems.
This assessment aims to provide actionable insights for laboratory managers, research directors, and procurement specialists seeking to optimize their cryogenic infrastructure investments while balancing performance requirements, operational constraints, and sustainability objectives.
Market Analysis of Laboratory Cooling Solutions
The laboratory cooling solutions market has experienced significant growth in recent years, driven by expanding research activities across pharmaceutical, biotechnology, and academic sectors. The global market for laboratory cooling technologies was valued at approximately $3.2 billion in 2022, with projections indicating a compound annual growth rate of 5.7% through 2028. This growth trajectory is primarily fueled by increasing demand for precise temperature control in sensitive experimental procedures and sample preservation.
Liquid nitrogen and solid CO2 (dry ice) represent the two dominant cryogenic cooling solutions in laboratory settings, collectively accounting for over 65% of the laboratory cooling market. Liquid nitrogen holds the larger market share at roughly 40%, owing to its extremely low temperature capabilities (-196°C) that enable specialized applications in cell preservation, genetic material storage, and superconductivity research. The liquid nitrogen segment has shown consistent annual growth of 6.3% over the past five years.
Solid CO2, while commanding a smaller market share of approximately 25%, maintains strong demand due to its cost-effectiveness and ease of handling for short-term cooling needs. The dry ice market segment has grown at a more modest 4.1% annually, finding particular application in shipping biological samples, temporary specimen preservation, and flash-freezing procedures.
Regional analysis reveals distinct market patterns, with North America leading consumption at 38% of the global market, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate at 7.8% annually, driven by expanding research infrastructure in China, India, and South Korea. Emerging markets in Latin America and Africa collectively represent about 9% of global consumption but show promising growth potential as research capabilities expand in these regions.
End-user segmentation indicates that pharmaceutical and biotechnology companies constitute the largest consumer base (42%), followed by academic and research institutions (31%), healthcare facilities (18%), and other industrial applications (9%). The pharmaceutical sector's dominance stems from stringent regulatory requirements for sample integrity and the increasing focus on temperature-sensitive biologics and personalized medicine.
Price sensitivity varies significantly across market segments, with academic institutions demonstrating higher price sensitivity compared to pharmaceutical companies. This has created distinct market tiers, with premium solutions featuring advanced monitoring capabilities commanding price premiums of 30-40% over basic systems. Cost considerations have also driven the growth of rental and service-based business models, particularly for liquid nitrogen supply, which now represents approximately 22% of the market.
Liquid nitrogen and solid CO2 (dry ice) represent the two dominant cryogenic cooling solutions in laboratory settings, collectively accounting for over 65% of the laboratory cooling market. Liquid nitrogen holds the larger market share at roughly 40%, owing to its extremely low temperature capabilities (-196°C) that enable specialized applications in cell preservation, genetic material storage, and superconductivity research. The liquid nitrogen segment has shown consistent annual growth of 6.3% over the past five years.
Solid CO2, while commanding a smaller market share of approximately 25%, maintains strong demand due to its cost-effectiveness and ease of handling for short-term cooling needs. The dry ice market segment has grown at a more modest 4.1% annually, finding particular application in shipping biological samples, temporary specimen preservation, and flash-freezing procedures.
Regional analysis reveals distinct market patterns, with North America leading consumption at 38% of the global market, followed by Europe (29%) and Asia-Pacific (24%). The Asia-Pacific region demonstrates the fastest growth rate at 7.8% annually, driven by expanding research infrastructure in China, India, and South Korea. Emerging markets in Latin America and Africa collectively represent about 9% of global consumption but show promising growth potential as research capabilities expand in these regions.
End-user segmentation indicates that pharmaceutical and biotechnology companies constitute the largest consumer base (42%), followed by academic and research institutions (31%), healthcare facilities (18%), and other industrial applications (9%). The pharmaceutical sector's dominance stems from stringent regulatory requirements for sample integrity and the increasing focus on temperature-sensitive biologics and personalized medicine.
Price sensitivity varies significantly across market segments, with academic institutions demonstrating higher price sensitivity compared to pharmaceutical companies. This has created distinct market tiers, with premium solutions featuring advanced monitoring capabilities commanding price premiums of 30-40% over basic systems. Cost considerations have also driven the growth of rental and service-based business models, particularly for liquid nitrogen supply, which now represents approximately 22% of the market.
Current Challenges in Cryogenic Applications
Despite significant advancements in cryogenic technologies, several persistent challenges continue to impact the effective utilization of both liquid nitrogen and solid CO2 in laboratory settings. The primary challenge remains temperature control precision, particularly when maintaining samples at specific temperatures within narrow ranges. Liquid nitrogen, operating at -196°C, often provides excessive cooling for applications requiring temperatures between -80°C and -20°C, while solid CO2 at -78.5°C may not achieve sufficiently low temperatures for certain biological preservation protocols.
Storage and handling safety presents another significant challenge, with liquid nitrogen posing serious risks of cryogenic burns, asphyxiation in poorly ventilated areas, and potential pressure build-up in sealed containers. Solid CO2 similarly presents hazards through sublimation, creating high-pressure conditions in enclosed spaces and displacing oxygen, though its slightly higher temperature makes it somewhat less dangerous to handle directly.
Cost efficiency and accessibility vary significantly by geographic location. While liquid nitrogen requires specialized vacuum-insulated dewars and regular replenishment due to continuous evaporation, solid CO2 sublimates more rapidly, necessitating more frequent replacement. In remote research facilities, the supply chain logistics for both cryogens remain problematic, with liquid nitrogen requiring specialized delivery infrastructure.
Energy consumption during production represents a growing concern as laboratories seek to reduce their carbon footprint. The liquefaction of nitrogen is particularly energy-intensive, requiring approximately 0.5 kWh per liter, while solid CO2 production, often a byproduct of industrial processes, may have a lower direct energy cost but higher transportation energy requirements.
Cross-contamination risks persist in both systems. Open liquid nitrogen containers can experience atmospheric condensation, potentially introducing contaminants, while solid CO2 may carry manufacturing impurities that could affect sensitive experimental results.
Equipment compatibility issues continue to challenge researchers, as many laboratory instruments are designed for specific cryogenic environments. The rapid temperature transitions when transferring samples between ambient conditions and cryogenic storage can cause thermal shock, potentially compromising sample integrity, particularly with fragile biological specimens.
Measurement and monitoring technologies remain inadequate for real-time temperature profiling throughout cryogenic processes, creating blind spots in quality assurance protocols. This is particularly problematic for pharmaceutical and biological research applications where temperature excursions can invalidate results or damage valuable samples.
Storage and handling safety presents another significant challenge, with liquid nitrogen posing serious risks of cryogenic burns, asphyxiation in poorly ventilated areas, and potential pressure build-up in sealed containers. Solid CO2 similarly presents hazards through sublimation, creating high-pressure conditions in enclosed spaces and displacing oxygen, though its slightly higher temperature makes it somewhat less dangerous to handle directly.
Cost efficiency and accessibility vary significantly by geographic location. While liquid nitrogen requires specialized vacuum-insulated dewars and regular replenishment due to continuous evaporation, solid CO2 sublimates more rapidly, necessitating more frequent replacement. In remote research facilities, the supply chain logistics for both cryogens remain problematic, with liquid nitrogen requiring specialized delivery infrastructure.
Energy consumption during production represents a growing concern as laboratories seek to reduce their carbon footprint. The liquefaction of nitrogen is particularly energy-intensive, requiring approximately 0.5 kWh per liter, while solid CO2 production, often a byproduct of industrial processes, may have a lower direct energy cost but higher transportation energy requirements.
Cross-contamination risks persist in both systems. Open liquid nitrogen containers can experience atmospheric condensation, potentially introducing contaminants, while solid CO2 may carry manufacturing impurities that could affect sensitive experimental results.
Equipment compatibility issues continue to challenge researchers, as many laboratory instruments are designed for specific cryogenic environments. The rapid temperature transitions when transferring samples between ambient conditions and cryogenic storage can cause thermal shock, potentially compromising sample integrity, particularly with fragile biological specimens.
Measurement and monitoring technologies remain inadequate for real-time temperature profiling throughout cryogenic processes, creating blind spots in quality assurance protocols. This is particularly problematic for pharmaceutical and biological research applications where temperature excursions can invalidate results or damage valuable samples.
Technical Comparison of LN2 and Solid CO2 Systems
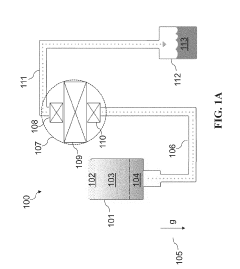
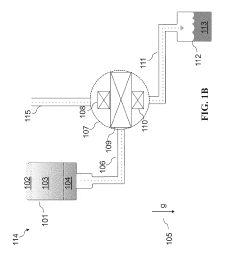
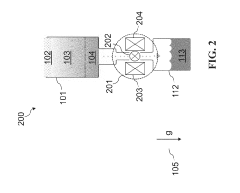
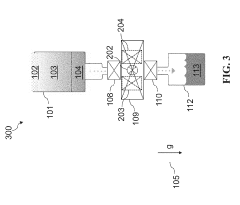
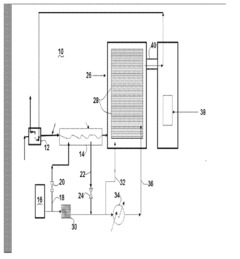
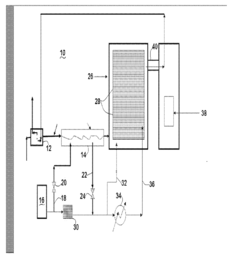
01 Cryogenic cooling systems using liquid nitrogen and solid CO2
Cryogenic cooling systems utilize liquid nitrogen and solid CO2 (dry ice) as refrigerants for achieving ultra-low temperatures. These systems are designed for various industrial applications requiring rapid cooling or maintaining extremely low temperatures. The combination of these cryogens offers efficient heat transfer properties and can be implemented in cascade refrigeration systems where different temperature stages are required.- Cryogenic cooling systems using liquid nitrogen and solid CO2: Cryogenic cooling systems utilize liquid nitrogen and solid CO2 (dry ice) as refrigerants for various industrial applications. These systems leverage the extremely low temperatures of these substances to achieve rapid cooling effects. Liquid nitrogen, with its boiling point of -196°C, provides deeper cooling capabilities, while solid CO2 sublimating at -78.5°C offers a more moderate cooling option. These systems are designed with specialized insulation and controlled release mechanisms to maintain temperature stability and efficiency.
- Gas separation and purification processes: Liquid nitrogen and solid CO2 are utilized in various gas separation and purification processes. Cryogenic distillation using liquid nitrogen enables the separation of air components and other gas mixtures based on different boiling points. Solid CO2 is employed in processes like carbon capture, where it helps in the separation and sequestration of carbon dioxide from industrial emissions. These techniques leverage the temperature and phase change properties of both substances to achieve high purity levels in the separated gases.
- Medical and biological preservation applications: Liquid nitrogen and solid CO2 play crucial roles in medical and biological preservation. Liquid nitrogen is widely used in cryopreservation of biological samples, cells, tissues, and reproductive materials at ultra-low temperatures (-196°C). Solid CO2 provides a less extreme but effective preservation environment at -78.5°C, suitable for short-term storage and transportation of biological materials. These cryogenic agents help maintain the viability and integrity of sensitive biological specimens by slowing down metabolic processes and preventing degradation.
- Industrial cleaning and surface treatment: Cryogenic cleaning and surface treatment methods employ liquid nitrogen and solid CO2 for various applications. Dry ice blasting uses accelerated solid CO2 particles to clean surfaces without chemicals or residues, as the CO2 sublimates upon impact. Liquid nitrogen is utilized for extreme cooling in certain surface hardening processes and for removing unwanted materials through thermal shock. These environmentally friendly cleaning methods are particularly valuable for sensitive equipment, food processing facilities, and historical preservation where traditional cleaning agents might cause damage.
- Enhanced oil recovery and well stimulation: Liquid nitrogen and solid CO2 are employed in enhanced oil recovery and well stimulation techniques. CO2 injection, often in its solid or liquid-to-supercritical state, helps maintain reservoir pressure and improves oil flow characteristics by reducing viscosity. Liquid nitrogen is used for creating fractures in formations through thermal shock and for displacing oxygen in wells to prevent combustion. These cryogenic fluids provide environmentally preferable alternatives to chemical treatments while improving hydrocarbon recovery rates from reservoirs.
02 Gas separation and purification processes
Liquid nitrogen and solid CO2 are utilized in gas separation and purification processes. These cryogenic substances facilitate the separation of gas mixtures through temperature-controlled phase changes, allowing for the selective condensation or solidification of different components. This technology is particularly valuable in industrial gas production, carbon capture systems, and purification of process streams where high purity gases are required.Expand Specific Solutions03 Food freezing and preservation applications
The use of liquid nitrogen and solid CO2 in food processing enables rapid freezing that preserves food quality by forming smaller ice crystals. These cryogenic freezing methods minimize cellular damage and nutrient loss while extending shelf life. The systems can be designed as continuous or batch processes for various food products, offering advantages over conventional freezing methods in terms of speed, efficiency, and product quality maintenance.Expand Specific Solutions04 Medical and laboratory cryopreservation techniques
Liquid nitrogen and solid CO2 are essential in medical and laboratory cryopreservation techniques for biological samples, tissues, and cells. These cryogenic agents provide controlled cooling rates necessary for preserving biological materials without damaging cellular structures. The technology includes specialized storage containers, handling systems, and temperature monitoring devices that maintain sample integrity during long-term storage at ultra-low temperatures.Expand Specific Solutions05 Industrial cleaning and surface treatment processes
Cryogenic cleaning and surface treatment processes utilize the properties of liquid nitrogen and solid CO2 for non-abrasive removal of contaminants from surfaces. Solid CO2 blasting (dry ice blasting) provides a non-toxic, residue-free cleaning method that works through thermal shock and kinetic impact. These techniques are particularly valuable for sensitive equipment, historical artifacts, and manufacturing equipment where traditional cleaning methods might cause damage or require lengthy downtime.Expand Specific Solutions
Major Suppliers and Manufacturers in Cryogenics
The laboratory cryogenics market is in a mature growth phase, with an estimated global value exceeding $2 billion. Liquid nitrogen and solid CO2 technologies represent complementary solutions with different application profiles. Air Liquide SA dominates the industrial gas sector, while specialized equipment manufacturers like Mayekawa MFG Co. focus on cryogenic systems development. Research institutions including Korea Institute of Energy Research, Japan Science & Technology Agency, and Council of Scientific & Industrial Research are advancing cryogenic applications through collaborative innovation. The technology landscape shows liquid nitrogen systems achieving higher technological maturity for ultra-low temperature applications, while solid CO2 solutions offer cost advantages and accessibility for moderate cooling needs, creating a segmented market where both technologies maintain distinct value propositions.
Air Liquide SA
Technical Solution: Air Liquide has developed comprehensive cryogenic solutions for laboratory settings comparing liquid nitrogen (LN2) and solid CO2 (dry ice). Their technology focuses on specialized cryogenic equipment that optimizes the delivery and usage of both cooling agents. For liquid nitrogen, they've engineered vacuum-insulated storage dewars with advanced phase separators that minimize evaporation losses to less than 1% per day, significantly improving efficiency compared to conventional systems. Their automated LN2 delivery systems maintain precise temperature control at -196°C with fluctuations of less than 0.5°C, critical for sensitive biological sample preservation. For solid CO2 applications, Air Liquide has developed proprietary pelletizers that produce standardized dry ice pellets with consistent density (1.5-1.6 g/cm³) and sublimation rates, addressing the historical variability issues in dry ice quality. Their comparative studies demonstrate that LN2 systems achieve 40% higher cooling rates than CO2-based alternatives, while their CO2 recovery systems capture and recycle up to 60% of used CO2, reducing operational costs and environmental impact.
Strengths: Air Liquide's solutions offer superior temperature stability with LN2 systems and improved sustainability through CO2 recovery technology. Their global supply chain ensures reliable access to both cooling agents. Weaknesses: Their LN2 systems require significant initial infrastructure investment and specialized handling protocols, while their dry ice solutions still face challenges with transport logistics and variable sublimation rates in different environments.
Texas Tech University System
Technical Solution: Texas Tech University System has conducted extensive research comparing liquid nitrogen and solid CO2 cooling methods in laboratory settings, particularly focusing on biospecimen preservation and materials science applications. Their research team developed a dual-cooling platform that allows direct comparison of both cryogens under identical experimental conditions. Their findings indicate that liquid nitrogen provides more consistent ultra-low temperature maintenance (-196°C) with temperature stability within ±0.3°C over extended periods, while solid CO2 systems (-78.5°C) showed temperature fluctuations of up to ±2.5°C under similar conditions. The university's cryogenic laboratory has engineered specialized containment vessels with advanced vacuum insulation that reduces LN2 evaporation rates by approximately 35% compared to standard dewars, extending holding times significantly. For solid CO2 applications, they've developed composite insulation materials that slow sublimation rates by up to 40% compared to conventional polystyrene containers. Their comparative analysis demonstrates that while LN2 systems require more complex safety protocols due to asphyxiation and cold burn risks, they offer superior temperature uniformity and are more cost-effective for long-term storage applications, with operational costs approximately 25% lower than equivalent CO2 systems when used continuously.
Strengths: Texas Tech's research provides empirical data on temperature stability differences between the two cooling methods and has developed improved containment technologies for both cryogens. Their dual-platform testing methodology allows for direct comparative analysis under identical conditions. Weaknesses: Their research has primarily focused on laboratory-scale applications and may not fully address industrial-scale implementation challenges. The improved containment systems still require specialized handling and training.
Key Patents and Innovations in Cryogenic Technology
Apparatus and method for separating liquid oxygen from liquified air
PatentActiveUS20190169026A1
Innovation
- The use of a strong magnetic field and gravity to separate LOX from liquefied air based on the different magnetic properties of LOX and liquid nitrogen (LN2), potentially combined with a leak valve system, allowing for the selective levitation of LN2 and acceleration of LOX, thereby eliminating the need for evaporation and achieving ultra-pure LOX without the risks associated with evaporation.
Liquid nitrogen (LIN) integrated lyophilization system for minimizing a carbon footprint
PatentActiveUS20170328634A1
Innovation
- The system replaces hot oil with liquid nitrogen or similar heat transfer media, allowing for external freezing of products before lyophilization, reducing the need for rotating parts and chamber cooling, and utilizing nitrogen for both freezing and sublimation processes to control ice crystal formation and enhance drying efficiency.
Safety Protocols and Risk Management
Safety protocols for handling liquid nitrogen and solid CO2 (dry ice) differ significantly due to their distinct physical properties and associated hazards. Liquid nitrogen, with its extremely low temperature of -196°C, presents severe frostbite risks upon skin contact, potentially causing tissue damage within seconds. Proper personal protective equipment (PPE) including cryogenic gloves, face shields, and lab coats is mandatory when handling liquid nitrogen to prevent cold burns and tissue damage.
Oxygen displacement represents a critical concern with both substances. In confined spaces, both nitrogen and carbon dioxide can rapidly displace oxygen, creating asphyxiation hazards. However, liquid nitrogen poses a greater risk due to its 1:694 liquid-to-gas expansion ratio, which can rapidly deplete oxygen levels in poorly ventilated areas. Laboratories must install oxygen level monitors and implement comprehensive ventilation systems when using either cryogen.
Storage requirements differ substantially between these substances. Liquid nitrogen necessitates specialized vacuum-insulated Dewar flasks to minimize evaporation, while solid CO2 requires insulated containers that allow for controlled sublimation. Both storage systems must include pressure relief mechanisms to prevent dangerous pressure buildup that could lead to container rupture or explosion.
Transportation protocols within laboratory settings require dedicated pathways and elevator usage restrictions. Many institutions prohibit transporting liquid nitrogen in passenger elevators due to asphyxiation risks in case of entrapment during a power failure. Solid CO2, while presenting similar concerns, typically has less stringent transportation requirements due to its slower sublimation rate.
Emergency response procedures must address specific incidents related to each substance. For liquid nitrogen spills, protocols focus on rapid evacuation, ventilation, and specialized first aid for cryogenic burns. For solid CO2 incidents, procedures emphasize carbon dioxide monitoring and respiratory support. Both require detailed documentation systems for near-misses and incidents to facilitate continuous improvement of safety protocols.
Training requirements constitute a fundamental component of risk management for both substances. Laboratory personnel must receive comprehensive training on proper handling techniques, emergency procedures, and recognition of hazard signs. Certification and regular refresher courses should be mandatory, with special emphasis on practical demonstrations rather than theoretical knowledge alone.
Oxygen displacement represents a critical concern with both substances. In confined spaces, both nitrogen and carbon dioxide can rapidly displace oxygen, creating asphyxiation hazards. However, liquid nitrogen poses a greater risk due to its 1:694 liquid-to-gas expansion ratio, which can rapidly deplete oxygen levels in poorly ventilated areas. Laboratories must install oxygen level monitors and implement comprehensive ventilation systems when using either cryogen.
Storage requirements differ substantially between these substances. Liquid nitrogen necessitates specialized vacuum-insulated Dewar flasks to minimize evaporation, while solid CO2 requires insulated containers that allow for controlled sublimation. Both storage systems must include pressure relief mechanisms to prevent dangerous pressure buildup that could lead to container rupture or explosion.
Transportation protocols within laboratory settings require dedicated pathways and elevator usage restrictions. Many institutions prohibit transporting liquid nitrogen in passenger elevators due to asphyxiation risks in case of entrapment during a power failure. Solid CO2, while presenting similar concerns, typically has less stringent transportation requirements due to its slower sublimation rate.
Emergency response procedures must address specific incidents related to each substance. For liquid nitrogen spills, protocols focus on rapid evacuation, ventilation, and specialized first aid for cryogenic burns. For solid CO2 incidents, procedures emphasize carbon dioxide monitoring and respiratory support. Both require detailed documentation systems for near-misses and incidents to facilitate continuous improvement of safety protocols.
Training requirements constitute a fundamental component of risk management for both substances. Laboratory personnel must receive comprehensive training on proper handling techniques, emergency procedures, and recognition of hazard signs. Certification and regular refresher courses should be mandatory, with special emphasis on practical demonstrations rather than theoretical knowledge alone.
Cost-Benefit Analysis and ROI Considerations
When evaluating the economic viability of liquid nitrogen versus solid CO2 (dry ice) in laboratory settings, initial acquisition costs represent only one dimension of the financial equation. Liquid nitrogen typically requires a higher upfront investment in specialized storage dewars and handling equipment, with prices ranging from $500 to $3,000 for standard laboratory dewars. In contrast, solid CO2 storage containers are generally less expensive, typically costing between $200 and $1,000, representing a lower barrier to entry for smaller laboratories.
Operational costs reveal significant differences between these cryogenic options. Liquid nitrogen, priced at approximately $0.50 to $2.00 per liter depending on location and supplier relationships, offers superior cooling efficiency per dollar when considering its lower temperature capability (-196°C vs -78.5°C for dry ice). Solid CO2 costs approximately $1.00 to $3.00 per kilogram, with the added consideration that it sublimates completely, requiring regular replenishment.
Infrastructure requirements further differentiate the return on investment profiles. Laboratories utilizing liquid nitrogen must factor in the cost of specialized plumbing and ventilation systems to mitigate asphyxiation risks, potentially adding $5,000 to $15,000 to initial setup costs. These safety systems require regular maintenance and certification, adding approximately $1,000 to $2,000 annually to operational expenses. Dry ice presents fewer infrastructure demands but incurs higher long-term costs due to more frequent replenishment and less efficient cooling capacity.
The ROI calculation must incorporate sample preservation efficacy and research outcome value. Liquid nitrogen's lower temperature provides superior preservation for sensitive biological materials, potentially reducing sample loss rates by 15-20% compared to dry ice. For laboratories working with high-value specimens or time-sensitive research, this preservation advantage can translate to thousands or even millions of dollars in protected research investments.
Energy efficiency considerations favor liquid nitrogen in high-volume applications. Modern liquid nitrogen generation systems can achieve efficiency rates of 0.4-0.7 kWh per liter of liquid nitrogen produced, representing significant cost advantages for facilities with consistent, high-volume cryogenic needs. Conversely, smaller laboratories with intermittent cooling requirements may find solid CO2 more economically viable due to lower infrastructure commitments and the ability to purchase only what is immediately needed.
The break-even analysis typically shows that liquid nitrogen becomes more economically advantageous at approximately 18-24 months of regular use in medium to large laboratory settings, while solid CO2 maintains cost advantages for smaller operations or those with sporadic cryogenic requirements. This timeline can shift significantly based on regional energy costs, supplier agreements, and specific application requirements.
Operational costs reveal significant differences between these cryogenic options. Liquid nitrogen, priced at approximately $0.50 to $2.00 per liter depending on location and supplier relationships, offers superior cooling efficiency per dollar when considering its lower temperature capability (-196°C vs -78.5°C for dry ice). Solid CO2 costs approximately $1.00 to $3.00 per kilogram, with the added consideration that it sublimates completely, requiring regular replenishment.
Infrastructure requirements further differentiate the return on investment profiles. Laboratories utilizing liquid nitrogen must factor in the cost of specialized plumbing and ventilation systems to mitigate asphyxiation risks, potentially adding $5,000 to $15,000 to initial setup costs. These safety systems require regular maintenance and certification, adding approximately $1,000 to $2,000 annually to operational expenses. Dry ice presents fewer infrastructure demands but incurs higher long-term costs due to more frequent replenishment and less efficient cooling capacity.
The ROI calculation must incorporate sample preservation efficacy and research outcome value. Liquid nitrogen's lower temperature provides superior preservation for sensitive biological materials, potentially reducing sample loss rates by 15-20% compared to dry ice. For laboratories working with high-value specimens or time-sensitive research, this preservation advantage can translate to thousands or even millions of dollars in protected research investments.
Energy efficiency considerations favor liquid nitrogen in high-volume applications. Modern liquid nitrogen generation systems can achieve efficiency rates of 0.4-0.7 kWh per liter of liquid nitrogen produced, representing significant cost advantages for facilities with consistent, high-volume cryogenic needs. Conversely, smaller laboratories with intermittent cooling requirements may find solid CO2 more economically viable due to lower infrastructure commitments and the ability to purchase only what is immediately needed.
The break-even analysis typically shows that liquid nitrogen becomes more economically advantageous at approximately 18-24 months of regular use in medium to large laboratory settings, while solid CO2 maintains cost advantages for smaller operations or those with sporadic cryogenic requirements. This timeline can shift significantly based on regional energy costs, supplier agreements, and specific application requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!