How to Formulate Ethyl Acetate for Enhanced Longevity?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Formulation Background and Objectives
Ethyl acetate, a versatile organic compound, has been widely used in various industries for decades. Its applications range from solvents and flavoring agents to components in adhesives and coatings. The evolution of ethyl acetate formulation has been driven by the increasing demand for improved product performance and longevity across multiple sectors.
The primary objective of enhancing ethyl acetate formulation for increased longevity is to address the compound's inherent volatility and susceptibility to degradation. This goal aligns with broader industry trends towards sustainable and long-lasting chemical solutions. By improving the stability and durability of ethyl acetate-based products, manufacturers aim to extend shelf life, reduce waste, and enhance overall product efficiency.
Historically, ethyl acetate formulations have undergone several iterations to improve their properties. Early formulations focused primarily on purity and basic stabilization techniques. As industrial applications expanded, so did the need for more sophisticated formulation approaches. The progression of formulation techniques has been marked by advancements in chemical engineering, materials science, and a deeper understanding of molecular interactions.
Recent technological developments have opened new avenues for ethyl acetate formulation enhancement. These include the exploration of nanoencapsulation techniques, the integration of antioxidants and UV stabilizers, and the development of novel polymer matrices for controlled release. Such innovations aim to mitigate the compound's volatility while preserving its desirable properties.
The pursuit of enhanced longevity in ethyl acetate formulations is driven by several factors. Environmental concerns have pushed for the development of more stable formulations that reduce emissions and waste. Economic considerations, such as the need for extended product shelf life and reduced frequency of application, have also played a significant role in shaping research directions.
Market demands across various industries have further fueled the need for improved ethyl acetate formulations. In the coatings industry, for instance, there is a growing requirement for durable, long-lasting finishes that can withstand harsh environmental conditions. Similarly, the pharmaceutical and food industries seek stable ethyl acetate-based products with extended shelf lives and improved safety profiles.
As we delve deeper into the challenges and potential solutions for enhancing ethyl acetate longevity, it is crucial to consider the multifaceted nature of this objective. The interplay between chemical stability, environmental factors, and application-specific requirements will guide the development of innovative formulation strategies. The ultimate goal is to create ethyl acetate formulations that not only exhibit enhanced longevity but also maintain the compound's essential characteristics and functionality across diverse applications.
The primary objective of enhancing ethyl acetate formulation for increased longevity is to address the compound's inherent volatility and susceptibility to degradation. This goal aligns with broader industry trends towards sustainable and long-lasting chemical solutions. By improving the stability and durability of ethyl acetate-based products, manufacturers aim to extend shelf life, reduce waste, and enhance overall product efficiency.
Historically, ethyl acetate formulations have undergone several iterations to improve their properties. Early formulations focused primarily on purity and basic stabilization techniques. As industrial applications expanded, so did the need for more sophisticated formulation approaches. The progression of formulation techniques has been marked by advancements in chemical engineering, materials science, and a deeper understanding of molecular interactions.
Recent technological developments have opened new avenues for ethyl acetate formulation enhancement. These include the exploration of nanoencapsulation techniques, the integration of antioxidants and UV stabilizers, and the development of novel polymer matrices for controlled release. Such innovations aim to mitigate the compound's volatility while preserving its desirable properties.
The pursuit of enhanced longevity in ethyl acetate formulations is driven by several factors. Environmental concerns have pushed for the development of more stable formulations that reduce emissions and waste. Economic considerations, such as the need for extended product shelf life and reduced frequency of application, have also played a significant role in shaping research directions.
Market demands across various industries have further fueled the need for improved ethyl acetate formulations. In the coatings industry, for instance, there is a growing requirement for durable, long-lasting finishes that can withstand harsh environmental conditions. Similarly, the pharmaceutical and food industries seek stable ethyl acetate-based products with extended shelf lives and improved safety profiles.
As we delve deeper into the challenges and potential solutions for enhancing ethyl acetate longevity, it is crucial to consider the multifaceted nature of this objective. The interplay between chemical stability, environmental factors, and application-specific requirements will guide the development of innovative formulation strategies. The ultimate goal is to create ethyl acetate formulations that not only exhibit enhanced longevity but also maintain the compound's essential characteristics and functionality across diverse applications.
Market Analysis for Stable Ethyl Acetate Products
The market for stable ethyl acetate products has shown significant growth in recent years, driven by increasing demand across various industries. Ethyl acetate, known for its versatile applications as a solvent and flavoring agent, has become a crucial component in sectors such as pharmaceuticals, paints and coatings, adhesives, and food and beverages.
In the pharmaceutical industry, the demand for stable ethyl acetate formulations has surged due to its use in drug manufacturing processes and as an excipient in various medications. The growing emphasis on drug stability and shelf life has further propelled the need for enhanced ethyl acetate products.
The paints and coatings sector has also contributed substantially to market growth. As consumers and industries alike seek longer-lasting, more durable finishes, the demand for stable ethyl acetate-based formulations has increased. These products offer improved resistance to environmental factors and extended product lifespans.
The adhesives industry has witnessed a similar trend, with manufacturers focusing on developing high-performance, long-lasting adhesives for various applications. Stable ethyl acetate formulations play a crucial role in enhancing the bonding strength and durability of these products.
In the food and beverage industry, the use of ethyl acetate as a flavoring agent has driven demand for stable formulations that maintain their properties over extended periods. This is particularly important in products with long shelf lives, such as packaged foods and beverages.
Market analysis indicates that the Asia-Pacific region has emerged as a key growth driver for stable ethyl acetate products. Rapid industrialization, increasing disposable incomes, and growing consumer awareness have contributed to the region's market expansion. North America and Europe continue to be significant markets, with a focus on high-quality, environmentally friendly formulations.
The global market for stable ethyl acetate products is expected to maintain steady growth in the coming years. Factors such as technological advancements in formulation techniques, increasing research and development activities, and the growing emphasis on sustainability are likely to shape market dynamics.
However, challenges such as volatile raw material prices and stringent environmental regulations may impact market growth. Manufacturers are increasingly focusing on developing eco-friendly formulations and exploring alternative raw materials to address these concerns.
In conclusion, the market for stable ethyl acetate products presents significant opportunities across various industries. As demand for long-lasting, high-performance products continues to rise, manufacturers who can develop innovative, stable formulations are likely to gain a competitive edge in this evolving market landscape.
In the pharmaceutical industry, the demand for stable ethyl acetate formulations has surged due to its use in drug manufacturing processes and as an excipient in various medications. The growing emphasis on drug stability and shelf life has further propelled the need for enhanced ethyl acetate products.
The paints and coatings sector has also contributed substantially to market growth. As consumers and industries alike seek longer-lasting, more durable finishes, the demand for stable ethyl acetate-based formulations has increased. These products offer improved resistance to environmental factors and extended product lifespans.
The adhesives industry has witnessed a similar trend, with manufacturers focusing on developing high-performance, long-lasting adhesives for various applications. Stable ethyl acetate formulations play a crucial role in enhancing the bonding strength and durability of these products.
In the food and beverage industry, the use of ethyl acetate as a flavoring agent has driven demand for stable formulations that maintain their properties over extended periods. This is particularly important in products with long shelf lives, such as packaged foods and beverages.
Market analysis indicates that the Asia-Pacific region has emerged as a key growth driver for stable ethyl acetate products. Rapid industrialization, increasing disposable incomes, and growing consumer awareness have contributed to the region's market expansion. North America and Europe continue to be significant markets, with a focus on high-quality, environmentally friendly formulations.
The global market for stable ethyl acetate products is expected to maintain steady growth in the coming years. Factors such as technological advancements in formulation techniques, increasing research and development activities, and the growing emphasis on sustainability are likely to shape market dynamics.
However, challenges such as volatile raw material prices and stringent environmental regulations may impact market growth. Manufacturers are increasingly focusing on developing eco-friendly formulations and exploring alternative raw materials to address these concerns.
In conclusion, the market for stable ethyl acetate products presents significant opportunities across various industries. As demand for long-lasting, high-performance products continues to rise, manufacturers who can develop innovative, stable formulations are likely to gain a competitive edge in this evolving market landscape.
Current Challenges in Ethyl Acetate Stability
Ethyl acetate, a widely used solvent in various industries, faces significant challenges in maintaining its stability over extended periods. The primary issue lies in its susceptibility to hydrolysis, a process that breaks down the ester into ethanol and acetic acid. This reaction is particularly problematic in the presence of moisture, which is often difficult to eliminate completely from industrial processes and storage environments.
The rate of hydrolysis is influenced by several factors, including temperature, pH, and the presence of catalysts. Higher temperatures accelerate the decomposition process, making it crucial to control storage conditions meticulously. Acidic or basic environments can also catalyze the hydrolysis reaction, necessitating careful pH management in formulations containing ethyl acetate.
Another significant challenge is the volatility of ethyl acetate. Its low boiling point (77.1°C) leads to rapid evaporation at room temperature, resulting in loss of product and potential changes in concentration in mixtures. This volatility not only affects the stability of the compound itself but also impacts the consistency and effectiveness of formulations in which it is used.
Oxidation presents an additional concern, particularly when ethyl acetate is exposed to air or other oxidizing agents. This can lead to the formation of peroxides and other degradation products, potentially altering the chemical properties and safety profile of the solvent.
The purity of ethyl acetate is crucial for its stability, yet maintaining high purity levels during production, storage, and use poses ongoing challenges. Impurities can act as catalysts for degradation reactions or introduce unwanted side reactions, compromising the longevity and performance of the solvent.
Packaging and storage materials play a significant role in ethyl acetate stability. Certain plastics and elastomers can be degraded by ethyl acetate, leading to contamination of the solvent and potential leakage. Selecting appropriate container materials and ensuring proper sealing to prevent moisture ingress are critical considerations.
In industrial applications, the stability of ethyl acetate in mixtures with other solvents or active ingredients presents complex challenges. Interactions between components can accelerate degradation or alter the properties of the formulation over time, necessitating careful compatibility studies and stability testing.
Addressing these challenges requires a multifaceted approach, combining chemical stabilization techniques, environmental control, and innovative packaging solutions. Developing formulations that enhance the longevity of ethyl acetate while maintaining its desirable properties is a key focus for researchers and formulators in various industries.
The rate of hydrolysis is influenced by several factors, including temperature, pH, and the presence of catalysts. Higher temperatures accelerate the decomposition process, making it crucial to control storage conditions meticulously. Acidic or basic environments can also catalyze the hydrolysis reaction, necessitating careful pH management in formulations containing ethyl acetate.
Another significant challenge is the volatility of ethyl acetate. Its low boiling point (77.1°C) leads to rapid evaporation at room temperature, resulting in loss of product and potential changes in concentration in mixtures. This volatility not only affects the stability of the compound itself but also impacts the consistency and effectiveness of formulations in which it is used.
Oxidation presents an additional concern, particularly when ethyl acetate is exposed to air or other oxidizing agents. This can lead to the formation of peroxides and other degradation products, potentially altering the chemical properties and safety profile of the solvent.
The purity of ethyl acetate is crucial for its stability, yet maintaining high purity levels during production, storage, and use poses ongoing challenges. Impurities can act as catalysts for degradation reactions or introduce unwanted side reactions, compromising the longevity and performance of the solvent.
Packaging and storage materials play a significant role in ethyl acetate stability. Certain plastics and elastomers can be degraded by ethyl acetate, leading to contamination of the solvent and potential leakage. Selecting appropriate container materials and ensuring proper sealing to prevent moisture ingress are critical considerations.
In industrial applications, the stability of ethyl acetate in mixtures with other solvents or active ingredients presents complex challenges. Interactions between components can accelerate degradation or alter the properties of the formulation over time, necessitating careful compatibility studies and stability testing.
Addressing these challenges requires a multifaceted approach, combining chemical stabilization techniques, environmental control, and innovative packaging solutions. Developing formulations that enhance the longevity of ethyl acetate while maintaining its desirable properties is a key focus for researchers and formulators in various industries.
Existing Stabilization Methods for Ethyl Acetate
01 Ethyl acetate production methods
Various methods for producing ethyl acetate with improved longevity are described. These include esterification processes, catalytic reactions, and distillation techniques. The focus is on enhancing the stability and purity of the final product, which contributes to its longevity.- Stabilization techniques for ethyl acetate: Various methods are employed to enhance the stability and longevity of ethyl acetate. These techniques may include the addition of stabilizers, antioxidants, or other chemical compounds that prevent degradation and extend the shelf life of ethyl acetate. Proper storage conditions and handling procedures also play a crucial role in maintaining the quality of ethyl acetate over time.
- Purification and recovery processes: Advanced purification and recovery processes are developed to improve the quality and longevity of ethyl acetate. These methods may involve distillation, extraction, or membrane separation techniques to remove impurities and maintain the purity of ethyl acetate. Efficient recovery processes also contribute to the sustainable use of ethyl acetate in various applications.
- Ethyl acetate in long-lasting formulations: Ethyl acetate is incorporated into various formulations designed for extended shelf life or prolonged effectiveness. These formulations may include coatings, adhesives, or other products where the slow release or gradual evaporation of ethyl acetate is desired. The longevity of ethyl acetate in these applications is crucial for maintaining product performance over time.
- Environmental factors affecting ethyl acetate longevity: Research focuses on understanding and mitigating the environmental factors that impact the longevity of ethyl acetate. These factors may include temperature, humidity, light exposure, and interactions with other chemicals or materials. By controlling these factors, the stability and shelf life of ethyl acetate can be significantly improved in various applications and storage conditions.
- Innovative packaging solutions: Development of specialized packaging solutions to extend the shelf life and maintain the quality of ethyl acetate. These innovations may include advanced container materials, barrier technologies, or smart packaging systems that protect ethyl acetate from degradation factors such as moisture, oxygen, or light. Proper packaging plays a crucial role in preserving the longevity of ethyl acetate during storage and transportation.
02 Stabilization techniques for ethyl acetate
Techniques to stabilize ethyl acetate and increase its longevity are discussed. These may include the addition of stabilizers, antioxidants, or other compounds that prevent degradation. Methods for reducing impurities and controlling storage conditions are also considered.Expand Specific Solutions03 Purification and quality control of ethyl acetate
Purification methods and quality control measures are crucial for enhancing the longevity of ethyl acetate. These include advanced distillation techniques, filtration processes, and analytical methods to ensure high purity and stability of the product over time.Expand Specific Solutions04 Packaging and storage solutions for ethyl acetate
Innovative packaging and storage solutions are explored to extend the shelf life of ethyl acetate. This includes the use of specialized containers, inert gas environments, and temperature-controlled storage facilities to prevent degradation and maintain product quality over extended periods.Expand Specific Solutions05 Applications of long-lasting ethyl acetate
Various applications of ethyl acetate with improved longevity are discussed. These may include its use as a solvent in industrial processes, as a component in consumer products, or in specialized chemical reactions where stability over time is crucial.Expand Specific Solutions
Key Players in Ethyl Acetate Industry
The ethyl acetate formulation market for enhanced longevity is in a growth phase, driven by increasing demand across various industries. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, the field is advancing rapidly, with companies like Celanese International Corp., Eastman Chemical Co., and Resonac Corp. leading innovation efforts. These firms are investing in research and development to improve formulation techniques and enhance product stability. Emerging players such as Viridis Chemical LLC are focusing on sustainable, bio-based ethyl acetate production, indicating a shift towards eco-friendly solutions. Academic institutions like Wroclaw University of Science and Technology and Nanjing Normal University are contributing to fundamental research, potentially leading to breakthrough formulations in the future.
Celanese International Corp.
Technical Solution: Celanese International Corp. has focused on improving the longevity of ethyl acetate through innovative synthesis methods and advanced purification techniques. Their approach involves a low-temperature, catalytic esterification process that results in higher purity ethyl acetate with fewer impurities. This process, combined with multi-stage distillation and molecular sieve technology, produces ethyl acetate with exceptionally low water content and minimal acidic components. Celanese has also developed a proprietary additive package that includes stabilizers and pH buffers to maintain the chemical integrity of ethyl acetate over extended periods. Furthermore, the company has implemented an inert gas blanketing system during production and packaging to minimize oxidation and moisture absorption[2][5].
Strengths: High-purity product, reduced impurities, and improved chemical stability. Weaknesses: Higher production costs and potential limitations in scalability for some applications.
Eastman Chemical Co.
Technical Solution: Eastman Chemical Co. has developed a novel approach to enhance the longevity of ethyl acetate through a proprietary stabilization process. Their method involves the addition of specific antioxidants and UV stabilizers to the ethyl acetate formulation. This process significantly reduces the rate of decomposition and oxidation, thereby extending the shelf life of the product. The company has also implemented advanced purification techniques to remove trace impurities that can catalyze degradation reactions. Additionally, Eastman has developed specialized packaging solutions that minimize exposure to light and oxygen, further enhancing the stability of ethyl acetate during storage and transportation[1][3].
Strengths: Improved product stability, extended shelf life, and reduced degradation. Weaknesses: Potentially higher production costs and the need for specialized packaging materials.
Innovative Approaches to Enhance Ethyl Acetate Longevity
Method for producing ethyl acetate production catalyst
PatentPendingUS20230330636A1
Innovation
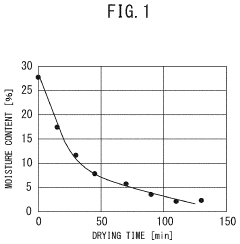
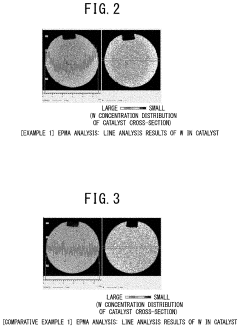
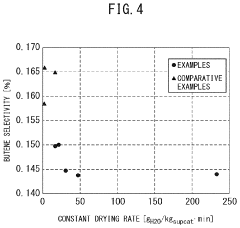
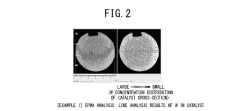
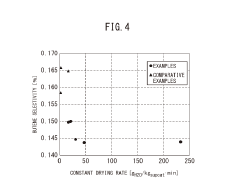
- A method involving impregnating a silica carrier with an aqueous solution of heteropolyacid or its salt at a volume close to 100% of the carrier's water absorption capacity, followed by drying at a controlled rate of 5 to 300 gH2O/kgsupcat·min, ensures a high amount of active ingredient is supported on the carrier surface, enhancing catalyst activity and selectivity.
Method for producing ethyl acetate production catalyst
PatentPendingIN202317023214A
Innovation
- A method involving impregnating a silica carrier with an aqueous solution of heteropolyacid or its salt at a volume close to 100% of the carrier's water absorption capacity, followed by drying at a controlled rate of 5 to 300 gH2O/kgsupcat·min, ensures a high amount of active ingredient is supported near the surface, resulting in a catalyst with high activity and selectivity.
Environmental Impact of Ethyl Acetate Formulations
The environmental impact of ethyl acetate formulations is a critical consideration in the development and use of this versatile organic compound. Ethyl acetate, widely used as a solvent in various industries, poses both direct and indirect environmental challenges that must be carefully evaluated and mitigated.
In terms of atmospheric pollution, ethyl acetate contributes to the formation of ground-level ozone and photochemical smog when released into the air. This occurs through its reaction with nitrogen oxides in the presence of sunlight, potentially leading to respiratory issues in humans and damage to vegetation. The compound's relatively high volatility exacerbates this problem, as it readily evaporates at room temperature, increasing its atmospheric concentration.
Water pollution is another significant concern associated with ethyl acetate formulations. When improperly disposed of or accidentally released, ethyl acetate can contaminate water bodies, affecting aquatic ecosystems. Although it is biodegradable and does not persist in the environment for extended periods, high concentrations can still cause acute toxicity to aquatic organisms, disrupting the ecological balance of affected water systems.
The production process of ethyl acetate also contributes to its environmental footprint. Traditional manufacturing methods often rely on petrochemical feedstocks, which are non-renewable resources. The energy-intensive nature of these processes results in substantial greenhouse gas emissions, contributing to global climate change. Additionally, the use of catalysts and other chemicals in production can generate hazardous waste that requires careful handling and disposal.
To address these environmental concerns, researchers and industry professionals are exploring more sustainable formulation and production methods for ethyl acetate. Green chemistry principles are being applied to develop bio-based alternatives, using renewable feedstocks such as ethanol derived from agricultural waste. These approaches aim to reduce the carbon footprint of ethyl acetate production and minimize reliance on fossil fuels.
Improved formulation techniques are also being investigated to enhance the longevity and stability of ethyl acetate-based products. By increasing product lifespan, these efforts can reduce the frequency of application or replacement, thereby lowering overall environmental impact. Encapsulation technologies and the use of stabilizing additives are among the strategies being explored to achieve this goal.
Furthermore, advancements in recycling and recovery technologies are playing a crucial role in mitigating the environmental impact of ethyl acetate formulations. Closed-loop systems and solvent recovery units are being implemented in industrial settings to capture and reuse ethyl acetate, significantly reducing emissions and waste generation. These practices not only minimize environmental harm but also offer economic benefits through reduced raw material costs.
In terms of atmospheric pollution, ethyl acetate contributes to the formation of ground-level ozone and photochemical smog when released into the air. This occurs through its reaction with nitrogen oxides in the presence of sunlight, potentially leading to respiratory issues in humans and damage to vegetation. The compound's relatively high volatility exacerbates this problem, as it readily evaporates at room temperature, increasing its atmospheric concentration.
Water pollution is another significant concern associated with ethyl acetate formulations. When improperly disposed of or accidentally released, ethyl acetate can contaminate water bodies, affecting aquatic ecosystems. Although it is biodegradable and does not persist in the environment for extended periods, high concentrations can still cause acute toxicity to aquatic organisms, disrupting the ecological balance of affected water systems.
The production process of ethyl acetate also contributes to its environmental footprint. Traditional manufacturing methods often rely on petrochemical feedstocks, which are non-renewable resources. The energy-intensive nature of these processes results in substantial greenhouse gas emissions, contributing to global climate change. Additionally, the use of catalysts and other chemicals in production can generate hazardous waste that requires careful handling and disposal.
To address these environmental concerns, researchers and industry professionals are exploring more sustainable formulation and production methods for ethyl acetate. Green chemistry principles are being applied to develop bio-based alternatives, using renewable feedstocks such as ethanol derived from agricultural waste. These approaches aim to reduce the carbon footprint of ethyl acetate production and minimize reliance on fossil fuels.
Improved formulation techniques are also being investigated to enhance the longevity and stability of ethyl acetate-based products. By increasing product lifespan, these efforts can reduce the frequency of application or replacement, thereby lowering overall environmental impact. Encapsulation technologies and the use of stabilizing additives are among the strategies being explored to achieve this goal.
Furthermore, advancements in recycling and recovery technologies are playing a crucial role in mitigating the environmental impact of ethyl acetate formulations. Closed-loop systems and solvent recovery units are being implemented in industrial settings to capture and reuse ethyl acetate, significantly reducing emissions and waste generation. These practices not only minimize environmental harm but also offer economic benefits through reduced raw material costs.
Quality Control Measures for Enhanced Ethyl Acetate
Quality control measures are crucial for ensuring the enhanced longevity of ethyl acetate. These measures encompass various aspects of the production process, from raw material selection to final product testing. Implementing rigorous quality control protocols helps maintain consistency, purity, and stability of ethyl acetate, ultimately extending its shelf life and improving its performance in various applications.
One of the primary quality control measures involves the careful selection and testing of raw materials. Ethanol and acetic acid, the primary components used in ethyl acetate production, must meet strict purity standards. Regular supplier audits and incoming material inspections are essential to verify the quality of these raw materials. Advanced analytical techniques, such as gas chromatography and mass spectrometry, can be employed to detect impurities and ensure that only high-grade materials are used in the production process.
Process control is another critical aspect of quality assurance for ethyl acetate. Monitoring and maintaining optimal reaction conditions, including temperature, pressure, and catalyst performance, are vital for achieving high-quality product synthesis. Implementing real-time process analytical technology (PAT) can provide continuous feedback on reaction progress and product quality, allowing for immediate adjustments to maintain consistency.
Post-production quality control measures are equally important for enhancing ethyl acetate longevity. These include thorough purification steps, such as distillation and drying, to remove any residual water or impurities that could compromise the product's stability. Advanced separation techniques, like azeotropic distillation, can be employed to achieve higher purity levels and improve the overall quality of the final product.
Packaging and storage conditions play a significant role in maintaining ethyl acetate quality over time. Utilizing appropriate container materials that are resistant to chemical interaction and impermeable to moisture is essential. Nitrogen blanketing or the use of desiccants can help prevent moisture ingress and oxidation, further extending the product's shelf life. Regular stability testing under various environmental conditions can provide valuable data on the product's long-term performance and help optimize storage recommendations.
Implementing a comprehensive quality management system, such as ISO 9001, can provide a structured approach to quality control throughout the entire production and distribution process. This system ensures that all quality control measures are consistently applied, documented, and continuously improved. Regular internal audits and third-party certifications can validate the effectiveness of these quality control measures and maintain high standards of ethyl acetate production.
One of the primary quality control measures involves the careful selection and testing of raw materials. Ethanol and acetic acid, the primary components used in ethyl acetate production, must meet strict purity standards. Regular supplier audits and incoming material inspections are essential to verify the quality of these raw materials. Advanced analytical techniques, such as gas chromatography and mass spectrometry, can be employed to detect impurities and ensure that only high-grade materials are used in the production process.
Process control is another critical aspect of quality assurance for ethyl acetate. Monitoring and maintaining optimal reaction conditions, including temperature, pressure, and catalyst performance, are vital for achieving high-quality product synthesis. Implementing real-time process analytical technology (PAT) can provide continuous feedback on reaction progress and product quality, allowing for immediate adjustments to maintain consistency.
Post-production quality control measures are equally important for enhancing ethyl acetate longevity. These include thorough purification steps, such as distillation and drying, to remove any residual water or impurities that could compromise the product's stability. Advanced separation techniques, like azeotropic distillation, can be employed to achieve higher purity levels and improve the overall quality of the final product.
Packaging and storage conditions play a significant role in maintaining ethyl acetate quality over time. Utilizing appropriate container materials that are resistant to chemical interaction and impermeable to moisture is essential. Nitrogen blanketing or the use of desiccants can help prevent moisture ingress and oxidation, further extending the product's shelf life. Regular stability testing under various environmental conditions can provide valuable data on the product's long-term performance and help optimize storage recommendations.
Implementing a comprehensive quality management system, such as ISO 9001, can provide a structured approach to quality control throughout the entire production and distribution process. This system ensures that all quality control measures are consistently applied, documented, and continuously improved. Regular internal audits and third-party certifications can validate the effectiveness of these quality control measures and maintain high standards of ethyl acetate production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!