How to Address HPLC System Contamination Issues
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Contamination Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical laboratories. The technology has progressed from basic isocratic systems to sophisticated ultra-high-performance instruments capable of separating complex mixtures with exceptional resolution and sensitivity. Despite these advancements, contamination remains one of the most persistent challenges affecting HPLC system performance and reliability.
System contamination in HPLC manifests through various symptoms including ghost peaks, baseline drift, pressure fluctuations, and reduced column efficiency. These issues compromise data integrity, method reproducibility, and ultimately the validity of analytical results. The economic impact of contamination extends beyond compromised data to include increased downtime, reduced instrument lifespan, higher maintenance costs, and wasted sample and solvent resources.
The evolution of HPLC technology has paradoxically made contamination issues more critical. As detection limits have decreased to parts-per-billion or even parts-per-trillion levels, even minute contaminants can significantly impact results. Modern HPLC systems with their intricate flow paths, specialized detectors, and high-pressure capabilities are particularly vulnerable to contamination effects that might have been negligible in earlier, less sensitive systems.
Contamination sources in HPLC systems are diverse and often interconnected. They include mobile phase impurities, sample matrix effects, microbial growth, particulate matter, leachables from system components, carryover from previous injections, and environmental contaminants. The complexity of these sources necessitates a comprehensive approach to contamination management rather than isolated troubleshooting efforts.
The primary objective of addressing HPLC contamination is to establish robust preventive protocols and effective remediation strategies that maintain system integrity across diverse analytical applications. This includes developing systematic approaches to identify contamination sources, implementing preventive maintenance procedures, optimizing system cleaning protocols, and establishing quality control measures to detect contamination before it affects analytical results.
Secondary objectives include quantifying the impact of different contamination types on various detector technologies, evaluating the effectiveness of different cleaning agents and procedures, developing predictive models for contamination risk assessment, and creating standardized protocols for contamination management that can be implemented across different laboratory environments and HPLC applications.
The ultimate goal is to transform HPLC contamination management from a reactive troubleshooting exercise to a proactive quality assurance component, thereby enhancing analytical reliability, extending system longevity, reducing operational costs, and improving laboratory productivity.
System contamination in HPLC manifests through various symptoms including ghost peaks, baseline drift, pressure fluctuations, and reduced column efficiency. These issues compromise data integrity, method reproducibility, and ultimately the validity of analytical results. The economic impact of contamination extends beyond compromised data to include increased downtime, reduced instrument lifespan, higher maintenance costs, and wasted sample and solvent resources.
The evolution of HPLC technology has paradoxically made contamination issues more critical. As detection limits have decreased to parts-per-billion or even parts-per-trillion levels, even minute contaminants can significantly impact results. Modern HPLC systems with their intricate flow paths, specialized detectors, and high-pressure capabilities are particularly vulnerable to contamination effects that might have been negligible in earlier, less sensitive systems.
Contamination sources in HPLC systems are diverse and often interconnected. They include mobile phase impurities, sample matrix effects, microbial growth, particulate matter, leachables from system components, carryover from previous injections, and environmental contaminants. The complexity of these sources necessitates a comprehensive approach to contamination management rather than isolated troubleshooting efforts.
The primary objective of addressing HPLC contamination is to establish robust preventive protocols and effective remediation strategies that maintain system integrity across diverse analytical applications. This includes developing systematic approaches to identify contamination sources, implementing preventive maintenance procedures, optimizing system cleaning protocols, and establishing quality control measures to detect contamination before it affects analytical results.
Secondary objectives include quantifying the impact of different contamination types on various detector technologies, evaluating the effectiveness of different cleaning agents and procedures, developing predictive models for contamination risk assessment, and creating standardized protocols for contamination management that can be implemented across different laboratory environments and HPLC applications.
The ultimate goal is to transform HPLC contamination management from a reactive troubleshooting exercise to a proactive quality assurance component, thereby enhancing analytical reliability, extending system longevity, reducing operational costs, and improving laboratory productivity.
Market Demand for Contamination-Free HPLC Analysis
The global market for High-Performance Liquid Chromatography (HPLC) systems continues to expand rapidly, with contamination-free analysis emerging as a critical demand driver across multiple industries. The worldwide HPLC market, valued at approximately 4.5 billion USD in 2022, is projected to grow at a compound annual growth rate of 5.8% through 2028, with contamination control solutions representing a significant growth segment.
Pharmaceutical and biotechnology sectors constitute the largest market segment demanding contamination-free HPLC analysis, accounting for nearly 60% of the total market share. These industries face stringent regulatory requirements from agencies like the FDA and EMA, which mandate precise analytical results for drug approval and quality control processes. Even minor contamination issues can lead to costly product recalls, regulatory penalties, and damaged brand reputation.
Clinical diagnostics represents another rapidly growing sector, where HPLC systems are increasingly utilized for analyzing biological samples. The accuracy of disease diagnosis and treatment monitoring depends heavily on contamination-free analysis. Healthcare providers are willing to invest in premium solutions that guarantee reliable results and minimize system downtime caused by contamination issues.
Environmental monitoring agencies and food safety laboratories form a substantial market segment with growing demand for contamination-free HPLC systems. These organizations require highly sensitive analysis of trace contaminants in environmental and food samples, where system contamination can lead to false positives or negatives with serious public health implications.
Market research indicates that end-users are increasingly prioritizing total cost of ownership over initial purchase price when selecting HPLC systems. Laboratories are willing to pay premium prices for systems with advanced contamination prevention features, as they recognize the long-term cost savings from reduced downtime, fewer failed analyses, and extended column life.
Regional market analysis reveals that North America and Europe currently lead in adoption of contamination-resistant HPLC technologies, while Asia-Pacific represents the fastest-growing market segment with increasing quality control requirements in developing economies. China and India, in particular, are experiencing rapid growth in pharmaceutical manufacturing and environmental monitoring sectors.
Customer surveys consistently highlight contamination prevention as a top-three purchasing consideration for new HPLC systems. End-users specifically demand solutions addressing carryover issues, ghost peaks, baseline drift, and system clogging. There is particular market interest in automated cleaning protocols, advanced filtration systems, and materials resistant to chemical degradation.
Pharmaceutical and biotechnology sectors constitute the largest market segment demanding contamination-free HPLC analysis, accounting for nearly 60% of the total market share. These industries face stringent regulatory requirements from agencies like the FDA and EMA, which mandate precise analytical results for drug approval and quality control processes. Even minor contamination issues can lead to costly product recalls, regulatory penalties, and damaged brand reputation.
Clinical diagnostics represents another rapidly growing sector, where HPLC systems are increasingly utilized for analyzing biological samples. The accuracy of disease diagnosis and treatment monitoring depends heavily on contamination-free analysis. Healthcare providers are willing to invest in premium solutions that guarantee reliable results and minimize system downtime caused by contamination issues.
Environmental monitoring agencies and food safety laboratories form a substantial market segment with growing demand for contamination-free HPLC systems. These organizations require highly sensitive analysis of trace contaminants in environmental and food samples, where system contamination can lead to false positives or negatives with serious public health implications.
Market research indicates that end-users are increasingly prioritizing total cost of ownership over initial purchase price when selecting HPLC systems. Laboratories are willing to pay premium prices for systems with advanced contamination prevention features, as they recognize the long-term cost savings from reduced downtime, fewer failed analyses, and extended column life.
Regional market analysis reveals that North America and Europe currently lead in adoption of contamination-resistant HPLC technologies, while Asia-Pacific represents the fastest-growing market segment with increasing quality control requirements in developing economies. China and India, in particular, are experiencing rapid growth in pharmaceutical manufacturing and environmental monitoring sectors.
Customer surveys consistently highlight contamination prevention as a top-three purchasing consideration for new HPLC systems. End-users specifically demand solutions addressing carryover issues, ghost peaks, baseline drift, and system clogging. There is particular market interest in automated cleaning protocols, advanced filtration systems, and materials resistant to chemical degradation.
Current Challenges in HPLC System Cleanliness
High-performance liquid chromatography (HPLC) systems face persistent contamination challenges that significantly impact analytical reliability and operational efficiency. These systems, comprising pumps, injectors, columns, detectors, and connecting tubing, are susceptible to various forms of contamination that compromise data integrity and instrument longevity.
Particulate contamination represents a primary concern, originating from mobile phase impurities, sample residues, or environmental dust. These particles can obstruct flow paths, damage pump seals, and create backpressure issues that degrade chromatographic performance. Even microscopic particulates can cause significant disruption to sensitive analytical procedures.
Chemical contamination presents another substantial challenge, occurring when residual analytes, mobile phase components, or sample matrix elements accumulate within the system. This contamination manifests as ghost peaks, baseline drift, or retention time shifts that undermine quantitative accuracy. Particularly problematic are strongly retained compounds that gradually leach from system components during subsequent analyses.
Microbial contamination emerges as an increasingly recognized issue, especially in systems using aqueous mobile phases or those left idle for extended periods. Bacterial and fungal growth can generate biofilms that release interfering compounds, block frits and filters, and potentially degrade stationary phases through enzymatic activity.
Carryover contamination occurs when residues from previous injections affect subsequent analyses, a particularly critical issue in trace analysis applications or when transitioning between samples of vastly different concentrations. This phenomenon can lead to false positives or inaccurate quantification, especially problematic in regulated environments like pharmaceutical quality control or forensic toxicology.
Cross-contamination between different methods sharing the same instrument presents additional complications, particularly in multi-user laboratory environments. Incompatible mobile phases or buffer systems can precipitate salts or create reactive species that damage system components and compromise analytical results.
Metal contamination from system components, particularly stainless steel parts interacting with certain mobile phases, can catalyze sample degradation or interfere with specific analyses. This issue becomes especially pronounced when working with metal-sensitive compounds or biological samples.
The economic impact of these contamination issues extends beyond analytical concerns, resulting in increased downtime, reduced column lifespans, higher maintenance costs, and compromised laboratory productivity. Modern HPLC applications demanding ever-greater sensitivity and reproducibility face heightened vulnerability to even minor contamination events.
Particulate contamination represents a primary concern, originating from mobile phase impurities, sample residues, or environmental dust. These particles can obstruct flow paths, damage pump seals, and create backpressure issues that degrade chromatographic performance. Even microscopic particulates can cause significant disruption to sensitive analytical procedures.
Chemical contamination presents another substantial challenge, occurring when residual analytes, mobile phase components, or sample matrix elements accumulate within the system. This contamination manifests as ghost peaks, baseline drift, or retention time shifts that undermine quantitative accuracy. Particularly problematic are strongly retained compounds that gradually leach from system components during subsequent analyses.
Microbial contamination emerges as an increasingly recognized issue, especially in systems using aqueous mobile phases or those left idle for extended periods. Bacterial and fungal growth can generate biofilms that release interfering compounds, block frits and filters, and potentially degrade stationary phases through enzymatic activity.
Carryover contamination occurs when residues from previous injections affect subsequent analyses, a particularly critical issue in trace analysis applications or when transitioning between samples of vastly different concentrations. This phenomenon can lead to false positives or inaccurate quantification, especially problematic in regulated environments like pharmaceutical quality control or forensic toxicology.
Cross-contamination between different methods sharing the same instrument presents additional complications, particularly in multi-user laboratory environments. Incompatible mobile phases or buffer systems can precipitate salts or create reactive species that damage system components and compromise analytical results.
Metal contamination from system components, particularly stainless steel parts interacting with certain mobile phases, can catalyze sample degradation or interfere with specific analyses. This issue becomes especially pronounced when working with metal-sensitive compounds or biological samples.
The economic impact of these contamination issues extends beyond analytical concerns, resulting in increased downtime, reduced column lifespans, higher maintenance costs, and compromised laboratory productivity. Modern HPLC applications demanding ever-greater sensitivity and reproducibility face heightened vulnerability to even minor contamination events.
Established Contamination Prevention Protocols
01 Prevention of contamination in HPLC systems
Various methods and devices are employed to prevent contamination in HPLC systems, including specialized filters, guard columns, and purification systems. These preventive measures help maintain system integrity by removing particulates, organic compounds, and other contaminants before they enter critical components of the chromatography system, thereby extending column life and ensuring accurate analytical results.- Prevention of contamination in HPLC systems: Various methods and devices are employed to prevent contamination in HPLC systems. These include specialized filters, guard columns, and purification systems that remove impurities before they enter the analytical column. Preventive measures focus on eliminating particulates, microbial growth, and chemical contaminants that could affect chromatographic performance and system integrity.
- Detection and monitoring of contaminants: Advanced detection systems are implemented to monitor and identify contaminants in HPLC systems. These include specialized sensors, spectroscopic methods, and automated monitoring systems that can detect impurities in real-time. Early detection allows for prompt intervention before contaminants can cause significant damage to the system or affect analytical results.
- Cleaning and maintenance protocols: Specific cleaning and maintenance protocols are essential for managing HPLC system contamination. These include regular flushing procedures with appropriate solvents, dedicated cleaning cycles, and systematic maintenance schedules. Proper cleaning methods target different types of contaminants including protein residues, salt deposits, and organic compounds that may accumulate in the system.
- Mobile phase and sample preparation techniques: Proper preparation of mobile phases and samples is critical to prevent contamination in HPLC systems. This includes filtration of solvents, degassing procedures, and appropriate sample clean-up methods. Techniques such as solid-phase extraction, liquid-liquid extraction, and protein precipitation help remove potential contaminants before samples are introduced into the HPLC system.
- System design improvements for contamination reduction: Innovative HPLC system designs incorporate features specifically aimed at reducing contamination risks. These include biocompatible materials, reduced dead volume, improved flow paths, and specialized connectors that minimize the potential for contamination. Advanced system designs also facilitate easier cleaning and maintenance, further reducing contamination issues.
02 Detection and monitoring of contamination
Advanced detection systems are used to monitor contamination levels in HPLC systems in real-time. These include specialized sensors, optical detection methods, and automated monitoring systems that can identify the presence of contaminants during chromatographic analysis. Early detection allows for prompt intervention before contamination affects analytical results or damages system components.Expand Specific Solutions03 Cleaning and decontamination procedures
Specific cleaning protocols and decontamination procedures are essential for maintaining HPLC system performance. These include flushing with appropriate solvents, chemical cleaning agents, and specialized washing sequences designed to remove accumulated contaminants from flow paths, columns, and detectors without damaging sensitive components.Expand Specific Solutions04 Material selection to minimize contamination
The choice of materials used in HPLC system components significantly impacts contamination levels. Advanced materials with properties such as chemical resistance, low leaching characteristics, and minimal interaction with analytes help reduce system contamination. These materials are used in tubing, connectors, seals, and other components that come into contact with mobile phases and samples.Expand Specific Solutions05 Innovative system designs to reduce contamination risk
Novel HPLC system architectures incorporate design features specifically aimed at minimizing contamination. These include closed-loop systems, reduced dead volume configurations, improved flow path designs, and modular components that can be easily replaced or cleaned. Such innovations help maintain system cleanliness and extend maintenance intervals while improving analytical performance.Expand Specific Solutions
Leading Manufacturers and Solution Providers
The HPLC system contamination market is currently in a growth phase, with increasing demand for effective contamination control solutions across pharmaceutical, biotechnology, and analytical chemistry sectors. The market is estimated to reach several billion dollars by 2025, driven by stringent regulatory requirements and growing analytical testing needs. From a technological maturity perspective, companies like Thermo Fisher Scientific (parent of Dionex Softron GmbH) and DuPont de Nemours lead with advanced contamination prevention technologies, while ARKRAY and United Laboratories offer specialized cleaning solutions. Applied Materials and Tokyo Electron contribute expertise in ultra-clean manufacturing processes that can be adapted to HPLC systems. Emerging players like Chengdu Giantech are developing innovative biocompatible materials to reduce contamination risks, creating a competitive landscape that balances established industry leaders with specialized solution providers.
DuPont de Nemours, Inc.
Technical Solution: DuPont addresses HPLC system contamination through their comprehensive materials science approach. Their strategy centers on specialized polymer technologies for HPLC components that minimize leaching and contamination. DuPont has developed high-purity PTFE and PFA tubing with ultra-smooth inner surfaces that reduce analyte adsorption and carryover between samples. Their proprietary surface treatments create hydrophobic or hydrophilic interfaces depending on application requirements, minimizing unwanted interactions with mobile phases and analytes. DuPont's contamination prevention extends to specialized filter membranes with precisely controlled pore sizes that effectively remove particulates while maintaining chemical compatibility with aggressive mobile phases. They've engineered specialized cleaning solutions formulated to dissolve and remove specific contaminant types without damaging sensitive system components. Their approach includes preventative strategies such as biofilm-resistant materials for water purification systems that feed HPLC instruments, addressing contamination at its source rather than just within the analytical system itself.
Strengths: Extensive materials science expertise applied to creating contamination-resistant components; comprehensive approach addressing both prevention and remediation; solutions based on fundamental understanding of chemical interactions at surfaces. Weaknesses: May focus more on materials and components rather than complete system solutions; requires integration with existing HPLC systems rather than offering turnkey contamination solutions; potentially higher cost for specialized materials compared to standard components.
Dionex Softron GmbH
Technical Solution: Dionex Softron GmbH has developed comprehensive HPLC system contamination solutions centered around their Thermo Scientific Dionex ICS systems. Their approach includes automated self-regenerating suppressors that continuously remove eluent ions, reducing background contamination. They've implemented intelligent predictive technologies that monitor system performance and detect early signs of contamination through pressure fluctuations and baseline shifts. Their SmartRinse technology provides targeted cleaning protocols based on detected contaminants, while their high-pressure capillary systems operate with minimal dead volume, reducing areas where contaminants can accumulate. Dionex's IC Cube technology integrates consumables into sealed modules to prevent external contamination during maintenance. Their systems feature specialized materials resistant to chemical degradation and particulate generation, with electrolytically regenerated suppressors that minimize chemical waste and contamination risks.
Strengths: Specialized expertise in ion chromatography with integrated contamination prevention systems; automated self-monitoring capabilities that detect contamination before it affects results; modular design allowing targeted maintenance without exposing the entire system. Weaknesses: Solutions may be proprietary and not compatible with other manufacturers' HPLC systems; higher initial investment compared to generic contamination prevention approaches; specialized consumables may have higher ongoing costs.
Key Innovations in HPLC Purification Technology
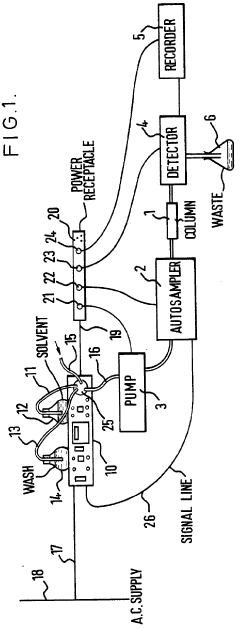
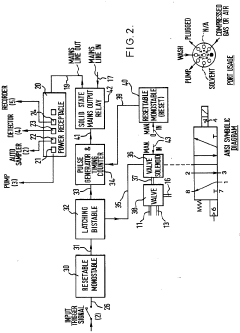
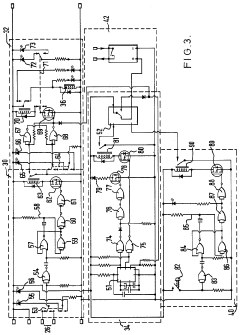
HPLC-system with variable flow rate
PatentActiveEP2210086A1
Innovation
- The HPLC system dynamically controls the flow rate based on variations in mobile phase pressure, allowing for operation at maximum available pressure while maintaining a constant control value, thereby optimizing resource utilization and reducing analysis times.
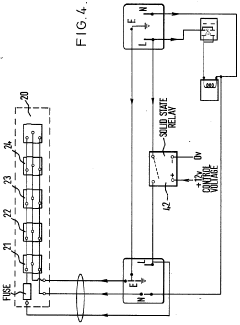
Improvements in or relating to high performance liquid chromatography systems
PatentInactiveGB2174016A
Innovation
- An ancillary apparatus that includes a valve system for selecting between mobile phase and wash liquid sources, a timing mechanism initiated by a trigger pulse from the HPLC system, and an electric switch to disconnect power to the HPLC system units after a predetermined wash liquid supply, ensuring a controlled shutdown and washout process.
Cost-Benefit Analysis of Contamination Solutions
When evaluating contamination solutions for HPLC systems, a comprehensive cost-benefit analysis is essential for making informed decisions. Initial investment costs for preventive measures such as advanced filtration systems, specialized column guards, and high-purity solvents must be weighed against their long-term benefits. While these solutions may require significant upfront expenditure, they typically reduce system downtime and extend the operational lifespan of critical components.
Preventive maintenance protocols represent a moderate ongoing cost but deliver substantial returns through reduced troubleshooting time and fewer emergency interventions. Regular system cleaning procedures, though labor-intensive, prevent accumulation of contaminants that could lead to more expensive repairs. Data indicates that laboratories implementing systematic preventive maintenance experience 40-60% fewer contamination-related failures compared to reactive approaches.
Automated cleaning systems present a compelling value proposition despite their higher initial cost. These systems can reduce manual labor by approximately 70% and minimize human error in cleaning procedures. The return on investment typically materializes within 12-18 months through improved analytical precision and reduced reagent waste.
Training programs for laboratory personnel represent another dimension in the cost-benefit equation. While requiring time and financial resources, well-trained staff can identify contamination issues earlier and implement appropriate remediation strategies more efficiently. Studies suggest that comprehensive training programs reduce contamination-related method failures by up to 35%.
The economic impact of system contamination extends beyond direct remediation costs. Analytical errors resulting from contaminated systems can lead to costly retesting, delayed product releases, and potential regulatory complications. For pharmaceutical companies, a single day of analytical system downtime can represent losses exceeding $10,000, making even expensive contamination solutions economically justifiable.
When comparing reactive versus proactive approaches, data consistently demonstrates that reactive contamination management ultimately costs 2-3 times more than preventive strategies. This multiplier effect stems from emergency service fees, expedited parts shipping, and the cascading impact of unplanned downtime on laboratory operations.
The optimal contamination management strategy balances immediate budget constraints with long-term operational efficiency. Organizations should consider their specific analytical requirements, sample throughput, and regulatory environment when determining the appropriate level of investment in contamination prevention and remediation technologies.
Preventive maintenance protocols represent a moderate ongoing cost but deliver substantial returns through reduced troubleshooting time and fewer emergency interventions. Regular system cleaning procedures, though labor-intensive, prevent accumulation of contaminants that could lead to more expensive repairs. Data indicates that laboratories implementing systematic preventive maintenance experience 40-60% fewer contamination-related failures compared to reactive approaches.
Automated cleaning systems present a compelling value proposition despite their higher initial cost. These systems can reduce manual labor by approximately 70% and minimize human error in cleaning procedures. The return on investment typically materializes within 12-18 months through improved analytical precision and reduced reagent waste.
Training programs for laboratory personnel represent another dimension in the cost-benefit equation. While requiring time and financial resources, well-trained staff can identify contamination issues earlier and implement appropriate remediation strategies more efficiently. Studies suggest that comprehensive training programs reduce contamination-related method failures by up to 35%.
The economic impact of system contamination extends beyond direct remediation costs. Analytical errors resulting from contaminated systems can lead to costly retesting, delayed product releases, and potential regulatory complications. For pharmaceutical companies, a single day of analytical system downtime can represent losses exceeding $10,000, making even expensive contamination solutions economically justifiable.
When comparing reactive versus proactive approaches, data consistently demonstrates that reactive contamination management ultimately costs 2-3 times more than preventive strategies. This multiplier effect stems from emergency service fees, expedited parts shipping, and the cascading impact of unplanned downtime on laboratory operations.
The optimal contamination management strategy balances immediate budget constraints with long-term operational efficiency. Organizations should consider their specific analytical requirements, sample throughput, and regulatory environment when determining the appropriate level of investment in contamination prevention and remediation technologies.
Environmental Impact of HPLC Cleaning Agents
The environmental impact of HPLC cleaning agents represents a growing concern in analytical laboratories worldwide. Traditional cleaning solutions often contain hazardous chemicals such as acetonitrile, methanol, and phosphoric acid, which pose significant environmental risks when improperly disposed. These solvents can contaminate water systems, contribute to air pollution through volatile organic compound (VOC) emissions, and potentially harm aquatic ecosystems even at low concentrations.
Recent studies indicate that approximately 60-70% of HPLC laboratories dispose of cleaning solvents through conventional drainage systems, despite regulatory guidelines advising against such practices. The cumulative effect of these disposal methods has led to detectable levels of acetonitrile and methanol in groundwater near facilities with high HPLC usage, particularly in industrial zones and academic research centers.
The persistence of these chemicals in the environment varies significantly. While acetonitrile has a relatively short environmental half-life of 1-2 weeks under aerobic conditions, certain phosphate buffers and ion-pairing reagents can persist for months, potentially disrupting aquatic nitrogen cycles and phosphorus balances in receiving water bodies.
Carbon footprint assessments of HPLC cleaning processes reveal that the production, transportation, and disposal of cleaning agents contribute approximately 15-20% of an HPLC system's total environmental impact. This calculation includes energy consumption during manufacturing and the greenhouse gas emissions associated with transportation of these often hazardous materials.
Emerging alternatives include biodegradable surfactants, enzymatic cleaners, and water-based solutions enhanced with environmentally benign additives. These green cleaning agents demonstrate promising efficacy against common HPLC contaminants while reducing environmental burden. For instance, citrus-based solvents have shown 85-90% effectiveness against protein and lipid residues compared to traditional organic solvents, with dramatically reduced ecotoxicity profiles.
Regulatory frameworks governing laboratory waste disposal are evolving rapidly. The European Union's REACH regulations and the EPA's Resource Conservation and Recovery Act in the United States have increasingly stringent requirements for handling and disposing of laboratory solvents. Non-compliance penalties have increased by an average of 35% over the past five years, creating additional financial incentives for laboratories to adopt environmentally responsible cleaning practices.
Cost-benefit analyses demonstrate that while environmentally friendly alternatives may have higher initial costs (typically 20-30% more expensive), the reduced waste disposal costs, lower regulatory compliance burden, and decreased environmental liability often result in net savings within 12-18 months of implementation. Furthermore, laboratories adopting green cleaning protocols report improved worker safety outcomes and reduced sick leave related to chemical exposure.
Recent studies indicate that approximately 60-70% of HPLC laboratories dispose of cleaning solvents through conventional drainage systems, despite regulatory guidelines advising against such practices. The cumulative effect of these disposal methods has led to detectable levels of acetonitrile and methanol in groundwater near facilities with high HPLC usage, particularly in industrial zones and academic research centers.
The persistence of these chemicals in the environment varies significantly. While acetonitrile has a relatively short environmental half-life of 1-2 weeks under aerobic conditions, certain phosphate buffers and ion-pairing reagents can persist for months, potentially disrupting aquatic nitrogen cycles and phosphorus balances in receiving water bodies.
Carbon footprint assessments of HPLC cleaning processes reveal that the production, transportation, and disposal of cleaning agents contribute approximately 15-20% of an HPLC system's total environmental impact. This calculation includes energy consumption during manufacturing and the greenhouse gas emissions associated with transportation of these often hazardous materials.
Emerging alternatives include biodegradable surfactants, enzymatic cleaners, and water-based solutions enhanced with environmentally benign additives. These green cleaning agents demonstrate promising efficacy against common HPLC contaminants while reducing environmental burden. For instance, citrus-based solvents have shown 85-90% effectiveness against protein and lipid residues compared to traditional organic solvents, with dramatically reduced ecotoxicity profiles.
Regulatory frameworks governing laboratory waste disposal are evolving rapidly. The European Union's REACH regulations and the EPA's Resource Conservation and Recovery Act in the United States have increasingly stringent requirements for handling and disposing of laboratory solvents. Non-compliance penalties have increased by an average of 35% over the past five years, creating additional financial incentives for laboratories to adopt environmentally responsible cleaning practices.
Cost-benefit analyses demonstrate that while environmentally friendly alternatives may have higher initial costs (typically 20-30% more expensive), the reduced waste disposal costs, lower regulatory compliance burden, and decreased environmental liability often result in net savings within 12-18 months of implementation. Furthermore, laboratories adopting green cleaning protocols report improved worker safety outcomes and reduced sick leave related to chemical exposure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!