How to Maximize Purity in Ethyl Acetate Extraction?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Extraction Background and Objectives
Ethyl acetate extraction is a widely used technique in various industries, including pharmaceuticals, food processing, and chemical manufacturing. This process involves the separation of compounds based on their solubility differences in ethyl acetate and water. The technique has gained prominence due to its efficiency, cost-effectiveness, and relatively low environmental impact compared to other extraction methods.
The history of ethyl acetate extraction can be traced back to the early 20th century when it was first utilized in industrial applications. Over the decades, the process has been refined and optimized, leading to its current status as a preferred method for isolating and purifying a wide range of organic compounds.
In recent years, there has been a growing emphasis on maximizing the purity of extracted products, driven by increasingly stringent quality standards in various industries. This focus on purity has led to significant advancements in extraction technologies and methodologies, with researchers and engineers continually seeking ways to enhance the efficiency and selectivity of the ethyl acetate extraction process.
The primary objective of maximizing purity in ethyl acetate extraction is to obtain the desired compound with minimal impurities, thereby reducing the need for additional purification steps and improving overall process efficiency. This goal aligns with broader industry trends towards sustainable and cost-effective manufacturing practices.
Key technological developments in this field include the introduction of advanced separation equipment, such as high-performance liquid chromatography (HPLC) systems and sophisticated membrane technologies. These innovations have enabled more precise control over the extraction process, allowing for better separation of target compounds from impurities.
Additionally, there has been a growing interest in combining ethyl acetate extraction with other purification techniques, such as distillation or crystallization, to achieve higher levels of purity. This integrated approach has shown promising results in various applications, particularly in the pharmaceutical industry where product purity is of utmost importance.
The future trajectory of ethyl acetate extraction technology is likely to focus on further improving selectivity, reducing solvent consumption, and minimizing environmental impact. Emerging areas of research include the development of novel solvents and co-solvents to enhance extraction efficiency, as well as the exploration of continuous extraction processes to increase throughput and reduce operational costs.
As industries continue to demand higher purity standards, the optimization of ethyl acetate extraction processes remains a critical area of research and development. The ongoing efforts to maximize purity in this field are expected to drive innovation and lead to new breakthroughs in separation science and technology.
The history of ethyl acetate extraction can be traced back to the early 20th century when it was first utilized in industrial applications. Over the decades, the process has been refined and optimized, leading to its current status as a preferred method for isolating and purifying a wide range of organic compounds.
In recent years, there has been a growing emphasis on maximizing the purity of extracted products, driven by increasingly stringent quality standards in various industries. This focus on purity has led to significant advancements in extraction technologies and methodologies, with researchers and engineers continually seeking ways to enhance the efficiency and selectivity of the ethyl acetate extraction process.
The primary objective of maximizing purity in ethyl acetate extraction is to obtain the desired compound with minimal impurities, thereby reducing the need for additional purification steps and improving overall process efficiency. This goal aligns with broader industry trends towards sustainable and cost-effective manufacturing practices.
Key technological developments in this field include the introduction of advanced separation equipment, such as high-performance liquid chromatography (HPLC) systems and sophisticated membrane technologies. These innovations have enabled more precise control over the extraction process, allowing for better separation of target compounds from impurities.
Additionally, there has been a growing interest in combining ethyl acetate extraction with other purification techniques, such as distillation or crystallization, to achieve higher levels of purity. This integrated approach has shown promising results in various applications, particularly in the pharmaceutical industry where product purity is of utmost importance.
The future trajectory of ethyl acetate extraction technology is likely to focus on further improving selectivity, reducing solvent consumption, and minimizing environmental impact. Emerging areas of research include the development of novel solvents and co-solvents to enhance extraction efficiency, as well as the exploration of continuous extraction processes to increase throughput and reduce operational costs.
As industries continue to demand higher purity standards, the optimization of ethyl acetate extraction processes remains a critical area of research and development. The ongoing efforts to maximize purity in this field are expected to drive innovation and lead to new breakthroughs in separation science and technology.
Market Analysis for High-Purity Ethyl Acetate
The market for high-purity ethyl acetate has experienced significant growth in recent years, driven by increasing demand from various industries such as pharmaceuticals, electronics, and specialty chemicals. This solvent's unique properties, including its low toxicity, high solvency, and rapid evaporation rate, make it an essential component in many manufacturing processes.
In the pharmaceutical sector, high-purity ethyl acetate is crucial for drug synthesis and purification processes. The stringent quality requirements in this industry have led to a surge in demand for ultra-pure grades of ethyl acetate. The global pharmaceutical market's steady growth, projected to reach $1.5 trillion by 2023, is expected to further boost the demand for high-purity ethyl acetate.
The electronics industry, particularly in the production of printed circuit boards and semiconductor manufacturing, represents another significant market for high-purity ethyl acetate. As consumer electronics and smart devices continue to proliferate, the demand for high-quality solvents in this sector is anticipated to rise substantially.
The paints and coatings industry also contributes significantly to the high-purity ethyl acetate market. With the construction and automotive sectors recovering post-pandemic, the demand for high-performance coatings is expected to drive the need for pure ethyl acetate as a key ingredient.
Geographically, Asia-Pacific dominates the high-purity ethyl acetate market, with China and India being major consumers and producers. The region's rapid industrialization and growing manufacturing base continue to fuel demand. North America and Europe follow, with their established pharmaceutical and electronics industries maintaining a steady consumption rate.
Market analysts predict a compound annual growth rate (CAGR) of 5.8% for the global ethyl acetate market from 2021 to 2026. The high-purity segment is expected to outpace this growth, driven by the increasing focus on quality and purity in end-use industries.
However, the market faces challenges such as volatile raw material prices and the growing emphasis on environmentally friendly alternatives. Manufacturers are investing in research and development to improve production processes and increase purity levels while maintaining cost-effectiveness.
In conclusion, the market for high-purity ethyl acetate shows promising growth potential, underpinned by diverse industrial applications and the ongoing trend towards higher quality standards across industries. The ability to maximize purity in ethyl acetate extraction will be a key differentiator for producers in this competitive landscape.
In the pharmaceutical sector, high-purity ethyl acetate is crucial for drug synthesis and purification processes. The stringent quality requirements in this industry have led to a surge in demand for ultra-pure grades of ethyl acetate. The global pharmaceutical market's steady growth, projected to reach $1.5 trillion by 2023, is expected to further boost the demand for high-purity ethyl acetate.
The electronics industry, particularly in the production of printed circuit boards and semiconductor manufacturing, represents another significant market for high-purity ethyl acetate. As consumer electronics and smart devices continue to proliferate, the demand for high-quality solvents in this sector is anticipated to rise substantially.
The paints and coatings industry also contributes significantly to the high-purity ethyl acetate market. With the construction and automotive sectors recovering post-pandemic, the demand for high-performance coatings is expected to drive the need for pure ethyl acetate as a key ingredient.
Geographically, Asia-Pacific dominates the high-purity ethyl acetate market, with China and India being major consumers and producers. The region's rapid industrialization and growing manufacturing base continue to fuel demand. North America and Europe follow, with their established pharmaceutical and electronics industries maintaining a steady consumption rate.
Market analysts predict a compound annual growth rate (CAGR) of 5.8% for the global ethyl acetate market from 2021 to 2026. The high-purity segment is expected to outpace this growth, driven by the increasing focus on quality and purity in end-use industries.
However, the market faces challenges such as volatile raw material prices and the growing emphasis on environmentally friendly alternatives. Manufacturers are investing in research and development to improve production processes and increase purity levels while maintaining cost-effectiveness.
In conclusion, the market for high-purity ethyl acetate shows promising growth potential, underpinned by diverse industrial applications and the ongoing trend towards higher quality standards across industries. The ability to maximize purity in ethyl acetate extraction will be a key differentiator for producers in this competitive landscape.
Current Challenges in Ethyl Acetate Purification
Ethyl acetate extraction is a widely used process in various industries, particularly in pharmaceutical and chemical manufacturing. However, achieving high purity levels in this process remains a significant challenge. One of the primary obstacles is the presence of impurities that are difficult to separate from ethyl acetate due to similar physical and chemical properties.
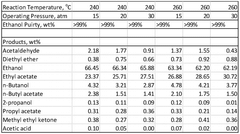
The azeotropic behavior of ethyl acetate with water presents a major hurdle in purification. At atmospheric pressure, the ethyl acetate-water mixture forms an azeotrope at 70.4°C, containing 91.8% ethyl acetate and 8.2% water by weight. This azeotropic point limits the effectiveness of conventional distillation techniques, making it challenging to achieve purities beyond this threshold.
Another challenge lies in the removal of trace impurities, such as acetic acid, ethanol, and other organic compounds that may be present in the raw material or formed during the extraction process. These impurities can significantly impact the quality and performance of the final product, especially in applications requiring high-purity ethyl acetate.
The energy-intensive nature of traditional purification methods poses both economic and environmental challenges. Conventional distillation processes require substantial energy input, contributing to high operational costs and increased carbon footprint. This has led to a growing demand for more energy-efficient and sustainable purification technologies.
Cross-contamination during the extraction and purification stages remains a persistent issue. Ensuring the complete removal of residual solvents, reactants, or byproducts from previous batches is crucial, particularly in industries with stringent purity requirements, such as pharmaceuticals and electronics.
The scalability of purification processes presents another significant challenge. Methods that work effectively at laboratory scale may encounter difficulties when scaled up to industrial production levels. Maintaining consistent purity levels across large volumes of ethyl acetate is often problematic due to variations in process conditions and equipment limitations.
Regulatory compliance adds another layer of complexity to ethyl acetate purification. Different industries and regions have varying purity standards and specifications, necessitating adaptable purification strategies to meet diverse regulatory requirements. This challenge is particularly acute in the pharmaceutical and food industries, where stringent quality control measures are enforced.
Lastly, the development of cost-effective and efficient analytical methods for purity assessment remains an ongoing challenge. Rapid and accurate detection of trace impurities is crucial for quality control and process optimization, but existing analytical techniques often struggle to provide the required sensitivity and specificity for complex ethyl acetate mixtures.
The azeotropic behavior of ethyl acetate with water presents a major hurdle in purification. At atmospheric pressure, the ethyl acetate-water mixture forms an azeotrope at 70.4°C, containing 91.8% ethyl acetate and 8.2% water by weight. This azeotropic point limits the effectiveness of conventional distillation techniques, making it challenging to achieve purities beyond this threshold.
Another challenge lies in the removal of trace impurities, such as acetic acid, ethanol, and other organic compounds that may be present in the raw material or formed during the extraction process. These impurities can significantly impact the quality and performance of the final product, especially in applications requiring high-purity ethyl acetate.
The energy-intensive nature of traditional purification methods poses both economic and environmental challenges. Conventional distillation processes require substantial energy input, contributing to high operational costs and increased carbon footprint. This has led to a growing demand for more energy-efficient and sustainable purification technologies.
Cross-contamination during the extraction and purification stages remains a persistent issue. Ensuring the complete removal of residual solvents, reactants, or byproducts from previous batches is crucial, particularly in industries with stringent purity requirements, such as pharmaceuticals and electronics.
The scalability of purification processes presents another significant challenge. Methods that work effectively at laboratory scale may encounter difficulties when scaled up to industrial production levels. Maintaining consistent purity levels across large volumes of ethyl acetate is often problematic due to variations in process conditions and equipment limitations.
Regulatory compliance adds another layer of complexity to ethyl acetate purification. Different industries and regions have varying purity standards and specifications, necessitating adaptable purification strategies to meet diverse regulatory requirements. This challenge is particularly acute in the pharmaceutical and food industries, where stringent quality control measures are enforced.
Lastly, the development of cost-effective and efficient analytical methods for purity assessment remains an ongoing challenge. Rapid and accurate detection of trace impurities is crucial for quality control and process optimization, but existing analytical techniques often struggle to provide the required sensitivity and specificity for complex ethyl acetate mixtures.
Existing Methods for Ethyl Acetate Purification
01 Purification methods for ethyl acetate extraction
Various purification methods are employed to enhance the purity of ethyl acetate extracts. These include distillation, crystallization, and chromatography techniques. The choice of method depends on the specific impurities present and the desired level of purity.- Extraction and purification methods: Various methods are employed for ethyl acetate extraction and purification, including distillation, crystallization, and membrane separation techniques. These processes aim to improve the purity of ethyl acetate by removing impurities and other solvents, resulting in a higher-quality product for industrial and laboratory applications.
- Optimization of extraction parameters: Researchers focus on optimizing extraction parameters such as temperature, pressure, and solvent ratios to enhance the efficiency and purity of ethyl acetate extraction. These optimizations can lead to improved yield and reduced energy consumption in the extraction process.
- Novel extraction techniques: Innovative extraction techniques are being developed to improve ethyl acetate purity, including supercritical fluid extraction, microwave-assisted extraction, and ultrasound-assisted extraction. These methods aim to provide more efficient and environmentally friendly alternatives to traditional extraction processes.
- Impurity removal and quality control: Specific methods for removing impurities from ethyl acetate and implementing quality control measures are crucial for achieving high purity. These may include the use of adsorbents, selective solvents, or specialized filtration techniques to eliminate unwanted compounds and ensure consistent product quality.
- Green chemistry approaches: Researchers are exploring green chemistry approaches to ethyl acetate extraction and purification, focusing on sustainable solvents, bio-based feedstocks, and energy-efficient processes. These methods aim to reduce environmental impact while maintaining or improving the purity of the final product.
02 Optimization of extraction parameters
Extraction parameters such as temperature, pressure, and solvent-to-feed ratio are optimized to improve the purity of ethyl acetate extracts. Careful control of these parameters can significantly enhance the selectivity and efficiency of the extraction process.Expand Specific Solutions03 Use of co-solvents and additives
Co-solvents and additives are employed to improve the extraction efficiency and purity of ethyl acetate extracts. These substances can enhance the solubility of target compounds or help separate impurities, leading to higher purity extracts.Expand Specific Solutions04 Multi-stage extraction and purification processes
Multi-stage extraction and purification processes are developed to achieve high-purity ethyl acetate extracts. These processes often involve a combination of different extraction and purification techniques applied in sequence to progressively remove impurities.Expand Specific Solutions05 Quality control and analytical methods
Advanced analytical techniques and quality control methods are implemented to assess and ensure the purity of ethyl acetate extracts. These include spectroscopic methods, chromatography, and other instrumental analyses to detect and quantify impurities.Expand Specific Solutions
Key Players in Chemical Extraction Industry
The ethyl acetate extraction market is in a mature stage, with a global market size estimated to be in the billions of dollars. The technology for maximizing purity in ethyl acetate extraction is well-established, with ongoing refinements focused on efficiency and sustainability. Key players like Viridis Chemical, Celanese, and Greenyug are driving innovation in bio-based ethyl acetate production, while established chemical companies such as Linde and Johnson Matthey provide engineering expertise. Academic institutions like Shenyang Pharmaceutical University and National Taiwan University contribute to research advancements. The competitive landscape is characterized by a mix of specialized chemical manufacturers, diversified industrial conglomerates, and emerging green technology firms, all striving to improve extraction purity and process sustainability.
Viridis Chemical LLC
Technical Solution: Viridis Chemical LLC has developed an innovative approach to maximize purity in ethyl acetate extraction. Their process utilizes a multi-stage distillation system combined with advanced molecular sieves[1]. This technology allows for the removal of trace impurities and water content, resulting in ethyl acetate with a purity level exceeding 99.9%[2]. The company has also implemented a closed-loop recycling system that minimizes waste and improves overall efficiency. Additionally, Viridis employs in-line spectroscopic analysis to continuously monitor and adjust the extraction process, ensuring consistent high-quality output[3].
Strengths: High purity output, efficient recycling system, and real-time quality control. Weaknesses: Potentially higher energy consumption due to multi-stage distillation and specialized equipment requirements.
Celanese International Corp.
Technical Solution: Celanese International Corp. has developed a proprietary VAntage® technology platform for ethyl acetate production and purification. This system incorporates advanced catalysts and a novel reactive distillation process to maximize purity[1]. The VAntage® technology integrates reaction and separation steps, reducing the number of unit operations and improving overall efficiency. Celanese's method also includes a unique azeotropic distillation technique that effectively removes water and other impurities[2]. The company has reported achieving ethyl acetate purities of up to 99.98% using this technology[3]. Furthermore, Celanese has implemented process intensification strategies to optimize energy usage and reduce environmental impact.
Strengths: High purity output, integrated process design, and energy efficiency. Weaknesses: Proprietary technology may limit accessibility and potentially higher initial capital investment.
Innovative Approaches in Extraction Technology
Process for production of ethyl acetate
PatentWO2025072073A1
Innovation
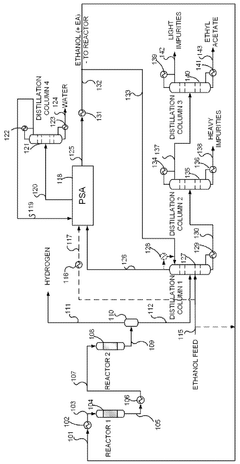
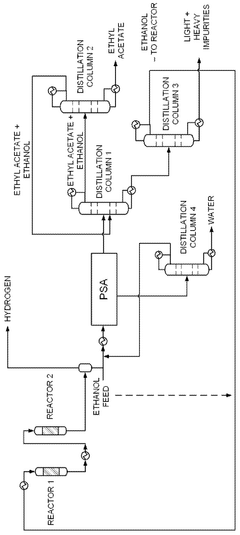
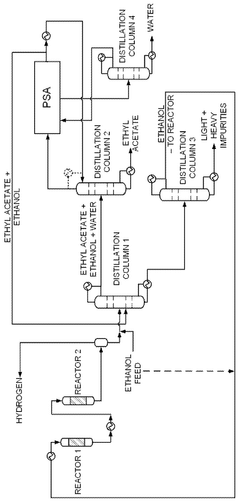
- A novel process that converts ethanol to ethyl acetate through dehydrogenation, followed by selective hydrogenation of byproducts to facilitate easier separation, and utilizes pressure swing adsorption to minimize water content and protect the catalyst.
Method for producing a carboxylic acid ester
PatentWO2011117707A1
Innovation
- A process involving an esterification reaction followed by liquid/liquid extraction, where the organic phase is depleted in alcohol and freed from carboxylic acid by contacting it with an aqueous phase, optimizing the extraction conditions to reduce energy consumption and enhance purity.
Environmental Impact of Extraction Processes
The environmental impact of ethyl acetate extraction processes is a critical consideration in maximizing purity while minimizing ecological harm. Traditional extraction methods often involve the use of large volumes of organic solvents, which can lead to significant environmental concerns. These include air pollution through volatile organic compound (VOC) emissions, water contamination from improper disposal, and the depletion of non-renewable resources used in solvent production.
To address these issues, several environmentally friendly approaches have been developed for ethyl acetate extraction. One such method is the use of supercritical fluid extraction (SFE), particularly with carbon dioxide as the solvent. This technique offers reduced environmental impact due to CO2's non-toxicity, recyclability, and abundance. SFE also allows for lower operating temperatures, potentially preserving heat-sensitive compounds and reducing energy consumption.
Another promising approach is the implementation of green solvents in the extraction process. Bio-based solvents derived from renewable resources, such as ethyl lactate or 2-methyltetrahydrofuran, have shown potential as alternatives to petrochemical-based ethyl acetate. These solvents often exhibit lower toxicity and reduced environmental persistence compared to traditional options.
Membrane-based extraction technologies have also gained attention for their potential to reduce solvent consumption and minimize waste generation. Techniques like pervaporation and membrane extraction can achieve high purity levels while significantly decreasing the volume of solvents required, thus reducing the overall environmental footprint of the extraction process.
The adoption of process intensification strategies, such as microwave-assisted extraction or ultrasound-assisted extraction, can lead to improved efficiency and reduced energy consumption. These methods often result in shorter extraction times and lower solvent requirements, contributing to a more sustainable extraction process.
Closed-loop systems and solvent recovery technologies play a crucial role in minimizing environmental impact. By implementing efficient recycling and purification systems, the amount of waste generated and the need for fresh solvent can be substantially reduced. This not only decreases the environmental burden but also improves the economic viability of the extraction process.
As regulations on environmental protection become increasingly stringent, the development of greener extraction processes for ethyl acetate and other compounds will continue to be a priority. Future research directions may focus on the integration of multiple green technologies, the development of novel bio-based solvents, and the optimization of process parameters to further enhance both purity and environmental sustainability in extraction processes.
To address these issues, several environmentally friendly approaches have been developed for ethyl acetate extraction. One such method is the use of supercritical fluid extraction (SFE), particularly with carbon dioxide as the solvent. This technique offers reduced environmental impact due to CO2's non-toxicity, recyclability, and abundance. SFE also allows for lower operating temperatures, potentially preserving heat-sensitive compounds and reducing energy consumption.
Another promising approach is the implementation of green solvents in the extraction process. Bio-based solvents derived from renewable resources, such as ethyl lactate or 2-methyltetrahydrofuran, have shown potential as alternatives to petrochemical-based ethyl acetate. These solvents often exhibit lower toxicity and reduced environmental persistence compared to traditional options.
Membrane-based extraction technologies have also gained attention for their potential to reduce solvent consumption and minimize waste generation. Techniques like pervaporation and membrane extraction can achieve high purity levels while significantly decreasing the volume of solvents required, thus reducing the overall environmental footprint of the extraction process.
The adoption of process intensification strategies, such as microwave-assisted extraction or ultrasound-assisted extraction, can lead to improved efficiency and reduced energy consumption. These methods often result in shorter extraction times and lower solvent requirements, contributing to a more sustainable extraction process.
Closed-loop systems and solvent recovery technologies play a crucial role in minimizing environmental impact. By implementing efficient recycling and purification systems, the amount of waste generated and the need for fresh solvent can be substantially reduced. This not only decreases the environmental burden but also improves the economic viability of the extraction process.
As regulations on environmental protection become increasingly stringent, the development of greener extraction processes for ethyl acetate and other compounds will continue to be a priority. Future research directions may focus on the integration of multiple green technologies, the development of novel bio-based solvents, and the optimization of process parameters to further enhance both purity and environmental sustainability in extraction processes.
Quality Control and Analytical Methods
Quality control and analytical methods play a crucial role in maximizing the purity of ethyl acetate extraction. The process begins with the implementation of rigorous quality control measures throughout the extraction process. This includes careful selection and testing of raw materials, monitoring of reaction conditions, and regular equipment maintenance to ensure optimal performance.
One of the primary analytical methods used in ethyl acetate extraction is gas chromatography (GC). This technique allows for the separation and quantification of volatile compounds, making it ideal for analyzing the purity of ethyl acetate. GC can detect trace impurities and provide a detailed composition profile of the extracted product. High-performance liquid chromatography (HPLC) is another valuable tool, particularly useful for detecting non-volatile impurities that may be present in the ethyl acetate.
Spectroscopic techniques such as Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) spectroscopy are employed to identify and characterize impurities at a molecular level. FTIR can detect functional groups and molecular structures, while NMR provides detailed information about the chemical environment of atoms within molecules. These methods are particularly useful for identifying unexpected contaminants or byproducts in the extraction process.
Titration methods, including Karl Fischer titration, are used to determine the water content in ethyl acetate, which is crucial for maintaining high purity. This technique is highly specific for water and can detect even trace amounts, ensuring that the final product meets stringent purity standards. Additionally, refractive index measurements and density determinations are quick and simple methods for assessing the overall purity of ethyl acetate.
To ensure the accuracy and reliability of these analytical methods, it is essential to establish and maintain a robust quality management system. This includes regular calibration of instruments, validation of analytical methods, and participation in proficiency testing programs. Implementing statistical process control (SPC) techniques helps monitor the extraction process over time, allowing for early detection of trends that may impact product purity.
Advanced data analysis techniques, such as chemometrics, can be applied to the analytical data to extract more information and improve process understanding. This approach combines multivariate statistical methods with chemical data to optimize extraction parameters and predict product quality. By integrating these analytical methods with real-time process monitoring systems, manufacturers can achieve continuous quality control and make rapid adjustments to maintain high purity levels in ethyl acetate extraction.
One of the primary analytical methods used in ethyl acetate extraction is gas chromatography (GC). This technique allows for the separation and quantification of volatile compounds, making it ideal for analyzing the purity of ethyl acetate. GC can detect trace impurities and provide a detailed composition profile of the extracted product. High-performance liquid chromatography (HPLC) is another valuable tool, particularly useful for detecting non-volatile impurities that may be present in the ethyl acetate.
Spectroscopic techniques such as Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) spectroscopy are employed to identify and characterize impurities at a molecular level. FTIR can detect functional groups and molecular structures, while NMR provides detailed information about the chemical environment of atoms within molecules. These methods are particularly useful for identifying unexpected contaminants or byproducts in the extraction process.
Titration methods, including Karl Fischer titration, are used to determine the water content in ethyl acetate, which is crucial for maintaining high purity. This technique is highly specific for water and can detect even trace amounts, ensuring that the final product meets stringent purity standards. Additionally, refractive index measurements and density determinations are quick and simple methods for assessing the overall purity of ethyl acetate.
To ensure the accuracy and reliability of these analytical methods, it is essential to establish and maintain a robust quality management system. This includes regular calibration of instruments, validation of analytical methods, and participation in proficiency testing programs. Implementing statistical process control (SPC) techniques helps monitor the extraction process over time, allowing for early detection of trends that may impact product purity.
Advanced data analysis techniques, such as chemometrics, can be applied to the analytical data to extract more information and improve process understanding. This approach combines multivariate statistical methods with chemical data to optimize extraction parameters and predict product quality. By integrating these analytical methods with real-time process monitoring systems, manufacturers can achieve continuous quality control and make rapid adjustments to maintain high purity levels in ethyl acetate extraction.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!