How to Minimize HPLC Analysis Time with Parallel Processing
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Parallel Processing Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming a cornerstone analytical technique in pharmaceutical, environmental, and food safety industries. The technology has progressed from basic isocratic systems to sophisticated ultra-high-performance instruments capable of complex separations. However, analysis time remains a critical bottleneck in laboratory workflows, particularly in high-throughput environments where hundreds of samples require processing daily.
Parallel processing in HPLC represents a paradigm shift in chromatographic analysis, aiming to maximize instrument utilization and minimize overall analysis time. This approach involves simultaneous operation of multiple chromatographic processes, either through multi-column configurations, multi-detector setups, or integrated sample preparation systems running concurrently with analysis.
The evolution of parallel processing techniques in HPLC can be traced through several technological milestones. Early attempts in the 1990s focused on column switching techniques, while the 2000s saw the development of comprehensive two-dimensional chromatography. Recent advancements include multi-channel systems and integrated automation platforms that coordinate multiple separation processes simultaneously.
Current technological trends point toward increased integration of artificial intelligence for predictive maintenance and method optimization, miniaturization of parallel systems, and enhanced compatibility with mass spectrometry detection. These developments collectively aim to transform HPLC from a sequential analytical technique to a parallel processing platform capable of handling complex analytical challenges with unprecedented efficiency.
The primary objective of implementing parallel processing in HPLC is to achieve a significant reduction in total analysis time without compromising analytical performance. Specific goals include increasing sample throughput by at least 200%, reducing instrument idle time to less than 10%, and maintaining or improving separation efficiency and detection sensitivity compared to conventional sequential methods.
Secondary objectives encompass developing scalable parallel processing solutions adaptable to various laboratory settings, from research facilities to quality control environments. Additionally, there is a focus on creating user-friendly interfaces that simplify the operation of complex parallel systems, making the technology accessible to analysts with varying levels of expertise.
The long-term vision for HPLC parallel processing extends beyond mere time efficiency to fundamentally transforming laboratory workflows. By enabling simultaneous multi-sample analysis, this technology aims to eliminate analytical bottlenecks, facilitate real-time decision-making in production environments, and ultimately contribute to faster drug development, more responsive quality control, and more efficient environmental monitoring programs.
Parallel processing in HPLC represents a paradigm shift in chromatographic analysis, aiming to maximize instrument utilization and minimize overall analysis time. This approach involves simultaneous operation of multiple chromatographic processes, either through multi-column configurations, multi-detector setups, or integrated sample preparation systems running concurrently with analysis.
The evolution of parallel processing techniques in HPLC can be traced through several technological milestones. Early attempts in the 1990s focused on column switching techniques, while the 2000s saw the development of comprehensive two-dimensional chromatography. Recent advancements include multi-channel systems and integrated automation platforms that coordinate multiple separation processes simultaneously.
Current technological trends point toward increased integration of artificial intelligence for predictive maintenance and method optimization, miniaturization of parallel systems, and enhanced compatibility with mass spectrometry detection. These developments collectively aim to transform HPLC from a sequential analytical technique to a parallel processing platform capable of handling complex analytical challenges with unprecedented efficiency.
The primary objective of implementing parallel processing in HPLC is to achieve a significant reduction in total analysis time without compromising analytical performance. Specific goals include increasing sample throughput by at least 200%, reducing instrument idle time to less than 10%, and maintaining or improving separation efficiency and detection sensitivity compared to conventional sequential methods.
Secondary objectives encompass developing scalable parallel processing solutions adaptable to various laboratory settings, from research facilities to quality control environments. Additionally, there is a focus on creating user-friendly interfaces that simplify the operation of complex parallel systems, making the technology accessible to analysts with varying levels of expertise.
The long-term vision for HPLC parallel processing extends beyond mere time efficiency to fundamentally transforming laboratory workflows. By enabling simultaneous multi-sample analysis, this technology aims to eliminate analytical bottlenecks, facilitate real-time decision-making in production environments, and ultimately contribute to faster drug development, more responsive quality control, and more efficient environmental monitoring programs.
Market Demand for High-Throughput Chromatography
The global market for high-throughput chromatography solutions has experienced substantial growth in recent years, driven primarily by increasing demands in pharmaceutical research, clinical diagnostics, and various industrial applications. This growth trajectory is expected to continue as laboratories face mounting pressure to process more samples in less time while maintaining analytical precision.
Pharmaceutical and biotechnology sectors represent the largest market segments for high-throughput chromatography technologies. These industries require rapid analytical methods for drug discovery, development, and quality control processes. The need to accelerate time-to-market for new therapeutics has created significant demand for parallel processing HPLC systems that can analyze multiple samples simultaneously.
Clinical laboratories constitute another major market driver, as they process thousands of samples daily for diagnostic purposes. The COVID-19 pandemic has further intensified this demand, highlighting the critical importance of rapid analytical capabilities in public health emergencies. Laboratories that have implemented high-throughput chromatography systems report significant improvements in workflow efficiency and diagnostic turnaround times.
Academic research institutions are increasingly adopting high-throughput chromatography solutions to support large-scale studies in proteomics, metabolomics, and other -omics fields. These applications generate massive datasets that require rapid analytical processing to derive meaningful insights within reasonable timeframes.
Market analysis indicates that the food and beverage industry is an emerging sector for high-throughput chromatography applications, particularly for quality control and safety testing. Regulatory requirements for comprehensive testing of food products have created new opportunities for parallel processing HPLC technologies in this sector.
Economic factors also drive market demand, as laboratories seek to maximize return on investment through increased sample throughput. Industry surveys reveal that laboratories implementing parallel processing HPLC systems typically achieve 3-4 times higher sample throughput compared to conventional sequential analysis methods, resulting in significant cost savings per analysis.
Regional market assessment shows North America and Europe currently dominating the high-throughput chromatography market, with Asia-Pacific representing the fastest-growing region. This growth is attributed to expanding pharmaceutical manufacturing capabilities and increasing research activities in countries like China, India, and South Korea.
Market forecasts suggest that demand for parallel processing HPLC technologies will continue to grow as industries increasingly prioritize analytical efficiency. The trend toward laboratory automation and integration with other analytical platforms further enhances the value proposition of high-throughput chromatography solutions, creating a robust market outlook for technologies that effectively minimize HPLC analysis time.
Pharmaceutical and biotechnology sectors represent the largest market segments for high-throughput chromatography technologies. These industries require rapid analytical methods for drug discovery, development, and quality control processes. The need to accelerate time-to-market for new therapeutics has created significant demand for parallel processing HPLC systems that can analyze multiple samples simultaneously.
Clinical laboratories constitute another major market driver, as they process thousands of samples daily for diagnostic purposes. The COVID-19 pandemic has further intensified this demand, highlighting the critical importance of rapid analytical capabilities in public health emergencies. Laboratories that have implemented high-throughput chromatography systems report significant improvements in workflow efficiency and diagnostic turnaround times.
Academic research institutions are increasingly adopting high-throughput chromatography solutions to support large-scale studies in proteomics, metabolomics, and other -omics fields. These applications generate massive datasets that require rapid analytical processing to derive meaningful insights within reasonable timeframes.
Market analysis indicates that the food and beverage industry is an emerging sector for high-throughput chromatography applications, particularly for quality control and safety testing. Regulatory requirements for comprehensive testing of food products have created new opportunities for parallel processing HPLC technologies in this sector.
Economic factors also drive market demand, as laboratories seek to maximize return on investment through increased sample throughput. Industry surveys reveal that laboratories implementing parallel processing HPLC systems typically achieve 3-4 times higher sample throughput compared to conventional sequential analysis methods, resulting in significant cost savings per analysis.
Regional market assessment shows North America and Europe currently dominating the high-throughput chromatography market, with Asia-Pacific representing the fastest-growing region. This growth is attributed to expanding pharmaceutical manufacturing capabilities and increasing research activities in countries like China, India, and South Korea.
Market forecasts suggest that demand for parallel processing HPLC technologies will continue to grow as industries increasingly prioritize analytical efficiency. The trend toward laboratory automation and integration with other analytical platforms further enhances the value proposition of high-throughput chromatography solutions, creating a robust market outlook for technologies that effectively minimize HPLC analysis time.
Current HPLC Throughput Limitations and Challenges
High-Performance Liquid Chromatography (HPLC) has become an indispensable analytical technique in pharmaceutical, environmental, and food safety industries. However, despite technological advancements, HPLC analysis continues to face significant throughput limitations that impact laboratory productivity and efficiency. These challenges have become increasingly critical as analytical demands grow in both volume and complexity.
The most prominent limitation in current HPLC systems is the inherently sequential nature of sample processing. Conventional HPLC platforms operate on a one-sample-at-a-time basis, creating inevitable bottlenecks in high-volume testing environments. This sequential processing paradigm means that laboratories must either invest in multiple instruments or accept extended analysis timelines, neither of which represents an optimal resource allocation.
Instrument idle time presents another significant challenge. During a typical HPLC analysis cycle, substantial portions of the workflow—including column equilibration, sample preparation, and system cleaning—result in productive downtime. Studies indicate that HPLC systems may spend 30-40% of operational time in these non-analytical states, dramatically reducing effective throughput capacity.
Method development and optimization processes further compound throughput challenges. The iterative nature of developing robust HPLC methods requires multiple experimental runs with varying parameters, consuming valuable instrument time that could otherwise be dedicated to routine analysis. This becomes particularly problematic when laboratories face diverse sample types requiring different methodological approaches.
Sample preparation remains a critical bottleneck in the HPLC workflow. Manual preparation steps are time-consuming, labor-intensive, and introduce variability that can necessitate repeat analyses. While automation solutions exist, they often operate in isolation from the chromatographic system, creating disconnected workflows that fail to address end-to-end throughput optimization.
Data processing and result interpretation also contribute significantly to throughput limitations. As analytical methods generate increasingly complex data sets, the computational demands for processing chromatograms, identifying peaks, and quantifying results have grown exponentially. Many laboratories still rely on semi-automated approaches that require substantial analyst intervention, creating delays between sample analysis and reportable results.
Regulatory and compliance requirements add another layer of complexity to throughput challenges. The need for system suitability testing, quality control samples, and calibration standards consumes significant instrument capacity that could otherwise be dedicated to sample analysis. These requirements, while essential for data integrity, can reduce effective throughput by 15-25% in regulated environments.
The economic implications of these throughput limitations are substantial. Laboratories face increasing pressure to maximize return on investment for expensive HPLC instrumentation while simultaneously reducing per-sample analysis costs and turnaround times. This economic reality drives the urgent need for innovative approaches to HPLC throughput optimization, particularly through parallel processing methodologies.
The most prominent limitation in current HPLC systems is the inherently sequential nature of sample processing. Conventional HPLC platforms operate on a one-sample-at-a-time basis, creating inevitable bottlenecks in high-volume testing environments. This sequential processing paradigm means that laboratories must either invest in multiple instruments or accept extended analysis timelines, neither of which represents an optimal resource allocation.
Instrument idle time presents another significant challenge. During a typical HPLC analysis cycle, substantial portions of the workflow—including column equilibration, sample preparation, and system cleaning—result in productive downtime. Studies indicate that HPLC systems may spend 30-40% of operational time in these non-analytical states, dramatically reducing effective throughput capacity.
Method development and optimization processes further compound throughput challenges. The iterative nature of developing robust HPLC methods requires multiple experimental runs with varying parameters, consuming valuable instrument time that could otherwise be dedicated to routine analysis. This becomes particularly problematic when laboratories face diverse sample types requiring different methodological approaches.
Sample preparation remains a critical bottleneck in the HPLC workflow. Manual preparation steps are time-consuming, labor-intensive, and introduce variability that can necessitate repeat analyses. While automation solutions exist, they often operate in isolation from the chromatographic system, creating disconnected workflows that fail to address end-to-end throughput optimization.
Data processing and result interpretation also contribute significantly to throughput limitations. As analytical methods generate increasingly complex data sets, the computational demands for processing chromatograms, identifying peaks, and quantifying results have grown exponentially. Many laboratories still rely on semi-automated approaches that require substantial analyst intervention, creating delays between sample analysis and reportable results.
Regulatory and compliance requirements add another layer of complexity to throughput challenges. The need for system suitability testing, quality control samples, and calibration standards consumes significant instrument capacity that could otherwise be dedicated to sample analysis. These requirements, while essential for data integrity, can reduce effective throughput by 15-25% in regulated environments.
The economic implications of these throughput limitations are substantial. Laboratories face increasing pressure to maximize return on investment for expensive HPLC instrumentation while simultaneously reducing per-sample analysis costs and turnaround times. This economic reality drives the urgent need for innovative approaches to HPLC throughput optimization, particularly through parallel processing methodologies.
Current Parallel HPLC Implementation Strategies
01 Rapid HPLC analysis methods
Various techniques have been developed to reduce HPLC analysis time while maintaining separation efficiency. These include the use of shorter columns, higher flow rates, optimized mobile phase compositions, and gradient elution programs. These methods can significantly decrease analysis time from traditional 30-60 minute runs to under 10 minutes, making them suitable for high-throughput applications in pharmaceutical, environmental, and food safety testing.- Optimization of HPLC analysis time through column selection and parameters: The selection of appropriate HPLC columns and optimization of parameters can significantly reduce analysis time while maintaining separation efficiency. This includes using shorter columns with smaller particle sizes, optimizing mobile phase composition, flow rate adjustments, and temperature control. These approaches can lead to faster chromatographic separations without compromising analytical quality.
- Fast HPLC methods for pharmaceutical analysis: Specialized fast HPLC methods have been developed specifically for pharmaceutical applications to reduce analysis time. These methods incorporate optimized gradient elution, specialized stationary phases designed for rapid separations, and higher pressure capabilities. Such techniques allow for high-throughput screening of pharmaceutical compounds and quality control testing with significantly reduced run times.
- Ultra-high performance liquid chromatography (UHPLC) techniques: UHPLC techniques utilize sub-2μm particle columns and higher system pressures to dramatically reduce analysis time compared to conventional HPLC. These systems can operate at pressures up to 15,000 psi, allowing for faster flow rates and shorter columns while maintaining or improving separation efficiency. UHPLC methods can reduce analysis times by up to 80% while providing comparable or superior resolution.
- Automated sample preparation to reduce overall analysis time: Integration of automated sample preparation systems with HPLC analysis can significantly reduce the total time required for analytical procedures. These systems include automated extraction, filtration, derivatization, and injection processes that minimize manual handling and preparation time. When combined with optimized HPLC methods, the overall analysis workflow can be streamlined for higher throughput and efficiency.
- Novel detection technologies for faster HPLC analysis: Advanced detection technologies can contribute to reduced HPLC analysis times by allowing faster data acquisition rates and improved sensitivity. These include high-speed diode array detectors, mass spectrometry interfaces optimized for fast chromatography, and multi-channel detectors that can simultaneously monitor multiple analytes. These detection systems support the faster separation techniques without becoming a bottleneck in the analytical process.
02 Ultra-high performance liquid chromatography (UHPLC)
UHPLC systems utilize columns packed with sub-2μm particles and equipment capable of handling higher pressures, resulting in dramatically reduced analysis times compared to conventional HPLC. These systems can achieve equivalent or better separation in a fraction of the time, often reducing analysis from 30 minutes to less than 5 minutes. The technology enables faster method development, increased sample throughput, and reduced solvent consumption.Expand Specific Solutions03 Monolithic column technology
Monolithic columns contain a single piece of porous material rather than packed particles, allowing for higher flow rates without excessive backpressure. This technology enables faster analysis times while maintaining good separation efficiency. The unique structure of monolithic columns permits rapid mass transfer and reduced diffusion paths, making them particularly suitable for the analysis of large biomolecules and applications requiring high-throughput screening.Expand Specific Solutions04 Automated sample preparation and analysis
Integration of automated sample preparation with HPLC analysis significantly reduces overall analysis time. Automated systems can perform multiple steps including filtration, dilution, derivatization, and injection in parallel with chromatographic runs. This approach minimizes manual handling, reduces human error, and allows for continuous operation, thereby increasing laboratory efficiency and sample throughput.Expand Specific Solutions05 Multi-dimensional HPLC techniques
Multi-dimensional HPLC techniques involve the coupling of two or more separation mechanisms to improve resolution while maintaining reasonable analysis times. These techniques can separate complex mixtures more efficiently than conventional single-dimension HPLC. By using orthogonal separation mechanisms, compounds that co-elute in one dimension can be separated in another, allowing for comprehensive analysis of complex samples in reduced overall time compared to sequential individual analyses.Expand Specific Solutions
Key Industry Players in Parallel HPLC Systems
The HPLC parallel processing market is in a growth phase, with increasing demand for high-throughput analytical solutions driving market expansion. The technology has reached moderate maturity, with established players like Shimadzu, Agilent Technologies, and Thermo Fisher Scientific offering commercial solutions. These companies have developed advanced parallel HPLC systems that significantly reduce analysis time through simultaneous sample processing. Emerging competitors like DENSO Corp and Fujitsu are bringing automation expertise from adjacent industries. The market is characterized by continuous innovation in hardware miniaturization, software integration, and workflow optimization, with recent developments focusing on AI-powered predictive maintenance and cloud connectivity for remote monitoring of parallel HPLC operations.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed the Infinity II 2D-LC Solution for parallel HPLC processing that incorporates multiple columns operating simultaneously with intelligent sample management. Their system utilizes a proprietary Multi-Column Thermostat (MCT) that can house up to eight columns with individual temperature control, allowing for parallel method development and analysis[1]. The Intelligent System Emulation Technology (ISET) enables seamless method transfer between different instruments while maintaining chromatographic results. Agilent's parallel processing approach includes the Multiple Heart-Cutting 2D-LC technology that can perform up to 12 heart-cuts per analysis, significantly reducing the overall analysis time by processing these cuts simultaneously in the second dimension[2]. Their software platform integrates automated method development tools that can optimize separation parameters across multiple columns in parallel, further reducing the time required for method development.
Strengths: Industry-leading column technology with superior separation efficiency; comprehensive software integration for automated parallel processing; established global support network. Weaknesses: Higher initial investment cost compared to conventional systems; requires specialized training for optimal utilization; complex setup may present challenges for laboratories with limited resources.
Exxonmobil Upstream Research Co.
Technical Solution: Exxonmobil has developed a proprietary High-Throughput HPLC (HT-HPLC) system that leverages parallel processing to dramatically reduce analysis time for complex hydrocarbon samples. Their approach incorporates a multi-channel injection system that can simultaneously prepare and inject up to eight samples into parallel analytical pathways[7]. The system utilizes custom-designed microfluidic manifolds that enable precise distribution of samples across multiple columns with minimal dead volume, maintaining chromatographic efficiency while increasing throughput. Exxonmobil's parallel processing technology includes specialized thermal management systems that independently control the temperature of each parallel column, allowing for simultaneous operation of different separation methods optimized for specific compound classes. Their proprietary data handling software employs machine learning algorithms to process and integrate chromatographic data from multiple parallel channels in real-time, automatically identifying and quantifying target compounds[8]. This integrated approach has enabled Exxonmobil to reduce analysis times by up to 75% for complex petroleum samples while maintaining or improving analytical precision.
Strengths: Highly specialized for complex hydrocarbon samples; advanced microfluidic technology minimizing dead volumes; sophisticated machine learning integration for automated data processing. Weaknesses: Limited application outside petroleum industry; requires specialized expertise to operate and maintain; significant customization needed for non-standard applications.
Core Innovations in Multi-Column Chromatography
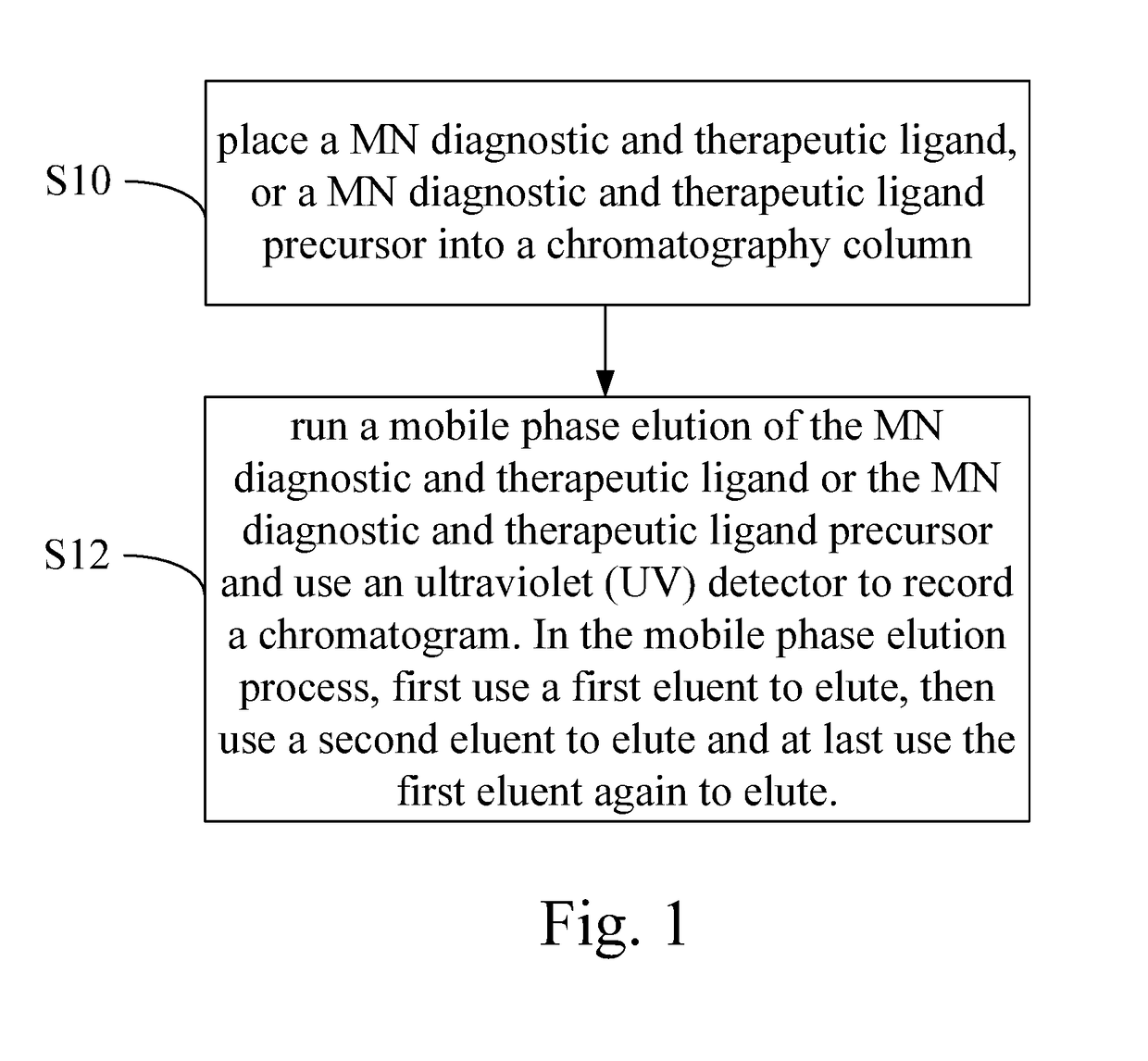
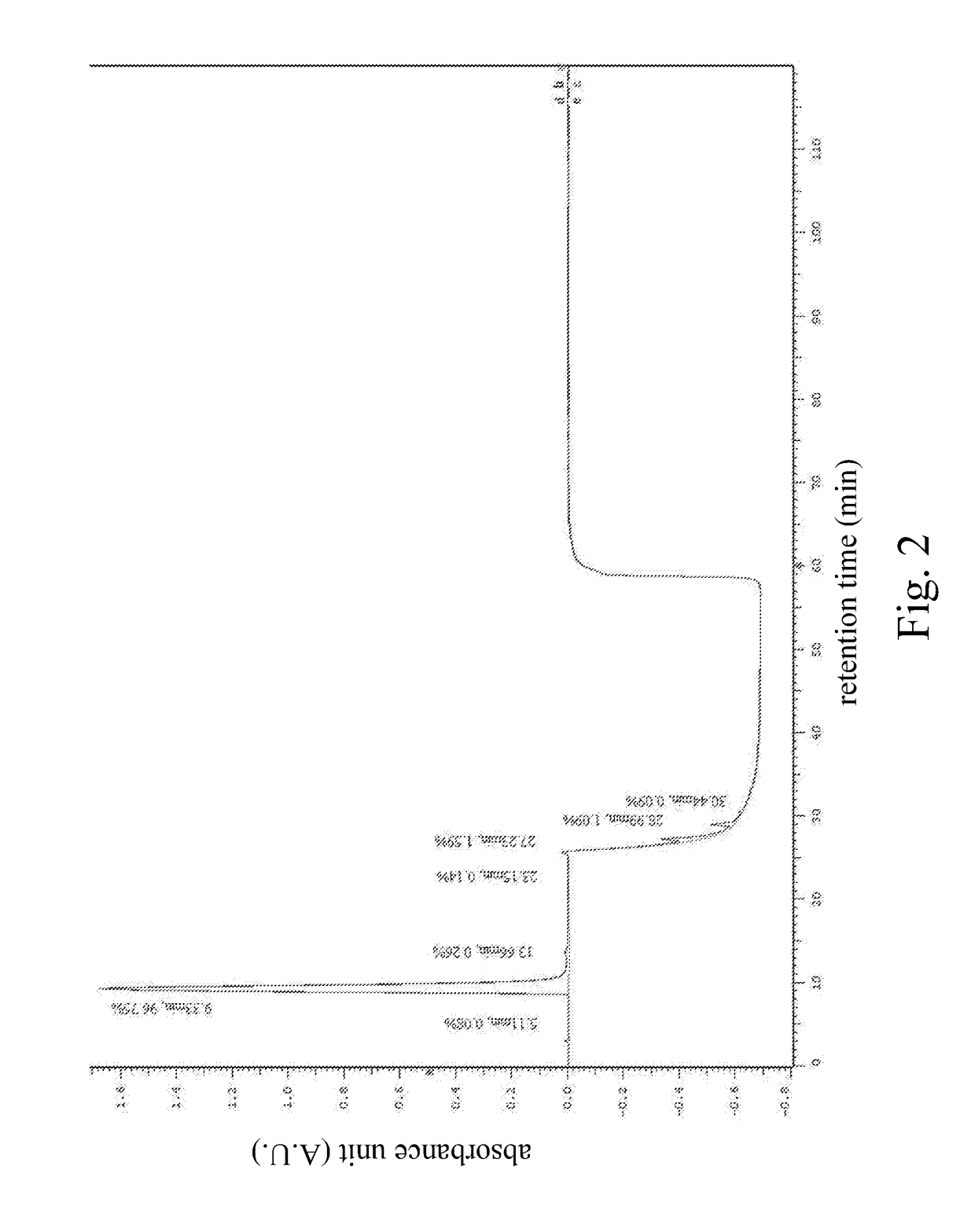
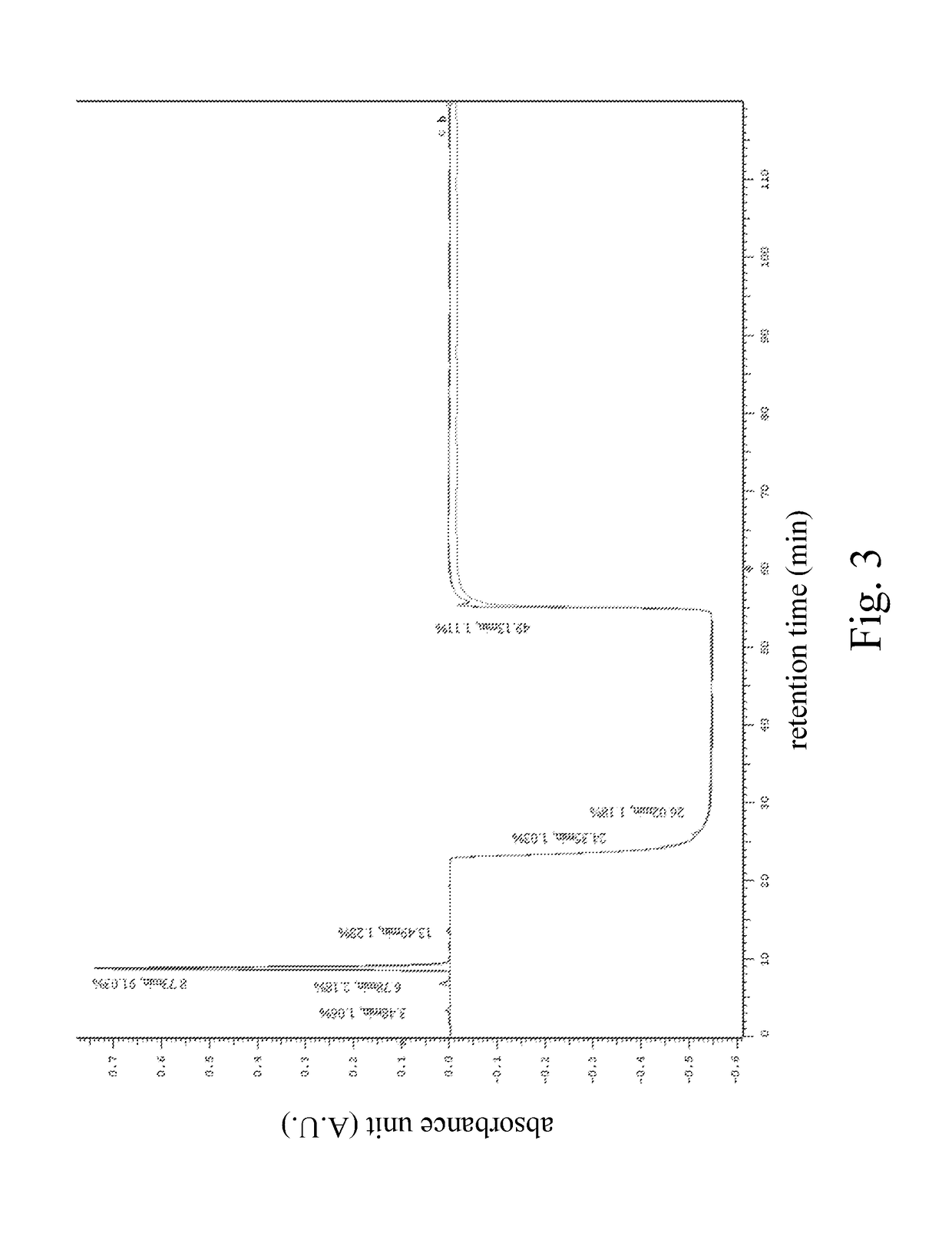
High performance liquid chromatography method for analysis of MN diagnostic and therapeutic ligand and precursor
PatentInactiveUS20180059072A1
Innovation
- A HPLC method utilizing a high ratio of acetonitrile in the mobile phase to wash out low-polarity impurities and employing gradient elution with a UV detector at 210 nm to improve detection accuracy, ensuring accurate analysis and reducing residual impurities in the column.
Cost-Benefit Analysis of Parallel HPLC Systems
Implementing parallel HPLC systems requires significant initial capital investment that must be carefully weighed against potential returns. The acquisition costs for multiple HPLC systems range from $30,000 to $100,000 per unit, depending on specifications and capabilities. Additional infrastructure expenses include laboratory space modifications, power supply upgrades, and specialized plumbing installations, potentially adding 15-25% to the base equipment cost.
Operational costs present another significant consideration, encompassing maintenance contracts (typically 10-15% of instrument cost annually), consumables (columns, solvents, reagents), and qualified personnel to operate multiple systems simultaneously. These recurring expenses can accumulate to approximately $15,000-$25,000 per system annually.
The benefits side of the equation reveals compelling advantages. Laboratory throughput increases nearly linearly with each additional parallel system, potentially multiplying sample analysis capacity by the number of systems deployed. Time-to-result metrics show dramatic improvements, with studies indicating 65-80% reduction in total analysis time for batch processing when using four parallel systems.
Resource utilization efficiency improves as parallel systems allow for continuous operation during maintenance periods. When one system requires service, others remain operational, minimizing downtime. This redundancy factor alone can justify additional capital expenditure in high-volume laboratories where analytical continuity is critical.
Return on investment calculations demonstrate that parallel HPLC systems typically achieve break-even within 1.5-3 years in high-throughput environments. Pharmaceutical quality control laboratories processing >100 samples daily show the fastest ROI, while research facilities with variable workloads may experience longer payback periods of 3-5 years.
Scalability considerations reveal that modular implementation approaches allow organizations to expand parallel capacity incrementally, distributing capital expenditure over time while gradually increasing throughput. This strategy minimizes financial risk while providing immediate productivity benefits with each additional unit.
The cost-benefit equation ultimately depends on laboratory-specific factors including sample volume, analysis complexity, and time sensitivity of results. Organizations must conduct thorough workflow analysis to determine the optimal number of parallel systems that maximizes return while avoiding excess capacity and unnecessary expenditure.
Operational costs present another significant consideration, encompassing maintenance contracts (typically 10-15% of instrument cost annually), consumables (columns, solvents, reagents), and qualified personnel to operate multiple systems simultaneously. These recurring expenses can accumulate to approximately $15,000-$25,000 per system annually.
The benefits side of the equation reveals compelling advantages. Laboratory throughput increases nearly linearly with each additional parallel system, potentially multiplying sample analysis capacity by the number of systems deployed. Time-to-result metrics show dramatic improvements, with studies indicating 65-80% reduction in total analysis time for batch processing when using four parallel systems.
Resource utilization efficiency improves as parallel systems allow for continuous operation during maintenance periods. When one system requires service, others remain operational, minimizing downtime. This redundancy factor alone can justify additional capital expenditure in high-volume laboratories where analytical continuity is critical.
Return on investment calculations demonstrate that parallel HPLC systems typically achieve break-even within 1.5-3 years in high-throughput environments. Pharmaceutical quality control laboratories processing >100 samples daily show the fastest ROI, while research facilities with variable workloads may experience longer payback periods of 3-5 years.
Scalability considerations reveal that modular implementation approaches allow organizations to expand parallel capacity incrementally, distributing capital expenditure over time while gradually increasing throughput. This strategy minimizes financial risk while providing immediate productivity benefits with each additional unit.
The cost-benefit equation ultimately depends on laboratory-specific factors including sample volume, analysis complexity, and time sensitivity of results. Organizations must conduct thorough workflow analysis to determine the optimal number of parallel systems that maximizes return while avoiding excess capacity and unnecessary expenditure.
Validation and Regulatory Considerations for Parallel HPLC Methods
Implementing parallel HPLC methods requires rigorous validation to ensure compliance with regulatory standards. Regulatory bodies such as the FDA, EMA, and ICH have established guidelines that must be followed when validating analytical methods, including those utilizing parallel processing technologies. These guidelines typically require demonstration of method specificity, accuracy, precision, linearity, range, and robustness—all of which must be carefully addressed in parallel HPLC systems.
Method validation for parallel HPLC presents unique challenges compared to traditional sequential analysis. Cross-contamination between parallel channels must be thoroughly evaluated and eliminated, as even minimal carryover can compromise data integrity. System suitability tests must be performed for each parallel channel independently to verify consistent performance across all processing paths.
Regulatory considerations also extend to data integrity and traceability. Parallel processing generates multiple data streams simultaneously, necessitating robust data management systems capable of accurately tracking and associating results with their respective samples. 21 CFR Part 11 compliance becomes particularly important, requiring secure electronic records and signatures for all parallel channels.
Calibration strategies require special attention in parallel systems. Each channel must demonstrate equivalent analytical performance, requiring comprehensive calibration protocols that account for potential inter-channel variability. Regulatory bodies typically expect documentation showing channel-to-channel consistency and equivalence testing results.
Quality control procedures must be adapted for parallel processing environments. This includes implementing appropriate system suitability tests, control samples, and monitoring protocols specific to multi-channel operations. Regulatory inspections will focus on evidence that parallel methods maintain the same level of quality assurance as traditional approaches.
Method transfer considerations are also critical when implementing parallel HPLC methods across different laboratories or manufacturing sites. Validation protocols must address how parallel processing configurations will be standardized across multiple locations while maintaining regulatory compliance.
Risk assessment frameworks should be employed to identify potential failure points specific to parallel processing. This includes evaluating risks associated with synchronization failures, differential channel performance, and complex maintenance requirements. Regulatory submissions should include comprehensive risk mitigation strategies addressing these parallel-specific concerns.
Method validation for parallel HPLC presents unique challenges compared to traditional sequential analysis. Cross-contamination between parallel channels must be thoroughly evaluated and eliminated, as even minimal carryover can compromise data integrity. System suitability tests must be performed for each parallel channel independently to verify consistent performance across all processing paths.
Regulatory considerations also extend to data integrity and traceability. Parallel processing generates multiple data streams simultaneously, necessitating robust data management systems capable of accurately tracking and associating results with their respective samples. 21 CFR Part 11 compliance becomes particularly important, requiring secure electronic records and signatures for all parallel channels.
Calibration strategies require special attention in parallel systems. Each channel must demonstrate equivalent analytical performance, requiring comprehensive calibration protocols that account for potential inter-channel variability. Regulatory bodies typically expect documentation showing channel-to-channel consistency and equivalence testing results.
Quality control procedures must be adapted for parallel processing environments. This includes implementing appropriate system suitability tests, control samples, and monitoring protocols specific to multi-channel operations. Regulatory inspections will focus on evidence that parallel methods maintain the same level of quality assurance as traditional approaches.
Method transfer considerations are also critical when implementing parallel HPLC methods across different laboratories or manufacturing sites. Validation protocols must address how parallel processing configurations will be standardized across multiple locations while maintaining regulatory compliance.
Risk assessment frameworks should be employed to identify potential failure points specific to parallel processing. This includes evaluating risks associated with synchronization failures, differential channel performance, and complex maintenance requirements. Regulatory submissions should include comprehensive risk mitigation strategies addressing these parallel-specific concerns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!