How to Implement Gradient HPLC for Biomolecule Separation
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomolecule HPLC Evolution and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the 1960s, transforming from a rudimentary analytical technique to a sophisticated method essential for biomolecule separation. The evolution of gradient HPLC specifically has been pivotal in addressing the complex challenges presented by biomolecules such as proteins, peptides, nucleic acids, and other biological compounds that exhibit diverse physicochemical properties.
The early development of HPLC focused primarily on isocratic elution methods, which proved inadequate for separating complex biomolecular mixtures. The introduction of gradient elution in the 1970s marked a significant advancement, allowing for the systematic variation of mobile phase composition during analysis. This innovation dramatically improved separation efficiency and resolution for biomolecules with varying hydrophobicity and charge characteristics.
Throughout the 1980s and 1990s, technological advancements in HPLC instrumentation, including high-pressure pumps, precise gradient controllers, and sensitive detectors, further enhanced the capabilities of gradient HPLC for biomolecule analysis. The development of specialized column chemistries, such as reversed-phase, ion-exchange, and size-exclusion materials, expanded the application scope of this technique in biochemical and pharmaceutical research.
The advent of ultra-high-performance liquid chromatography (UHPLC) in the early 2000s represented another milestone, offering superior resolution, sensitivity, and speed compared to conventional HPLC. This advancement has been particularly beneficial for the analysis of complex biological samples, enabling the detection and quantification of biomolecules at increasingly lower concentrations.
Recent years have witnessed the integration of gradient HPLC with mass spectrometry and other advanced detection techniques, creating powerful analytical platforms for proteomics, genomics, and metabolomics research. These hyphenated techniques have revolutionized our ability to identify and characterize biomolecules in complex biological matrices.
The primary objectives of implementing gradient HPLC for biomolecule separation include achieving high-resolution separation of complex mixtures, maintaining the structural integrity and biological activity of sensitive biomolecules during analysis, and developing reproducible and robust analytical methods suitable for routine applications in research and quality control settings.
Additional goals encompass enhancing detection sensitivity to accommodate the often limited sample quantities available in biological research, reducing analysis time to increase throughput, and developing environmentally sustainable methods that minimize solvent consumption and waste generation. The ultimate aim is to establish gradient HPLC as a reliable, versatile, and efficient analytical tool that can address the diverse challenges presented by biomolecular analysis across various scientific disciplines.
The early development of HPLC focused primarily on isocratic elution methods, which proved inadequate for separating complex biomolecular mixtures. The introduction of gradient elution in the 1970s marked a significant advancement, allowing for the systematic variation of mobile phase composition during analysis. This innovation dramatically improved separation efficiency and resolution for biomolecules with varying hydrophobicity and charge characteristics.
Throughout the 1980s and 1990s, technological advancements in HPLC instrumentation, including high-pressure pumps, precise gradient controllers, and sensitive detectors, further enhanced the capabilities of gradient HPLC for biomolecule analysis. The development of specialized column chemistries, such as reversed-phase, ion-exchange, and size-exclusion materials, expanded the application scope of this technique in biochemical and pharmaceutical research.
The advent of ultra-high-performance liquid chromatography (UHPLC) in the early 2000s represented another milestone, offering superior resolution, sensitivity, and speed compared to conventional HPLC. This advancement has been particularly beneficial for the analysis of complex biological samples, enabling the detection and quantification of biomolecules at increasingly lower concentrations.
Recent years have witnessed the integration of gradient HPLC with mass spectrometry and other advanced detection techniques, creating powerful analytical platforms for proteomics, genomics, and metabolomics research. These hyphenated techniques have revolutionized our ability to identify and characterize biomolecules in complex biological matrices.
The primary objectives of implementing gradient HPLC for biomolecule separation include achieving high-resolution separation of complex mixtures, maintaining the structural integrity and biological activity of sensitive biomolecules during analysis, and developing reproducible and robust analytical methods suitable for routine applications in research and quality control settings.
Additional goals encompass enhancing detection sensitivity to accommodate the often limited sample quantities available in biological research, reducing analysis time to increase throughput, and developing environmentally sustainable methods that minimize solvent consumption and waste generation. The ultimate aim is to establish gradient HPLC as a reliable, versatile, and efficient analytical tool that can address the diverse challenges presented by biomolecular analysis across various scientific disciplines.
Market Analysis for Gradient HPLC Applications
The global market for gradient HPLC applications in biomolecule separation continues to experience robust growth, driven by increasing demand in pharmaceutical research, biotechnology, and academic institutions. Current market valuation stands at approximately 4.5 billion USD, with projections indicating a compound annual growth rate of 6.8% through 2028, significantly outpacing the broader analytical instrumentation market.
Pharmaceutical and biopharmaceutical sectors represent the largest market segments, collectively accounting for over 65% of gradient HPLC applications. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where precise biomolecule separation is critical. The expanding pipeline of biologic drugs, including monoclonal antibodies and recombinant proteins, has substantially increased demand for advanced separation technologies.
Academic and research institutions constitute the second-largest market segment at 18%, followed by food safety testing at 8%, environmental analysis at 5%, and other applications comprising the remaining 4%. Geographically, North America leads with 38% market share, followed by Europe (32%), Asia-Pacific (25%), and rest of the world (5%). Notably, China and India are experiencing the fastest growth rates, exceeding 10% annually, as their pharmaceutical and biotechnology sectors rapidly expand.
The consumables segment, including columns, solvents, and sample preparation products, generates recurring revenue streams that exceed instrument sales by a factor of 2.3 over the typical product lifecycle. High-performance columns designed specifically for biomolecule separation command premium pricing, with profit margins averaging 65% compared to 40% for standard HPLC columns.
Key market drivers include increasing R&D investments in biopharmaceuticals, growing adoption of precision medicine approaches, and technological advancements enabling higher resolution separations. The trend toward miniaturization and automation in HPLC systems is gaining momentum, with integrated systems offering reduced sample volume requirements and higher throughput capabilities.
Customer demand patterns reveal growing preference for modular systems that can be upgraded as needs evolve, rather than complete system replacements. Additionally, software integration capabilities have become a significant differentiating factor, with customers prioritizing platforms that offer seamless data management and compliance with regulatory requirements.
Market challenges include high initial investment costs, technical complexity requiring specialized training, and competition from alternative separation technologies such as ultra-high performance liquid chromatography (UHPLC) and capillary electrophoresis. However, gradient HPLC maintains competitive advantages in versatility, reliability, and established validation protocols, particularly for complex biomolecule separations.
Pharmaceutical and biopharmaceutical sectors represent the largest market segments, collectively accounting for over 65% of gradient HPLC applications. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where precise biomolecule separation is critical. The expanding pipeline of biologic drugs, including monoclonal antibodies and recombinant proteins, has substantially increased demand for advanced separation technologies.
Academic and research institutions constitute the second-largest market segment at 18%, followed by food safety testing at 8%, environmental analysis at 5%, and other applications comprising the remaining 4%. Geographically, North America leads with 38% market share, followed by Europe (32%), Asia-Pacific (25%), and rest of the world (5%). Notably, China and India are experiencing the fastest growth rates, exceeding 10% annually, as their pharmaceutical and biotechnology sectors rapidly expand.
The consumables segment, including columns, solvents, and sample preparation products, generates recurring revenue streams that exceed instrument sales by a factor of 2.3 over the typical product lifecycle. High-performance columns designed specifically for biomolecule separation command premium pricing, with profit margins averaging 65% compared to 40% for standard HPLC columns.
Key market drivers include increasing R&D investments in biopharmaceuticals, growing adoption of precision medicine approaches, and technological advancements enabling higher resolution separations. The trend toward miniaturization and automation in HPLC systems is gaining momentum, with integrated systems offering reduced sample volume requirements and higher throughput capabilities.
Customer demand patterns reveal growing preference for modular systems that can be upgraded as needs evolve, rather than complete system replacements. Additionally, software integration capabilities have become a significant differentiating factor, with customers prioritizing platforms that offer seamless data management and compliance with regulatory requirements.
Market challenges include high initial investment costs, technical complexity requiring specialized training, and competition from alternative separation technologies such as ultra-high performance liquid chromatography (UHPLC) and capillary electrophoresis. However, gradient HPLC maintains competitive advantages in versatility, reliability, and established validation protocols, particularly for complex biomolecule separations.
Current Gradient HPLC Technologies and Limitations
Gradient High-Performance Liquid Chromatography (HPLC) represents a cornerstone technology in biomolecule separation, offering superior resolution and flexibility compared to isocratic methods. Current gradient HPLC systems typically employ binary or quaternary pump configurations, with binary systems providing higher precision for challenging separations while quaternary systems offer greater versatility for method development. Modern instruments feature advanced capabilities including ultra-high pressure tolerance (up to 1500 bar), enabling the use of smaller particle size columns and faster analysis times.
Despite these advancements, several limitations persist in gradient HPLC applications for biomolecule separation. Gradient delay volume remains a significant challenge, particularly in conventional systems where it can range from 0.5-2.0 mL, causing reproducibility issues when transferring methods between instruments. This delay becomes especially problematic when working with small-volume columns or when rapid gradient changes are required for complex biomolecule mixtures.
Temperature control represents another critical limitation, as biomolecules often exhibit temperature-dependent retention behaviors. While current systems offer column temperature control, many lack precise temperature regulation for mobile phases before they enter the column, potentially causing peak broadening and reduced separation efficiency for temperature-sensitive biomolecules like proteins and nucleic acids.
Mobile phase compatibility issues present ongoing challenges, particularly when separating diverse biomolecule classes. Current gradient systems struggle with highly aqueous mobile phases needed for hydrophilic biomolecules, often experiencing check valve failures and pumping irregularities. Additionally, the limited pH range (typically 2-12) of conventional column materials restricts separation options for charged biomolecules.
Detector technology limitations affect comprehensive biomolecule analysis, with UV detectors lacking sensitivity for non-chromophoric biomolecules and mass spectrometers facing challenges with buffer compatibility. Current coupling technologies between gradient HPLC and mass spectrometry require compromises in either chromatographic performance or detection sensitivity.
Automation capabilities, while advanced, still present bottlenecks in high-throughput biomolecule analysis. Current systems offer autosampling and fraction collection but often lack integrated sample preparation capabilities critical for complex biological matrices. The software interfaces, though improved, frequently require significant expertise for method optimization, limiting accessibility for routine users.
Carryover effects remain particularly problematic for biomolecule analysis, with adsorption of proteins and peptides to system components causing sample cross-contamination. Despite advances in biocompatible materials, current systems still exhibit measurable carryover (typically 0.01-0.1%) that can compromise quantitative accuracy when analyzing low-abundance biomolecules in complex mixtures.
Despite these advancements, several limitations persist in gradient HPLC applications for biomolecule separation. Gradient delay volume remains a significant challenge, particularly in conventional systems where it can range from 0.5-2.0 mL, causing reproducibility issues when transferring methods between instruments. This delay becomes especially problematic when working with small-volume columns or when rapid gradient changes are required for complex biomolecule mixtures.
Temperature control represents another critical limitation, as biomolecules often exhibit temperature-dependent retention behaviors. While current systems offer column temperature control, many lack precise temperature regulation for mobile phases before they enter the column, potentially causing peak broadening and reduced separation efficiency for temperature-sensitive biomolecules like proteins and nucleic acids.
Mobile phase compatibility issues present ongoing challenges, particularly when separating diverse biomolecule classes. Current gradient systems struggle with highly aqueous mobile phases needed for hydrophilic biomolecules, often experiencing check valve failures and pumping irregularities. Additionally, the limited pH range (typically 2-12) of conventional column materials restricts separation options for charged biomolecules.
Detector technology limitations affect comprehensive biomolecule analysis, with UV detectors lacking sensitivity for non-chromophoric biomolecules and mass spectrometers facing challenges with buffer compatibility. Current coupling technologies between gradient HPLC and mass spectrometry require compromises in either chromatographic performance or detection sensitivity.
Automation capabilities, while advanced, still present bottlenecks in high-throughput biomolecule analysis. Current systems offer autosampling and fraction collection but often lack integrated sample preparation capabilities critical for complex biological matrices. The software interfaces, though improved, frequently require significant expertise for method optimization, limiting accessibility for routine users.
Carryover effects remain particularly problematic for biomolecule analysis, with adsorption of proteins and peptides to system components causing sample cross-contamination. Despite advances in biocompatible materials, current systems still exhibit measurable carryover (typically 0.01-0.1%) that can compromise quantitative accuracy when analyzing low-abundance biomolecules in complex mixtures.
Established Gradient HPLC Methodologies
01 Mobile phase composition and gradient optimization
Optimization of mobile phase composition and gradient profiles is crucial for effective HPLC separation. This involves carefully selecting solvent combinations and designing gradient elution programs that change solvent strength over time to improve separation of complex mixtures. Proper gradient design can enhance resolution, reduce analysis time, and improve peak shape for compounds with varying polarities.- Mobile phase composition and gradient optimization: Gradient HPLC separation involves the systematic variation of mobile phase composition during analysis to improve separation efficiency. By optimizing the gradient profile, including parameters such as initial and final solvent compositions, gradient slope, and time, chromatographers can achieve better resolution of complex mixtures. This approach allows for the separation of compounds with varying polarities within a single run, reducing analysis time while maintaining separation quality.
- Column technology for gradient separations: Specialized column technologies enhance gradient HPLC separation performance. These include columns with optimized particle sizes, pore structures, and stationary phase chemistries designed specifically for gradient applications. Modern columns feature improved stability under changing mobile phase conditions, reduced peak broadening, and compatibility with steep gradients. Advanced bonding technologies provide better peak shape and resolution while maintaining column longevity during repeated gradient cycles.
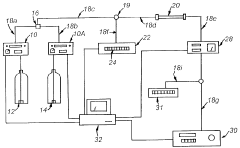
- Instrumentation and system design for gradient HPLC: Specialized instrumentation for gradient HPLC includes high-precision pumping systems capable of delivering accurate solvent mixtures with minimal delay volume and pulse-free operation. Modern systems incorporate advanced features such as automatic gradient formation, solvent degassing, and precise flow control. System designs focus on minimizing gradient delay volume, improving mixing efficiency, and ensuring reproducible gradient delivery to enhance separation performance and method transferability.
- Method development strategies for gradient separations: Systematic approaches to gradient HPLC method development involve strategic selection of initial conditions followed by methodical optimization. This includes screening of stationary phases, evaluation of different organic modifiers, pH adjustment, and temperature control. Advanced method development strategies incorporate computer-assisted modeling to predict retention behavior under various gradient conditions, reducing the experimental work required to achieve optimal separation. Quality by Design principles are applied to ensure robust and transferable gradient methods.
- Applications of gradient HPLC in specialized analyses: Gradient HPLC separation techniques are applied across diverse analytical challenges, including pharmaceutical analysis, environmental monitoring, food safety testing, and biological sample analysis. Specialized gradient approaches have been developed for complex matrices, chiral separations, and high-throughput screening. Recent innovations include ultra-high pressure gradient separations, multi-dimensional chromatography, and hyphenated techniques combining gradient HPLC with various detection methods to enhance sensitivity and specificity.
02 Column technology for gradient separations
Specialized column technologies designed specifically for gradient HPLC separations can significantly improve chromatographic performance. These columns feature optimized stationary phases, particle sizes, and pore structures that maintain stability under changing mobile phase conditions. Advanced column technologies provide better peak capacity, reduced band broadening, and improved resolution during gradient elution.Expand Specific Solutions03 Automated gradient HPLC systems
Automated systems for gradient HPLC separation incorporate programmable pumps, sample injectors, and detectors to ensure precise and reproducible analyses. These systems can automatically adjust flow rates, mixing ratios, and gradient profiles according to predefined methods. Advanced automation features include self-diagnosis, remote operation capabilities, and integration with data analysis software for improved efficiency and reliability.Expand Specific Solutions04 Method development for complex sample matrices
Specialized gradient HPLC methods for complex biological, pharmaceutical, and environmental samples require careful optimization to overcome matrix effects and achieve adequate separation. These methods involve strategic selection of gradient steepness, temperature control, pH adjustment, and detection parameters tailored to specific sample types. Advanced method development approaches may incorporate experimental design and modeling to efficiently optimize multiple separation parameters simultaneously.Expand Specific Solutions05 Novel detection techniques for gradient separations
Integration of advanced detection technologies with gradient HPLC separations enhances sensitivity, selectivity, and information content. These detection approaches include multi-wavelength UV detection, mass spectrometry, charged aerosol detection, and fluorescence techniques that can accommodate changing mobile phase compositions during gradient elution. Some systems incorporate multiple detection methods in series to provide complementary information about separated compounds.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Gradient HPLC for biomolecule separation is in a mature growth phase with an expanding market driven by biopharmaceutical and research applications. The global market is estimated at $3-4 billion, growing at 6-8% annually due to increasing demand for protein therapeutics and personalized medicine. Leading companies demonstrate varying technological maturity: Sepiatec, Jordi Labs, and PureHoney Technologies offer specialized HPLC solutions with advanced gradient capabilities; pharmaceutical giants like Sanofi, Biogen, and DuPont have integrated these systems into their R&D workflows; while academic institutions (Peking University, University of Michigan) and research organizations (CNRS) contribute significant methodological innovations, particularly in complex biomolecule analysis and high-throughput applications.
Biogen MA, Inc.
Technical Solution: Biogen has developed advanced gradient HPLC methodologies specifically optimized for therapeutic protein characterization and quality control. Their approach incorporates multi-dimensional chromatography combining ion-exchange and reversed-phase HPLC with specialized gradient profiles tailored for monoclonal antibody analysis. The company employs patented buffer systems with controlled pH gradients that enhance resolution of closely related protein variants. Their platform integrates automated sample preparation with specialized column technologies featuring core-shell particles that provide superior mass transfer properties for large biomolecules. Biogen's system incorporates real-time UV and mass spectrometric detection allowing for comprehensive characterization of post-translational modifications in biotherapeutics. The company has published extensively on their optimization of gradient steepness and temperature parameters to maximize separation efficiency while maintaining protein stability during analysis[1][3].
Strengths: Highly specialized for therapeutic protein analysis with superior resolution of protein variants and post-translational modifications. Integrated multi-detection platform provides comprehensive characterization. Weaknesses: System complexity requires significant expertise to operate and maintain. Higher implementation costs compared to standard HPLC systems. Limited flexibility for diverse biomolecule types beyond proteins.
Sanofi-Aventis Deutschland GmbH
Technical Solution: Sanofi-Aventis has pioneered a comprehensive gradient HPLC platform specifically designed for complex biomolecule separation in pharmaceutical development. Their approach features a quaternary pump system capable of delivering precise multi-component gradients with deviation control below 0.1%. The company has developed proprietary stationary phases with controlled surface chemistry and pore architecture optimized for different biomolecule classes including proteins, peptides, and oligonucleotides. Their method incorporates temperature-controlled column compartments with precision of ±0.1°C to ensure reproducibility in biomolecule separations that are highly temperature-dependent. Sanofi's platform includes specialized gradient profiles with segment-specific slope changes to enhance resolution in targeted regions of complex biomolecule mixtures. The system incorporates multiple detection modalities including UV-array, fluorescence, and mass spectrometry with automated method switching based on sample characteristics[2][5].
Strengths: Exceptional gradient precision and reproducibility critical for regulatory compliance in pharmaceutical development. Versatile platform applicable across multiple biomolecule classes. Comprehensive validation protocols ensure data reliability. Weaknesses: Significant capital investment required for implementation. Complex method development process with multiple parameters requiring optimization. Higher operational costs compared to isocratic systems.
Critical Patents and Innovations in Biomolecule Separation
Rapid method for separation of small molecules using reverse phase high performance liquid chromatography
PatentInactiveUS5968361A
Innovation
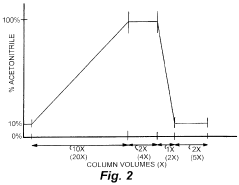
- The method involves minimizing the total volume of eluant applied to the column, maximizing the linear flow velocity, and compressing the gradient time to achieve peak resolution every 2 seconds, allowing for rapid separation of small organic compounds using a full gradient reverse phase HPLC system with a flow rate of at least 5 column volumes/min and a maximum total volume of 10 column volumes.
Separation of pre-peak in fusion protein sample by using size exclusion high performance liquid chromatography
PatentPendingUS20250236640A1
Innovation
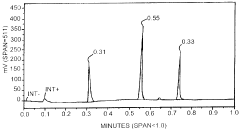
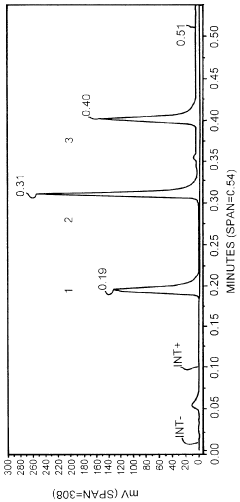
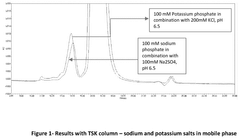
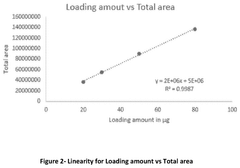
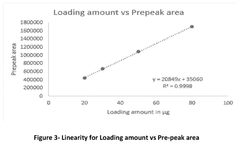
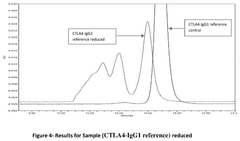
- A method involving SE-HPLC with specific mobile phase compositions and pH conditions, flow rates, and column types is employed to separate and quantify pre-peak impurities, achieving resolutions greater than 1.3 and pre-peak areas not less than 1.0, using columns like TSKgel G3000SWXL and mobile phases with salts such as potassium phosphate and sodium sulphate.
Method Validation and Regulatory Compliance
Method validation is a critical component in the implementation of gradient HPLC for biomolecule separation, ensuring that analytical procedures consistently deliver reliable and accurate results. The validation process must adhere to guidelines established by regulatory bodies such as the FDA, EMA, and ICH, particularly ICH Q2(R1) for analytical method validation.
For gradient HPLC methods targeting biomolecules, validation parameters must include specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. Specificity validation is particularly challenging for biomolecules due to their complex structures and potential for degradation products or impurities with similar properties.
System suitability tests (SSTs) serve as an ongoing verification mechanism, confirming that the chromatographic system performs adequately during routine analysis. For biomolecule separations, SST parameters typically include retention time reproducibility, peak resolution, column efficiency, and peak asymmetry factor, with acceptance criteria established during method development.
Regulatory compliance extends beyond method validation to encompass documentation requirements. Laboratories must maintain comprehensive records of instrument qualification (IQ/OQ/PQ), analyst training, reference standard certification, and method transfer protocols when applicable. For biomolecule analysis, additional documentation regarding sample stability and handling conditions is essential due to their susceptibility to degradation.
Risk assessment methodologies, such as Failure Mode and Effects Analysis (FMEA), should be incorporated into validation protocols to identify critical method parameters that could impact the reliability of biomolecule separation. This approach aligns with modern quality-by-design principles advocated by regulatory agencies.
For methods intended for release testing of biopharmaceuticals, validation must also address method transferability between laboratories and demonstrate robustness against variations in sample matrices. Comparative studies with orthogonal techniques may be required to confirm method specificity for complex biomolecules.
Ongoing method performance verification through trend analysis of system suitability data and periodic revalidation ensures continued compliance with regulatory expectations. For biomolecule separations, particular attention must be paid to column aging effects and potential changes in selectivity over time, which may necessitate more frequent performance verification than traditional small molecule methods.
For gradient HPLC methods targeting biomolecules, validation parameters must include specificity, linearity, range, accuracy, precision, detection limit, quantitation limit, and robustness. Specificity validation is particularly challenging for biomolecules due to their complex structures and potential for degradation products or impurities with similar properties.
System suitability tests (SSTs) serve as an ongoing verification mechanism, confirming that the chromatographic system performs adequately during routine analysis. For biomolecule separations, SST parameters typically include retention time reproducibility, peak resolution, column efficiency, and peak asymmetry factor, with acceptance criteria established during method development.
Regulatory compliance extends beyond method validation to encompass documentation requirements. Laboratories must maintain comprehensive records of instrument qualification (IQ/OQ/PQ), analyst training, reference standard certification, and method transfer protocols when applicable. For biomolecule analysis, additional documentation regarding sample stability and handling conditions is essential due to their susceptibility to degradation.
Risk assessment methodologies, such as Failure Mode and Effects Analysis (FMEA), should be incorporated into validation protocols to identify critical method parameters that could impact the reliability of biomolecule separation. This approach aligns with modern quality-by-design principles advocated by regulatory agencies.
For methods intended for release testing of biopharmaceuticals, validation must also address method transferability between laboratories and demonstrate robustness against variations in sample matrices. Comparative studies with orthogonal techniques may be required to confirm method specificity for complex biomolecules.
Ongoing method performance verification through trend analysis of system suitability data and periodic revalidation ensures continued compliance with regulatory expectations. For biomolecule separations, particular attention must be paid to column aging effects and potential changes in selectivity over time, which may necessitate more frequent performance verification than traditional small molecule methods.
Scale-up Strategies for Industrial Applications
Scaling up gradient HPLC methods from analytical to industrial scale requires careful consideration of multiple factors to maintain separation efficiency while increasing throughput. The transition from laboratory to production scale involves significant engineering challenges that must be addressed systematically to ensure consistent biomolecule separation performance.
Column dimensions represent a primary consideration in scale-up strategies. When moving from analytical columns (typically 4.6 mm internal diameter) to preparative or process-scale columns (50-1000 mm), the linear velocity must be maintained rather than flow rate alone. This requires adjusting flow rates proportionally to the square of the column diameter ratio, ensuring comparable separation kinetics across scales.
Buffer systems must be reformulated for industrial applications, with considerations for cost, availability, and disposal requirements. While laboratory-grade reagents are acceptable for analytical work, industrial-scale operations demand pharmaceutical-grade or bulk chemicals that maintain comparable separation performance while reducing operational expenses. Environmental impact assessments become increasingly important at larger scales.
Pressure limitations present significant challenges during scale-up. As column dimensions increase, the pressure drop across the column changes according to Darcy's law. Industrial systems often operate at lower pressures than analytical instruments, necessitating adjustments to gradient profiles, particle size selection, and column length to maintain separation efficiency without exceeding equipment pressure limits.
Gradient delay volumes increase dramatically in industrial systems due to larger pump heads, mixers, and connection tubing. This requires recalibration of gradient programs to account for these system-specific delays. Implementing pre-column mixers and reducing unnecessary tubing volume can help minimize these effects and maintain gradient accuracy.
Heat dissipation becomes critical at industrial scale. The friction generated as mobile phase moves through the packed bed creates heat that can degrade thermally sensitive biomolecules and create radial temperature gradients, reducing separation efficiency. Column jacketing, mobile phase pre-heating, and optimized flow rates help mitigate these thermal effects.
Economic considerations ultimately drive industrial scale-up decisions. A comprehensive cost analysis must balance throughput, resolution requirements, and resource utilization. Often, slightly reduced resolution is acceptable if it enables significantly higher throughput and lower operational costs. Continuous processing approaches, such as simulated moving bed chromatography, may offer advantages for large-scale biomolecule purification by maximizing stationary phase utilization and reducing solvent consumption.
Column dimensions represent a primary consideration in scale-up strategies. When moving from analytical columns (typically 4.6 mm internal diameter) to preparative or process-scale columns (50-1000 mm), the linear velocity must be maintained rather than flow rate alone. This requires adjusting flow rates proportionally to the square of the column diameter ratio, ensuring comparable separation kinetics across scales.
Buffer systems must be reformulated for industrial applications, with considerations for cost, availability, and disposal requirements. While laboratory-grade reagents are acceptable for analytical work, industrial-scale operations demand pharmaceutical-grade or bulk chemicals that maintain comparable separation performance while reducing operational expenses. Environmental impact assessments become increasingly important at larger scales.
Pressure limitations present significant challenges during scale-up. As column dimensions increase, the pressure drop across the column changes according to Darcy's law. Industrial systems often operate at lower pressures than analytical instruments, necessitating adjustments to gradient profiles, particle size selection, and column length to maintain separation efficiency without exceeding equipment pressure limits.
Gradient delay volumes increase dramatically in industrial systems due to larger pump heads, mixers, and connection tubing. This requires recalibration of gradient programs to account for these system-specific delays. Implementing pre-column mixers and reducing unnecessary tubing volume can help minimize these effects and maintain gradient accuracy.
Heat dissipation becomes critical at industrial scale. The friction generated as mobile phase moves through the packed bed creates heat that can degrade thermally sensitive biomolecules and create radial temperature gradients, reducing separation efficiency. Column jacketing, mobile phase pre-heating, and optimized flow rates help mitigate these thermal effects.
Economic considerations ultimately drive industrial scale-up decisions. A comprehensive cost analysis must balance throughput, resolution requirements, and resource utilization. Often, slightly reduced resolution is acceptable if it enables significantly higher throughput and lower operational costs. Continuous processing approaches, such as simulated moving bed chromatography, may offer advantages for large-scale biomolecule purification by maximizing stationary phase utilization and reducing solvent consumption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!