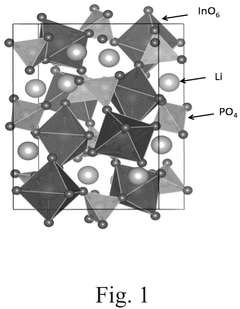
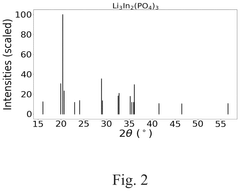
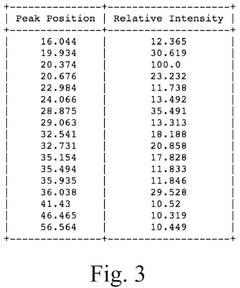
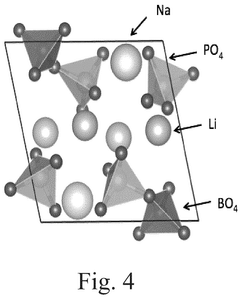
How to Measure Lithium Phosphate Conductive Properties Accurately
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Phosphate Conductivity Measurement Background and Objectives
Lithium phosphate materials have emerged as critical components in the development of advanced energy storage systems, particularly in the realm of solid-state batteries. The evolution of these materials spans several decades, beginning with fundamental research in the 1970s on lithium-ion conductors, followed by significant breakthroughs in the 1990s with the discovery of NASICON-type structures and lithium phosphate olivines. Recent years have witnessed an acceleration in research activities focused on enhancing the conductive properties of these materials to meet the growing demands of next-generation energy storage technologies.
The technological trajectory of lithium phosphate conductivity measurement has been characterized by continuous refinement of methodologies. Early approaches relied primarily on basic impedance spectroscopy techniques with limited frequency ranges and temperature controls. As understanding of ion transport mechanisms deepened, measurement techniques evolved to incorporate more sophisticated electrochemical methods, including advanced AC impedance spectroscopy, galvanostatic intermittent titration technique (GITT), and potentiostatic intermittent titration technique (PITT).
Current research trends indicate a shift toward multi-modal measurement approaches that combine electrochemical, spectroscopic, and computational methods to achieve comprehensive characterization of conductive properties. This integration of techniques allows for more accurate determination of bulk, grain boundary, and interfacial conductivities—parameters critical for optimizing material performance in practical applications.
The primary technical objectives in this field center on developing standardized, reproducible measurement protocols that can accurately quantify the intrinsic ionic conductivity of lithium phosphate materials across varying compositions, microstructures, and environmental conditions. Specifically, there is a pressing need to establish methods that can distinguish between electronic and ionic contributions to total conductivity, as well as techniques capable of measuring conductivity under operational conditions relevant to actual battery systems.
Additionally, researchers aim to correlate measured conductivity values with structural features and processing parameters to establish predictive models for material optimization. This includes understanding how factors such as crystallinity, grain size, dopant concentration, and synthesis routes influence the conductive properties of lithium phosphate materials.
Looking forward, the field is moving toward in-situ and operando measurement techniques that can monitor conductivity changes during battery cycling, thereby providing insights into degradation mechanisms and performance limitations. The ultimate goal is to develop measurement methodologies that can accelerate the discovery and optimization of high-performance lithium phosphate materials for next-generation energy storage systems, supporting the broader transition to sustainable energy technologies.
The technological trajectory of lithium phosphate conductivity measurement has been characterized by continuous refinement of methodologies. Early approaches relied primarily on basic impedance spectroscopy techniques with limited frequency ranges and temperature controls. As understanding of ion transport mechanisms deepened, measurement techniques evolved to incorporate more sophisticated electrochemical methods, including advanced AC impedance spectroscopy, galvanostatic intermittent titration technique (GITT), and potentiostatic intermittent titration technique (PITT).
Current research trends indicate a shift toward multi-modal measurement approaches that combine electrochemical, spectroscopic, and computational methods to achieve comprehensive characterization of conductive properties. This integration of techniques allows for more accurate determination of bulk, grain boundary, and interfacial conductivities—parameters critical for optimizing material performance in practical applications.
The primary technical objectives in this field center on developing standardized, reproducible measurement protocols that can accurately quantify the intrinsic ionic conductivity of lithium phosphate materials across varying compositions, microstructures, and environmental conditions. Specifically, there is a pressing need to establish methods that can distinguish between electronic and ionic contributions to total conductivity, as well as techniques capable of measuring conductivity under operational conditions relevant to actual battery systems.
Additionally, researchers aim to correlate measured conductivity values with structural features and processing parameters to establish predictive models for material optimization. This includes understanding how factors such as crystallinity, grain size, dopant concentration, and synthesis routes influence the conductive properties of lithium phosphate materials.
Looking forward, the field is moving toward in-situ and operando measurement techniques that can monitor conductivity changes during battery cycling, thereby providing insights into degradation mechanisms and performance limitations. The ultimate goal is to develop measurement methodologies that can accelerate the discovery and optimization of high-performance lithium phosphate materials for next-generation energy storage systems, supporting the broader transition to sustainable energy technologies.
Market Applications and Demand Analysis for Lithium Phosphate Materials
The lithium phosphate materials market is experiencing unprecedented growth, primarily driven by the rapid expansion of the electric vehicle (EV) industry. As governments worldwide implement stringent emission regulations and offer incentives for EV adoption, the demand for high-performance lithium iron phosphate (LFP) batteries has surged dramatically. Market research indicates that the global LFP battery market is projected to grow at a compound annual growth rate of 15% through 2030, with the EV segment accounting for approximately two-thirds of this demand.
Beyond automotive applications, lithium phosphate materials are gaining significant traction in stationary energy storage systems. The push for renewable energy integration into power grids has created substantial demand for large-scale battery storage solutions, where LFP's safety characteristics and longer cycle life provide compelling advantages over alternative chemistries. Utility companies and renewable energy developers are increasingly deploying LFP-based storage systems for grid stabilization, peak shaving, and backup power applications.
Consumer electronics represents another growing market segment for lithium phosphate materials. Manufacturers are exploring LFP batteries for devices requiring enhanced safety and longevity, particularly in applications where the higher energy density of traditional lithium-ion batteries is not critical. This includes power tools, medical devices, and certain portable electronics where operational safety is paramount.
The industrial sector has also begun adopting lithium phosphate materials for specialized applications. Material handling equipment, including forklifts and automated guided vehicles in warehouses and manufacturing facilities, increasingly utilize LFP batteries due to their fast-charging capabilities and operational safety in confined environments. Similarly, telecommunications infrastructure and data centers are adopting LFP-based backup power systems.
Regional market analysis reveals that Asia-Pacific, particularly China, dominates both production and consumption of lithium phosphate materials. However, significant manufacturing capacity expansion is underway in North America and Europe as part of strategic initiatives to reduce dependency on Asian supply chains. This regionalization trend is reshaping the global market landscape and driving innovation in manufacturing processes.
Market demand is increasingly focused on lithium phosphate materials with enhanced conductive properties, as these directly impact battery performance metrics including charge/discharge rates, energy efficiency, and power density. Consequently, accurate measurement of conductive properties has become a critical factor in material selection and quality control processes across the supply chain, highlighting the importance of standardized and reliable measurement methodologies.
Beyond automotive applications, lithium phosphate materials are gaining significant traction in stationary energy storage systems. The push for renewable energy integration into power grids has created substantial demand for large-scale battery storage solutions, where LFP's safety characteristics and longer cycle life provide compelling advantages over alternative chemistries. Utility companies and renewable energy developers are increasingly deploying LFP-based storage systems for grid stabilization, peak shaving, and backup power applications.
Consumer electronics represents another growing market segment for lithium phosphate materials. Manufacturers are exploring LFP batteries for devices requiring enhanced safety and longevity, particularly in applications where the higher energy density of traditional lithium-ion batteries is not critical. This includes power tools, medical devices, and certain portable electronics where operational safety is paramount.
The industrial sector has also begun adopting lithium phosphate materials for specialized applications. Material handling equipment, including forklifts and automated guided vehicles in warehouses and manufacturing facilities, increasingly utilize LFP batteries due to their fast-charging capabilities and operational safety in confined environments. Similarly, telecommunications infrastructure and data centers are adopting LFP-based backup power systems.
Regional market analysis reveals that Asia-Pacific, particularly China, dominates both production and consumption of lithium phosphate materials. However, significant manufacturing capacity expansion is underway in North America and Europe as part of strategic initiatives to reduce dependency on Asian supply chains. This regionalization trend is reshaping the global market landscape and driving innovation in manufacturing processes.
Market demand is increasingly focused on lithium phosphate materials with enhanced conductive properties, as these directly impact battery performance metrics including charge/discharge rates, energy efficiency, and power density. Consequently, accurate measurement of conductive properties has become a critical factor in material selection and quality control processes across the supply chain, highlighting the importance of standardized and reliable measurement methodologies.
Current Measurement Techniques and Technical Challenges
The accurate measurement of lithium phosphate conductive properties presents significant challenges due to the complex nature of these materials and their sensitivity to environmental conditions. Currently, several established techniques are employed in research and industrial settings, each with distinct advantages and limitations.
Electrochemical Impedance Spectroscopy (EIS) remains the gold standard for measuring ionic conductivity in solid electrolytes. This technique applies an AC voltage of varying frequencies to the sample and measures the resulting current response, allowing researchers to determine the bulk, grain boundary, and total conductivity. However, EIS requires careful sample preparation and electrode contact optimization to avoid introducing measurement artifacts.
Direct Current (DC) polarization methods provide complementary data to EIS by applying a constant voltage across the sample and measuring the resulting steady-state current. While conceptually simpler than EIS, DC methods often struggle with electrode polarization effects that can significantly distort measurements of fast ion conductors like lithium phosphates.
The four-point probe technique has gained popularity for its ability to eliminate contact resistance issues. By using separate current and voltage electrodes, this method provides more accurate bulk conductivity measurements but requires precise probe placement and can be challenging to implement with thin-film or irregularly shaped samples.
Temperature-dependent conductivity measurements represent another critical approach, as they enable the determination of activation energies for ion transport. These measurements typically involve specialized equipment capable of maintaining stable temperatures while simultaneously performing electrical measurements, adding complexity to the experimental setup.
A significant technical challenge in all these methods is the extreme sensitivity of lithium phosphate materials to moisture and air exposure. Even brief contact with atmospheric conditions can alter surface properties and introduce measurement errors. Consequently, advanced glove box systems with controlled atmospheres are essential but add substantial complexity and cost to measurement protocols.
Sample preparation inconsistencies represent another major obstacle. Variations in compaction density, grain size distribution, and crystallinity significantly impact measured conductivity values, making standardization difficult across different research groups. This challenge is particularly pronounced when comparing results from different laboratories or literature sources.
The interface between the electrolyte material and electrodes introduces additional complications. Contact issues, interfacial reactions, and space charge effects can all contribute to measurement errors that are difficult to decouple from the intrinsic material properties. Advanced surface characterization techniques are often required alongside conductivity measurements to properly interpret results.
Recent developments in in-situ and operando measurement techniques show promise for overcoming some of these challenges by allowing conductivity measurements under realistic operating conditions, but these approaches remain primarily in the research domain rather than standard practice.
Electrochemical Impedance Spectroscopy (EIS) remains the gold standard for measuring ionic conductivity in solid electrolytes. This technique applies an AC voltage of varying frequencies to the sample and measures the resulting current response, allowing researchers to determine the bulk, grain boundary, and total conductivity. However, EIS requires careful sample preparation and electrode contact optimization to avoid introducing measurement artifacts.
Direct Current (DC) polarization methods provide complementary data to EIS by applying a constant voltage across the sample and measuring the resulting steady-state current. While conceptually simpler than EIS, DC methods often struggle with electrode polarization effects that can significantly distort measurements of fast ion conductors like lithium phosphates.
The four-point probe technique has gained popularity for its ability to eliminate contact resistance issues. By using separate current and voltage electrodes, this method provides more accurate bulk conductivity measurements but requires precise probe placement and can be challenging to implement with thin-film or irregularly shaped samples.
Temperature-dependent conductivity measurements represent another critical approach, as they enable the determination of activation energies for ion transport. These measurements typically involve specialized equipment capable of maintaining stable temperatures while simultaneously performing electrical measurements, adding complexity to the experimental setup.
A significant technical challenge in all these methods is the extreme sensitivity of lithium phosphate materials to moisture and air exposure. Even brief contact with atmospheric conditions can alter surface properties and introduce measurement errors. Consequently, advanced glove box systems with controlled atmospheres are essential but add substantial complexity and cost to measurement protocols.
Sample preparation inconsistencies represent another major obstacle. Variations in compaction density, grain size distribution, and crystallinity significantly impact measured conductivity values, making standardization difficult across different research groups. This challenge is particularly pronounced when comparing results from different laboratories or literature sources.
The interface between the electrolyte material and electrodes introduces additional complications. Contact issues, interfacial reactions, and space charge effects can all contribute to measurement errors that are difficult to decouple from the intrinsic material properties. Advanced surface characterization techniques are often required alongside conductivity measurements to properly interpret results.
Recent developments in in-situ and operando measurement techniques show promise for overcoming some of these challenges by allowing conductivity measurements under realistic operating conditions, but these approaches remain primarily in the research domain rather than standard practice.
State-of-the-Art Measurement Solutions and Protocols
01 Lithium phosphate as solid electrolyte material
Lithium phosphate compounds serve as effective solid electrolyte materials in batteries due to their high ionic conductivity. These materials facilitate lithium ion transport through their crystal structure, enabling efficient electrochemical reactions. The conductivity properties can be enhanced through various synthesis methods and compositional modifications, making them suitable for applications in solid-state batteries where safety and stability are paramount.- Lithium phosphate composition for enhanced conductivity: Lithium phosphate materials can be modified with specific compositions to enhance their conductive properties. These compositions often include doping with other elements or creating specific structural arrangements to improve ionic conductivity. The enhanced conductive properties make these materials suitable for various applications in energy storage and conversion technologies.
- Lithium iron phosphate as cathode material: Lithium iron phosphate (LiFePO4) exhibits unique conductive properties that make it an excellent cathode material for lithium-ion batteries. Its olivine structure provides stable lithium-ion transport pathways, contributing to good electrochemical performance. Various methods to enhance the conductivity of lithium iron phosphate include carbon coating, particle size reduction, and doping with conductive elements.
- Solid electrolyte applications of lithium phosphate: Lithium phosphate-based materials serve as solid electrolytes in various energy storage devices. These solid electrolytes offer advantages such as improved safety, thermal stability, and electrochemical performance. The conductive properties of lithium phosphate solid electrolytes can be tailored through compositional modifications and processing techniques to achieve optimal ionic conductivity for specific applications.
- Nano-structured lithium phosphate for improved conductivity: Nano-structuring of lithium phosphate materials significantly enhances their conductive properties. By reducing particle size to the nanoscale and controlling morphology, the ionic and electronic conductivity can be substantially improved. Techniques such as sol-gel synthesis, hydrothermal methods, and template-assisted growth are employed to create nano-structured lithium phosphate with optimized conductive properties for energy storage applications.
- Composite materials with lithium phosphate for enhanced conductivity: Composite materials incorporating lithium phosphate show enhanced conductive properties compared to pure lithium phosphate. These composites often combine lithium phosphate with conductive additives such as carbon materials, conductive polymers, or other ionic conductors. The synergistic effects in these composite structures lead to improved ionic and electronic conductivity, making them suitable for advanced energy storage and conversion applications.
02 Doping strategies to enhance conductivity
Doping lithium phosphate materials with various elements such as aluminum, magnesium, or transition metals significantly improves their conductive properties. These dopants create defects in the crystal structure that facilitate faster lithium ion movement. The introduction of specific dopants can increase ionic conductivity by orders of magnitude, optimize grain boundary conductivity, and improve overall electrochemical performance in battery applications.Expand Specific Solutions03 Nanostructured lithium phosphate for improved conductivity
Nanostructuring lithium phosphate materials enhances their conductive properties by reducing diffusion paths for lithium ions. Techniques such as creating nanoparticles, nanowires, or porous structures increase the surface area and active sites for ion transport. These nanostructured materials exhibit superior rate capability and conductivity compared to their bulk counterparts, making them valuable for high-performance energy storage applications.Expand Specific Solutions04 Composite materials with lithium phosphate
Combining lithium phosphate with conductive additives such as carbon materials, polymers, or other ionic conductors creates composite materials with enhanced conductive properties. These composites leverage the synergistic effects of different components to overcome the inherent limitations of lithium phosphate. The resulting materials demonstrate improved electronic conductivity, mechanical stability, and electrochemical performance suitable for advanced battery technologies.Expand Specific Solutions05 Temperature and pressure effects on conductivity
The conductive properties of lithium phosphate materials are significantly influenced by temperature and pressure conditions. Elevated temperatures typically enhance ionic mobility and conductivity, while specific pressure treatments can modify crystal structure and grain boundaries. Understanding these relationships allows for optimized operating conditions and material processing techniques that maximize the conductive performance of lithium phosphate in various applications.Expand Specific Solutions
Leading Research Institutions and Industry Players
The lithium phosphate conductive properties measurement landscape is evolving rapidly, with the market currently in a growth phase driven by increasing demand for advanced battery technologies. Major players include established corporations like BYD, LG Chem, and Murata Manufacturing, alongside research institutions such as Japan Science & Technology Agency and Fraunhofer-Gesellschaft. The technology maturity varies significantly, with companies like Panasonic and Robert Bosch demonstrating advanced measurement capabilities, while emerging players like Hubei Yiwei Power are developing innovative approaches. Academic institutions including MIT, Shanghai Jiao Tong University, and Northwestern University are contributing fundamental research that bridges current technical gaps, creating a competitive environment where collaboration between industry and academia is increasingly vital for accurate measurement solutions.
BYD Co., Ltd.
Technical Solution: BYD has implemented an integrated conductivity measurement system specifically designed for lithium phosphate materials used in their LFP battery production. Their approach combines traditional AC impedance spectroscopy with proprietary in-line quality control methods that enable rapid assessment of conductive properties during manufacturing. BYD's system utilizes specially designed spring-loaded four-point probe arrays that maintain consistent contact pressure while accommodating variations in sample geometry. Their methodology incorporates real-time temperature compensation algorithms that normalize conductivity measurements to standard conditions, enabling meaningful comparison between batches. The company has developed specialized sample preparation techniques that minimize surface contamination and oxidation, critical factors affecting measurement accuracy for lithium phosphate materials[7]. BYD's approach includes correlation analysis between measured conductivity parameters and actual battery performance metrics, allowing them to identify the most relevant electrical characteristics for quality control purposes. Their system also incorporates machine learning algorithms that can detect subtle patterns in impedance spectra that correlate with specific material defects or processing issues.
Strengths: Direct integration with manufacturing processes enables immediate feedback for quality control; optimized for high-throughput screening; established correlations between measurements and actual device performance. Weaknesses: Primarily focused on production-relevant parameters rather than fundamental material properties; limited frequency range compared to research-grade systems; proprietary nature restricts methodological transparency and academic validation.
Forschungszentrum Jülich GmbH
Technical Solution: Forschungszentrum Jülich has pioneered a comprehensive approach to lithium phosphate conductivity measurement combining multiple complementary techniques. Their methodology centers on a custom-built variable-temperature impedance spectroscopy system capable of measurements from -150°C to 500°C under controlled atmospheres. This system is integrated with in-situ X-ray diffraction capabilities, allowing simultaneous structural and conductivity analysis during temperature cycling. The institute has developed specialized sample preparation protocols that include precision polishing techniques and sputter-deposited blocking electrodes to minimize interfacial contributions. Their approach incorporates advanced mathematical modeling using distribution of relaxation times (DRT) analysis to deconvolute complex impedance spectra into discrete physical processes[4]. Additionally, Jülich researchers have implemented pulsed-field gradient nuclear magnetic resonance (PFG-NMR) measurements as a complementary technique to directly measure lithium ion diffusion coefficients, providing correlation between macroscopic conductivity and microscopic ion mobility[5]. This multi-technique approach enables comprehensive validation of conductivity measurements.
Strengths: Exceptional measurement accuracy through multi-technique validation; ability to correlate structural changes with conductivity properties; sophisticated data analysis capabilities for complex materials. Weaknesses: Extremely specialized equipment requirements limit accessibility; time-intensive measurement protocols; requires significant expertise in multiple analytical techniques for proper data interpretation.
Critical Patents and Literature on Conductivity Measurement
Lithium phosphate derivative compounds as Li super-ionic conductor, solid electrolyte and coating layer for lithium metal battery and lithium-ion battery
PatentActiveUS12206069B2
Innovation
- Development of novel lithium phosphate derivative compounds and their incorporation into solid-state lithium ion electrolytes and electrode coating layers, which exhibit high Li+ conductivity, low activation energy, and stability against electrochemical degradation.
Method, measurement circuit and measuring device for measuring the dielectric properties of conductive media
PatentInactiveEP2555005A2
Innovation
- The method involves using phase diagrams derived from equivalent circuit diagrams to modify the measuring cell's wiring and calculation methods, allowing for the determination of dielectric properties by adjusting alternating voltage to match direct voltage across electrodes, and employing auxiliary resistors, capacitances, or inductances to enhance measurement accuracy at low frequencies.
Standardization and Calibration Procedures
Accurate measurement of lithium phosphate conductive properties requires robust standardization and calibration procedures to ensure reliability and reproducibility of results. The establishment of standardized testing protocols begins with the selection of reference materials that exhibit well-documented conductivity values across various temperature ranges. These reference materials, such as NIST-certified standards, provide benchmarks against which measurement systems can be calibrated.
Equipment calibration represents a critical foundation for accurate conductivity measurements. Impedance analyzers, potentiostats, and other measurement devices must undergo regular calibration using certified reference resistors and capacitors. This calibration should follow a documented schedule, typically quarterly or bi-annually, with verification checks performed before each significant measurement campaign.
Temperature control and calibration deserve particular attention, as lithium phosphate conductivity exhibits strong temperature dependence. Measurement chambers require calibration using certified thermocouples or resistance temperature detectors (RTDs) with traceability to national standards. Temperature gradients within sample holders must be mapped and minimized through careful thermal design and insulation.
Sample preparation standardization represents another crucial aspect of the calibration framework. Procedures must specify precise parameters for powder compaction pressure, sintering conditions, electrode deposition methods, and surface treatment protocols. These parameters significantly influence measured conductivity values and must be controlled to ensure meaningful comparisons between different research groups and industrial facilities.
Electrode-sample interface calibration procedures address contact resistance issues that can severely distort conductivity measurements. Standard protocols should include methods for quantifying and compensating for these interface effects, such as multi-point probe techniques or extrapolation methods that separate bulk and interface contributions to the measured impedance.
Interlaboratory calibration programs provide essential validation of measurement procedures. These programs involve multiple facilities measuring identical reference samples according to standardized protocols, with statistical analysis of the results to identify systematic errors and establish uncertainty budgets. Such collaborative efforts have proven invaluable in refining measurement techniques and establishing confidence in reported conductivity values.
Data processing standardization completes the calibration framework. Procedures must specify consistent methods for extracting conductivity values from raw impedance data, including equivalent circuit modeling approaches, frequency range selection criteria, and statistical methods for uncertainty quantification. Software validation using synthetic data sets with known properties ensures computational accuracy in the analysis pipeline.
Equipment calibration represents a critical foundation for accurate conductivity measurements. Impedance analyzers, potentiostats, and other measurement devices must undergo regular calibration using certified reference resistors and capacitors. This calibration should follow a documented schedule, typically quarterly or bi-annually, with verification checks performed before each significant measurement campaign.
Temperature control and calibration deserve particular attention, as lithium phosphate conductivity exhibits strong temperature dependence. Measurement chambers require calibration using certified thermocouples or resistance temperature detectors (RTDs) with traceability to national standards. Temperature gradients within sample holders must be mapped and minimized through careful thermal design and insulation.
Sample preparation standardization represents another crucial aspect of the calibration framework. Procedures must specify precise parameters for powder compaction pressure, sintering conditions, electrode deposition methods, and surface treatment protocols. These parameters significantly influence measured conductivity values and must be controlled to ensure meaningful comparisons between different research groups and industrial facilities.
Electrode-sample interface calibration procedures address contact resistance issues that can severely distort conductivity measurements. Standard protocols should include methods for quantifying and compensating for these interface effects, such as multi-point probe techniques or extrapolation methods that separate bulk and interface contributions to the measured impedance.
Interlaboratory calibration programs provide essential validation of measurement procedures. These programs involve multiple facilities measuring identical reference samples according to standardized protocols, with statistical analysis of the results to identify systematic errors and establish uncertainty budgets. Such collaborative efforts have proven invaluable in refining measurement techniques and establishing confidence in reported conductivity values.
Data processing standardization completes the calibration framework. Procedures must specify consistent methods for extracting conductivity values from raw impedance data, including equivalent circuit modeling approaches, frequency range selection criteria, and statistical methods for uncertainty quantification. Software validation using synthetic data sets with known properties ensures computational accuracy in the analysis pipeline.
Environmental Factors Affecting Measurement Accuracy
The accuracy of lithium phosphate conductivity measurements is significantly influenced by various environmental factors that must be carefully controlled and accounted for during experimental procedures. Temperature stands as the most critical environmental variable, with conductivity properties of lithium phosphate materials exhibiting strong temperature dependence. Research indicates that even minor temperature fluctuations of ±1°C can result in measurement variations exceeding 5%, necessitating precise temperature control systems and equilibration periods before data collection.
Humidity represents another crucial factor affecting measurement precision, particularly for hygroscopic lithium phosphate materials. Exposure to moisture can alter surface properties and introduce parasitic conduction pathways, potentially leading to overestimated conductivity values. Studies have demonstrated that relative humidity variations between 30% and 70% can modify measured conductivity by up to 15% in certain lithium phosphate compositions, highlighting the importance of controlled atmosphere testing environments.
Atmospheric contamination, especially exposure to carbon dioxide, can trigger surface reactions with lithium phosphate materials, forming resistive carbonate layers that compromise measurement accuracy. These surface modifications may not be immediately apparent but can progressively influence results during extended measurement sessions. Implementation of inert gas environments, typically argon or nitrogen with purity levels exceeding 99.999%, has been shown to mitigate these effects.
Electromagnetic interference (EMI) from laboratory equipment and external sources can introduce significant noise into sensitive impedance measurements. This is particularly problematic when measuring high-resistance lithium phosphate samples where signal-to-noise ratios are inherently challenging. Proper electromagnetic shielding, including Faraday cages for measurement setups and appropriate grounding protocols, has demonstrated improvement in measurement reproducibility by factors of 3-5 in controlled studies.
Mechanical stress and sample mounting pressure have emerged as often overlooked factors affecting conductivity measurements. Research indicates that contact pressure variations between electrodes and lithium phosphate samples can alter measured conductivity by up to 20%, particularly for powder-based or composite materials. Standardized sample preparation protocols and controlled-pressure sample holders are essential for achieving reproducible results across different measurement sessions and laboratories.
Radiation exposure, including ambient light, can influence measurement accuracy through photoelectric effects in certain lithium phosphate compositions, particularly those containing transition metal dopants. These effects are most pronounced in materials designed for photoelectrochemical applications, where light-induced conductivity changes can reach 30-50% under standard laboratory lighting conditions.
Humidity represents another crucial factor affecting measurement precision, particularly for hygroscopic lithium phosphate materials. Exposure to moisture can alter surface properties and introduce parasitic conduction pathways, potentially leading to overestimated conductivity values. Studies have demonstrated that relative humidity variations between 30% and 70% can modify measured conductivity by up to 15% in certain lithium phosphate compositions, highlighting the importance of controlled atmosphere testing environments.
Atmospheric contamination, especially exposure to carbon dioxide, can trigger surface reactions with lithium phosphate materials, forming resistive carbonate layers that compromise measurement accuracy. These surface modifications may not be immediately apparent but can progressively influence results during extended measurement sessions. Implementation of inert gas environments, typically argon or nitrogen with purity levels exceeding 99.999%, has been shown to mitigate these effects.
Electromagnetic interference (EMI) from laboratory equipment and external sources can introduce significant noise into sensitive impedance measurements. This is particularly problematic when measuring high-resistance lithium phosphate samples where signal-to-noise ratios are inherently challenging. Proper electromagnetic shielding, including Faraday cages for measurement setups and appropriate grounding protocols, has demonstrated improvement in measurement reproducibility by factors of 3-5 in controlled studies.
Mechanical stress and sample mounting pressure have emerged as often overlooked factors affecting conductivity measurements. Research indicates that contact pressure variations between electrodes and lithium phosphate samples can alter measured conductivity by up to 20%, particularly for powder-based or composite materials. Standardized sample preparation protocols and controlled-pressure sample holders are essential for achieving reproducible results across different measurement sessions and laboratories.
Radiation exposure, including ambient light, can influence measurement accuracy through photoelectric effects in certain lithium phosphate compositions, particularly those containing transition metal dopants. These effects are most pronounced in materials designed for photoelectrochemical applications, where light-induced conductivity changes can reach 30-50% under standard laboratory lighting conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!