How to Optimize Microfluidics for Lab-on-Chip Efficiency
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics Evolution and Optimization Goals
Microfluidics emerged in the early 1990s as a revolutionary approach to manipulating fluids at the microscale level. This technology evolved from the convergence of microelectronics fabrication techniques and fluid dynamics principles, enabling the precise control of small volumes of liquids within channels typically ranging from tens to hundreds of micrometers. The initial development focused primarily on creating basic components such as micropumps, microvalves, and micromixers that could be integrated into more complex systems.
Throughout the 2000s, microfluidics technology witnessed significant advancements in fabrication methods, transitioning from silicon-based approaches to more cost-effective polymer-based materials like polydimethylsiloxane (PDMS). This shift dramatically reduced production costs and accelerated prototyping capabilities, making the technology more accessible to researchers across various disciplines. Concurrently, the integration of additional functionalities such as optical detection, electrochemical sensing, and thermal control expanded the application scope of microfluidic devices.
The evolution of microfluidics has been driven by the vision of creating fully integrated lab-on-chip (LOC) systems capable of performing complex analytical procedures with minimal human intervention. This concept promises numerous advantages including reduced reagent consumption, faster analysis times, enhanced sensitivity, portability, and automation of laboratory processes. The trajectory has moved from simple single-function devices toward multifunctional integrated platforms that can execute complete analytical workflows.
Current optimization goals for microfluidics in lab-on-chip applications center around several key objectives. First, enhancing fluid control precision to enable more complex and reliable operations, particularly for applications requiring precise timing and sequencing of reactions. Second, improving integration density to incorporate more functionality within smaller footprints, thereby increasing analytical capabilities while maintaining device portability.
Third, developing standardized interfaces and modular designs to facilitate easier assembly and reconfiguration of microfluidic components, which would accelerate innovation and adoption across different application domains. Fourth, reducing energy consumption to enable truly portable, battery-operated devices suitable for point-of-care diagnostics and field applications.
Fifth, improving manufacturing scalability to transition from laboratory prototypes to mass-produced commercial products, which requires addressing challenges in materials, fabrication processes, and quality control. Finally, enhancing robustness and reliability to ensure consistent performance under various operating conditions, particularly important for clinical and industrial applications where device failure could have significant consequences.
Throughout the 2000s, microfluidics technology witnessed significant advancements in fabrication methods, transitioning from silicon-based approaches to more cost-effective polymer-based materials like polydimethylsiloxane (PDMS). This shift dramatically reduced production costs and accelerated prototyping capabilities, making the technology more accessible to researchers across various disciplines. Concurrently, the integration of additional functionalities such as optical detection, electrochemical sensing, and thermal control expanded the application scope of microfluidic devices.
The evolution of microfluidics has been driven by the vision of creating fully integrated lab-on-chip (LOC) systems capable of performing complex analytical procedures with minimal human intervention. This concept promises numerous advantages including reduced reagent consumption, faster analysis times, enhanced sensitivity, portability, and automation of laboratory processes. The trajectory has moved from simple single-function devices toward multifunctional integrated platforms that can execute complete analytical workflows.
Current optimization goals for microfluidics in lab-on-chip applications center around several key objectives. First, enhancing fluid control precision to enable more complex and reliable operations, particularly for applications requiring precise timing and sequencing of reactions. Second, improving integration density to incorporate more functionality within smaller footprints, thereby increasing analytical capabilities while maintaining device portability.
Third, developing standardized interfaces and modular designs to facilitate easier assembly and reconfiguration of microfluidic components, which would accelerate innovation and adoption across different application domains. Fourth, reducing energy consumption to enable truly portable, battery-operated devices suitable for point-of-care diagnostics and field applications.
Fifth, improving manufacturing scalability to transition from laboratory prototypes to mass-produced commercial products, which requires addressing challenges in materials, fabrication processes, and quality control. Finally, enhancing robustness and reliability to ensure consistent performance under various operating conditions, particularly important for clinical and industrial applications where device failure could have significant consequences.
Market Analysis for Lab-on-Chip Applications
The global lab-on-chip (LOC) market has been experiencing robust growth, with a market value reaching $8.2 billion in 2022 and projected to grow at a CAGR of 10.5% through 2030. This growth is primarily driven by increasing demand for point-of-care diagnostics, personalized medicine, and the need for more efficient drug discovery processes. The COVID-19 pandemic has further accelerated market expansion by highlighting the importance of rapid, portable diagnostic solutions.
Healthcare applications currently dominate the LOC market, accounting for approximately 65% of total market share. Within this segment, diagnostic applications represent the largest portion, followed by drug discovery and genomics research. The pharmaceutical industry has increasingly adopted microfluidic technologies to reduce drug development costs and accelerate time-to-market for new therapeutics.
Regionally, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, growing research infrastructure, and rising adoption of advanced diagnostic technologies in countries like China, Japan, and India.
Consumer demand trends indicate a strong preference for devices offering faster results, higher sensitivity, lower sample volume requirements, and reduced costs per test. The market is also seeing increased demand for multiplexed assays that can detect multiple analytes simultaneously, enhancing diagnostic efficiency and reducing overall testing time.
Key market drivers include the aging global population, rising incidence of chronic diseases, increasing healthcare costs driving the need for cost-effective solutions, and technological advancements in microfluidics. The shift toward personalized medicine has created significant opportunities for LOC technologies that enable rapid genetic analysis and biomarker detection.
Barriers to market adoption include high initial development costs, technical challenges in system integration, regulatory hurdles, and the need for standardization across platforms. Additionally, many healthcare systems face challenges in implementing new technologies due to reimbursement issues and the need for clinical validation.
Emerging application areas showing significant growth potential include environmental monitoring, food safety testing, veterinary diagnostics, and forensic analysis. These diversified applications are expected to create new revenue streams for microfluidic technology providers and expand the overall market footprint beyond traditional healthcare applications.
Healthcare applications currently dominate the LOC market, accounting for approximately 65% of total market share. Within this segment, diagnostic applications represent the largest portion, followed by drug discovery and genomics research. The pharmaceutical industry has increasingly adopted microfluidic technologies to reduce drug development costs and accelerate time-to-market for new therapeutics.
Regionally, North America leads the market with approximately 40% share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is expected to witness the fastest growth rate due to increasing healthcare expenditure, growing research infrastructure, and rising adoption of advanced diagnostic technologies in countries like China, Japan, and India.
Consumer demand trends indicate a strong preference for devices offering faster results, higher sensitivity, lower sample volume requirements, and reduced costs per test. The market is also seeing increased demand for multiplexed assays that can detect multiple analytes simultaneously, enhancing diagnostic efficiency and reducing overall testing time.
Key market drivers include the aging global population, rising incidence of chronic diseases, increasing healthcare costs driving the need for cost-effective solutions, and technological advancements in microfluidics. The shift toward personalized medicine has created significant opportunities for LOC technologies that enable rapid genetic analysis and biomarker detection.
Barriers to market adoption include high initial development costs, technical challenges in system integration, regulatory hurdles, and the need for standardization across platforms. Additionally, many healthcare systems face challenges in implementing new technologies due to reimbursement issues and the need for clinical validation.
Emerging application areas showing significant growth potential include environmental monitoring, food safety testing, veterinary diagnostics, and forensic analysis. These diversified applications are expected to create new revenue streams for microfluidic technology providers and expand the overall market footprint beyond traditional healthcare applications.
Current Microfluidic Technologies and Limitations
Microfluidic technologies have evolved significantly over the past two decades, with current systems employing various mechanisms for fluid manipulation at the microscale. Continuous-flow microfluidics represents the most established approach, utilizing pressure-driven or electrokinetic forces to move fluids through microfabricated channels. These systems excel in applications requiring consistent flow rates but face challenges in precise control of individual fluid packets.
Droplet-based microfluidics has emerged as a powerful alternative, enabling the generation and manipulation of discrete droplets within immiscible carrier fluids. This technology offers advantages in high-throughput screening and single-cell analysis but requires sophisticated control systems for droplet generation, sorting, and merging. Surface tension effects at this scale can lead to unpredictable behavior, limiting reproducibility.
Digital microfluidics, based on electrowetting principles, allows for individual droplet manipulation on planar surfaces without requiring complex channel networks. While offering unprecedented flexibility in fluid routing, these systems struggle with scalability and integration with detection components. The high voltages required for operation can also affect sensitive biological samples.
Paper-based microfluidics presents a low-cost alternative utilizing capillary action in porous substrates. Though advantageous for point-of-care diagnostics in resource-limited settings, these platforms suffer from limited sensitivity, poor quantification capabilities, and restricted complexity of operations.
Current microfluidic technologies face several critical limitations. Fabrication challenges persist, with most systems requiring cleanroom facilities and specialized equipment, driving up costs and limiting accessibility. Material compatibility issues arise when working with biological samples, as surface adsorption can lead to sample loss and cross-contamination. Many polymers used in microfluidic devices exhibit problematic gas permeability and solvent compatibility.
Integration remains a significant hurdle, with difficulties in combining multiple functionalities (sample preparation, reaction, detection) on a single chip. The "world-to-chip" interface problem—connecting macroscale laboratory equipment to microscale devices—continues to impede practical implementation. Standardization is notably lacking across the field, with most academic and commercial systems using proprietary designs that hinder interoperability.
Scaling limitations affect throughput capabilities, particularly for applications requiring parallel processing. Detection sensitivity is often compromised by the reduced optical path lengths inherent to microfluidic channels. Additionally, most current systems require external pumps, power sources, and control equipment, limiting true portability and point-of-use applications.
Droplet-based microfluidics has emerged as a powerful alternative, enabling the generation and manipulation of discrete droplets within immiscible carrier fluids. This technology offers advantages in high-throughput screening and single-cell analysis but requires sophisticated control systems for droplet generation, sorting, and merging. Surface tension effects at this scale can lead to unpredictable behavior, limiting reproducibility.
Digital microfluidics, based on electrowetting principles, allows for individual droplet manipulation on planar surfaces without requiring complex channel networks. While offering unprecedented flexibility in fluid routing, these systems struggle with scalability and integration with detection components. The high voltages required for operation can also affect sensitive biological samples.
Paper-based microfluidics presents a low-cost alternative utilizing capillary action in porous substrates. Though advantageous for point-of-care diagnostics in resource-limited settings, these platforms suffer from limited sensitivity, poor quantification capabilities, and restricted complexity of operations.
Current microfluidic technologies face several critical limitations. Fabrication challenges persist, with most systems requiring cleanroom facilities and specialized equipment, driving up costs and limiting accessibility. Material compatibility issues arise when working with biological samples, as surface adsorption can lead to sample loss and cross-contamination. Many polymers used in microfluidic devices exhibit problematic gas permeability and solvent compatibility.
Integration remains a significant hurdle, with difficulties in combining multiple functionalities (sample preparation, reaction, detection) on a single chip. The "world-to-chip" interface problem—connecting macroscale laboratory equipment to microscale devices—continues to impede practical implementation. Standardization is notably lacking across the field, with most academic and commercial systems using proprietary designs that hinder interoperability.
Scaling limitations affect throughput capabilities, particularly for applications requiring parallel processing. Detection sensitivity is often compromised by the reduced optical path lengths inherent to microfluidic channels. Additionally, most current systems require external pumps, power sources, and control equipment, limiting true portability and point-of-use applications.
Current Approaches to Microfluidic Efficiency
01 Microfluidic channel design optimization
Optimizing the design of microfluidic channels can significantly improve efficiency in microfluidic systems. This includes considerations for channel geometry, surface treatments, and flow dynamics to reduce resistance and enhance fluid transport. Advanced channel designs incorporate features like herringbone patterns or specialized junctions that promote mixing while minimizing pressure drops, resulting in more efficient operations with lower energy requirements.- Microfluidic device design optimization: Optimizing the design of microfluidic devices can significantly improve efficiency. This includes considerations for channel geometry, flow path design, and integration of functional components. Advanced designs incorporate features that minimize dead volumes, reduce pressure drops, and enhance mixing capabilities. These optimizations lead to improved throughput, reduced sample consumption, and more reliable results in applications ranging from diagnostics to chemical synthesis.
- Flow control and manipulation techniques: Various techniques for controlling and manipulating fluid flow in microfluidic systems enhance operational efficiency. These include electrokinetic methods, pressure-driven systems, and surface tension-based approaches. Advanced flow control mechanisms allow for precise regulation of fluid movement, droplet generation, and mixing processes. Implementation of these techniques results in improved reaction kinetics, reduced processing times, and enhanced analytical performance.
- Integration of sensing and detection systems: Incorporating sensing and detection systems directly into microfluidic platforms improves analytical efficiency. These integrated systems enable real-time monitoring of processes, immediate feedback control, and on-chip analysis. Technologies such as optical, electrochemical, and acoustic sensors provide enhanced detection sensitivity and specificity. This integration reduces the need for external equipment, minimizes sample handling, and accelerates analytical workflows.
- Surface modification and material selection: Strategic surface modification and material selection play crucial roles in enhancing microfluidic efficiency. Treatments that alter surface properties can prevent non-specific adsorption, control wettability, and improve flow characteristics. Advanced materials with optimized properties provide benefits such as reduced fouling, enhanced biocompatibility, and improved chemical resistance. These approaches extend device lifetime, maintain consistent performance, and enable more efficient operation across diverse applications.
- Automation and parallelization strategies: Implementing automation and parallelization strategies significantly increases throughput and operational efficiency in microfluidic systems. Automated sample handling, reagent dispensing, and process control reduce human error and labor requirements. Parallel processing architectures enable simultaneous execution of multiple operations, dramatically increasing analytical capacity. These approaches are particularly valuable for high-throughput screening, multiplexed assays, and industrial-scale applications where processing efficiency is paramount.
02 Integration of sensing and control systems
Incorporating advanced sensing and control systems into microfluidic platforms enables real-time monitoring and adjustment of process parameters. These integrated systems use feedback mechanisms to optimize flow rates, reaction conditions, and mixing efficiency. Sensors embedded within the microfluidic architecture provide continuous data on temperature, pressure, and chemical composition, allowing for automated adjustments that maintain optimal operating conditions and improve overall system efficiency.Expand Specific Solutions03 Novel materials for microfluidic device fabrication
The development and application of novel materials in microfluidic device fabrication can enhance operational efficiency. Materials with specific surface properties, such as hydrophobic or hydrophilic characteristics, can control fluid behavior within channels. Advanced polymers, glass formulations, and hybrid materials offer improved chemical resistance, thermal stability, and optical transparency, enabling more efficient and reliable microfluidic operations across a wider range of applications and conditions.Expand Specific Solutions04 Droplet-based microfluidic technologies
Droplet-based microfluidic systems improve efficiency through compartmentalization of reactions in discrete volumes. This approach enables high-throughput processing with minimal reagent consumption and reduced cross-contamination. The generation, manipulation, and analysis of uniform droplets allow for parallel processing of thousands of reactions simultaneously, significantly increasing throughput while maintaining precision. These systems are particularly valuable for applications requiring high efficiency in screening or analytical processes.Expand Specific Solutions05 Energy-efficient pumping and fluid actuation methods
Innovative pumping and fluid actuation methods can substantially improve the energy efficiency of microfluidic systems. These include electrokinetic techniques, acoustic wave propulsion, and magnetically-driven flows that require less power than conventional pressure-driven systems. Advanced valve designs and passive flow control mechanisms further reduce energy consumption while maintaining precise fluid handling capabilities, making microfluidic platforms more sustainable and suitable for portable or point-of-care applications.Expand Specific Solutions
Leading Companies in Lab-on-Chip Industry
The microfluidics lab-on-chip market is currently in a growth phase, with an expanding market size driven by increasing demand for point-of-care diagnostics and personalized medicine. The technology maturity varies across applications, with established players like Agilent Technologies and Robert Bosch GmbH leading commercial development through robust engineering capabilities and standardized manufacturing processes. Academic institutions including Vanderbilt University and EPFL are advancing fundamental research, while companies like Samsung Electronics and IBM are leveraging their semiconductor expertise to enhance miniaturization and integration. Emerging players such as Lansion Biotechnology and Kromek are focusing on specialized applications, creating a competitive landscape that balances established industrial capabilities with innovative research approaches to overcome challenges in scalability and system integration.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed advanced microfluidic platforms that integrate multiple laboratory functions on a single chip. Their technology utilizes precision flow control systems with integrated pressure sensors that dynamically adjust flow rates based on real-time feedback. Agilent's lab-on-chip solutions incorporate digital microfluidics for droplet manipulation, allowing precise control of nanoliter-scale samples through electrowetting techniques. Their systems feature multilayer fabrication processes that enable complex 3D microfluidic architectures with optimized channel geometries to minimize dead volumes and reduce sample dispersion. Agilent has also pioneered surface modification techniques that prevent non-specific binding and reduce biofouling, significantly improving device longevity and reproducibility in biological applications[1][3].
Strengths: Superior precision in fluid handling with industry-leading sensitivity for bioanalytical applications; extensive integration capabilities with analytical instruments. Weaknesses: Higher cost compared to simpler systems; proprietary interfaces may limit compatibility with third-party components.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed microfluidic technologies that leverage their semiconductor manufacturing expertise. Their approach focuses on digital microfluidics using electrowetting-on-dielectric (EWOD) principles for precise droplet manipulation. Samsung's lab-on-chip platforms incorporate high-density electrode arrays fabricated using advanced photolithography techniques, enabling complex droplet routing protocols with minimal cross-contamination. Their systems feature integrated CMOS sensors for real-time monitoring of biochemical reactions, with specialized signal processing algorithms to enhance detection sensitivity. Samsung has also pioneered low-power microfluidic control systems that optimize energy consumption through adaptive duty cycling of actuation voltages. Additionally, they've developed specialized surface coatings that maintain hydrophobic properties over thousands of actuation cycles, significantly improving device reliability for long-term operation[4][7].
Strengths: Exceptional manufacturing capabilities leveraging semiconductor expertise; strong integration of electronic components with microfluidics. Weaknesses: Relatively newer entrant to the biological applications market; less established presence in life science research communities.
Key Patents in Microfluidic Flow Control
Microfluidic devices for the controlled manipulation of small volumes
PatentInactiveUS20070227890A1
Innovation
- A method and apparatus for forming and transporting minute volume segments using a fluidic microchip with interconnected channels and electrodes, allowing for precise control of transport and segmenting fluids, enabling serial registration and storage of reagents, cells, or reaction beads for later analysis.
Microfluidic phase-change membrane microvalves
PatentActiveUS20230149923A1
Innovation
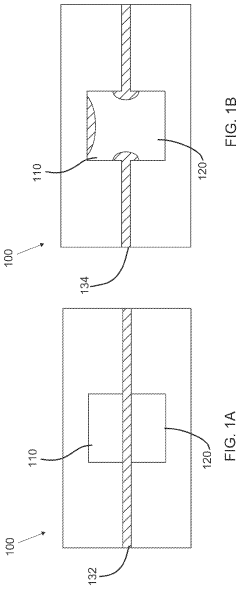
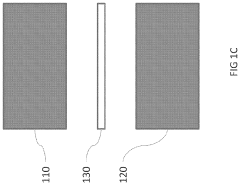
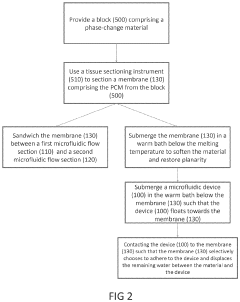
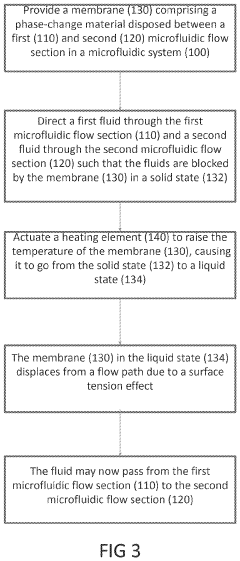
- The development of phase-change microvalves using a thin paraffin membrane sandwiched between microfluidic flow sections, actuated by heat to control fluid flow without pressure differentials, allowing for efficient fabrication and operation with tissue sectioning instruments, and featuring a unique property where the entire membrane melts to displace within the system while remaining sealed outside, preventing leakage.
Manufacturing Challenges and Solutions
Manufacturing microfluidic lab-on-chip devices presents significant challenges that impact overall system efficiency. Traditional fabrication methods often struggle with the precision required for microscale channels and structures. Photolithography, while offering high resolution, involves complex multi-step processes that increase production costs and limit scalability. The need for cleanroom facilities further restricts widespread manufacturing adoption, creating barriers for smaller research institutions and startups.
Material selection represents another critical manufacturing challenge. PDMS (polydimethylsiloxane), commonly used for prototyping, exhibits limitations in mass production scenarios due to its absorption properties and potential for chemical leaching. Alternative materials like thermoplastics offer better manufacturing scalability but present different surface chemistry considerations that can affect fluid behavior and biological compatibility.
Bonding and sealing processes remain particularly problematic in microfluidic device production. Creating leak-proof connections between different device layers requires precise alignment and uniform pressure application. Current techniques often result in channel deformation or incomplete sealing, leading to device failure and reduced manufacturing yields. The integration of functional components such as sensors, electrodes, and optical elements further complicates the manufacturing process.
Recent advancements in additive manufacturing technologies offer promising solutions to these challenges. 3D printing approaches, particularly stereolithography and two-photon polymerization, enable direct fabrication of complex microfluidic structures without requiring extensive cleanroom infrastructure. These methods significantly reduce prototyping cycles and allow for rapid design iterations. However, resolution limitations and surface roughness issues must be addressed before widespread adoption for high-precision applications.
Roll-to-roll manufacturing represents another emerging solution for high-volume production. This continuous process technology, adapted from the printing industry, enables fabrication of microfluidic devices on flexible substrates at significantly higher throughput than traditional batch processes. Combined with laser ablation or hot embossing techniques, roll-to-roll manufacturing offers cost-effective scaling potential for disposable diagnostic devices.
Standardization efforts are increasingly important in addressing manufacturing variability. The development of modular design approaches and standardized interconnects facilitates more reliable production and assembly processes. Organizations like the Microfluidics Association are working to establish industry-wide manufacturing standards that could reduce production inconsistencies and improve device reliability across different manufacturing facilities.
Quality control methodologies specifically adapted for microfluidic devices represent another crucial development area. Advanced imaging techniques, including optical coherence tomography and confocal microscopy, enable non-destructive inspection of internal channel geometries and surface properties. Automated testing platforms that verify fluid flow characteristics and functional performance help identify manufacturing defects before final device assembly.
Material selection represents another critical manufacturing challenge. PDMS (polydimethylsiloxane), commonly used for prototyping, exhibits limitations in mass production scenarios due to its absorption properties and potential for chemical leaching. Alternative materials like thermoplastics offer better manufacturing scalability but present different surface chemistry considerations that can affect fluid behavior and biological compatibility.
Bonding and sealing processes remain particularly problematic in microfluidic device production. Creating leak-proof connections between different device layers requires precise alignment and uniform pressure application. Current techniques often result in channel deformation or incomplete sealing, leading to device failure and reduced manufacturing yields. The integration of functional components such as sensors, electrodes, and optical elements further complicates the manufacturing process.
Recent advancements in additive manufacturing technologies offer promising solutions to these challenges. 3D printing approaches, particularly stereolithography and two-photon polymerization, enable direct fabrication of complex microfluidic structures without requiring extensive cleanroom infrastructure. These methods significantly reduce prototyping cycles and allow for rapid design iterations. However, resolution limitations and surface roughness issues must be addressed before widespread adoption for high-precision applications.
Roll-to-roll manufacturing represents another emerging solution for high-volume production. This continuous process technology, adapted from the printing industry, enables fabrication of microfluidic devices on flexible substrates at significantly higher throughput than traditional batch processes. Combined with laser ablation or hot embossing techniques, roll-to-roll manufacturing offers cost-effective scaling potential for disposable diagnostic devices.
Standardization efforts are increasingly important in addressing manufacturing variability. The development of modular design approaches and standardized interconnects facilitates more reliable production and assembly processes. Organizations like the Microfluidics Association are working to establish industry-wide manufacturing standards that could reduce production inconsistencies and improve device reliability across different manufacturing facilities.
Quality control methodologies specifically adapted for microfluidic devices represent another crucial development area. Advanced imaging techniques, including optical coherence tomography and confocal microscopy, enable non-destructive inspection of internal channel geometries and surface properties. Automated testing platforms that verify fluid flow characteristics and functional performance help identify manufacturing defects before final device assembly.
Integration with IoT and AI Systems
The integration of microfluidic Lab-on-Chip (LOC) systems with Internet of Things (IoT) and Artificial Intelligence (AI) represents a transformative approach to enhancing the efficiency and capabilities of these miniaturized analytical platforms. This convergence creates smart microfluidic systems capable of autonomous operation, real-time data analysis, and remote monitoring, significantly expanding their utility in clinical diagnostics, environmental monitoring, and pharmaceutical research.
IoT connectivity enables microfluidic devices to transmit data to cloud platforms, facilitating remote operation and monitoring of experiments. This connectivity allows researchers to control experimental parameters, receive alerts about system status, and access results from anywhere in the world. The implementation of wireless communication protocols such as Bluetooth Low Energy (BLE), Wi-Fi, or cellular networks provides the necessary infrastructure for seamless data transfer between microfluidic devices and central data repositories.
AI algorithms, particularly machine learning and deep learning approaches, enhance microfluidic LOC systems by automating data interpretation and decision-making processes. These algorithms can identify patterns in complex datasets generated by microfluidic experiments, enabling more accurate diagnostics and predictive analytics. For instance, neural networks can analyze microscopic images from microfluidic channels to detect rare cells or pathogens with higher sensitivity than traditional methods, while reinforcement learning algorithms can optimize fluid flow parameters in real-time to maximize reaction efficiency.
Edge computing architectures are increasingly being integrated with microfluidic systems to process data locally before transmission, reducing bandwidth requirements and enabling faster response times. This approach is particularly valuable for point-of-care applications where immediate results are critical. By implementing lightweight AI models directly on microcontroller units attached to microfluidic chips, complex analytical tasks can be performed without constant connectivity to cloud resources.
Digital twin technology represents another promising integration pathway, creating virtual replicas of physical microfluidic systems that can simulate experiments, predict outcomes, and optimize protocols before physical implementation. These digital models continuously update based on real-time data from the physical device, enabling predictive maintenance and performance optimization through AI-driven simulations.
Security considerations must be addressed when integrating IoT and AI with microfluidic systems, particularly for applications involving sensitive medical data. Implementing encryption protocols, secure authentication mechanisms, and privacy-preserving AI techniques ensures that data integrity and patient confidentiality are maintained throughout the analytical workflow.
The convergence of these technologies is creating a new generation of intelligent microfluidic systems that not only perform analytical tasks but also learn from each experiment, adapt to changing conditions, and integrate seamlessly with broader healthcare and research ecosystems. This integration pathway represents a significant opportunity to overcome current efficiency limitations in microfluidic LOC platforms.
IoT connectivity enables microfluidic devices to transmit data to cloud platforms, facilitating remote operation and monitoring of experiments. This connectivity allows researchers to control experimental parameters, receive alerts about system status, and access results from anywhere in the world. The implementation of wireless communication protocols such as Bluetooth Low Energy (BLE), Wi-Fi, or cellular networks provides the necessary infrastructure for seamless data transfer between microfluidic devices and central data repositories.
AI algorithms, particularly machine learning and deep learning approaches, enhance microfluidic LOC systems by automating data interpretation and decision-making processes. These algorithms can identify patterns in complex datasets generated by microfluidic experiments, enabling more accurate diagnostics and predictive analytics. For instance, neural networks can analyze microscopic images from microfluidic channels to detect rare cells or pathogens with higher sensitivity than traditional methods, while reinforcement learning algorithms can optimize fluid flow parameters in real-time to maximize reaction efficiency.
Edge computing architectures are increasingly being integrated with microfluidic systems to process data locally before transmission, reducing bandwidth requirements and enabling faster response times. This approach is particularly valuable for point-of-care applications where immediate results are critical. By implementing lightweight AI models directly on microcontroller units attached to microfluidic chips, complex analytical tasks can be performed without constant connectivity to cloud resources.
Digital twin technology represents another promising integration pathway, creating virtual replicas of physical microfluidic systems that can simulate experiments, predict outcomes, and optimize protocols before physical implementation. These digital models continuously update based on real-time data from the physical device, enabling predictive maintenance and performance optimization through AI-driven simulations.
Security considerations must be addressed when integrating IoT and AI with microfluidic systems, particularly for applications involving sensitive medical data. Implementing encryption protocols, secure authentication mechanisms, and privacy-preserving AI techniques ensures that data integrity and patient confidentiality are maintained throughout the analytical workflow.
The convergence of these technologies is creating a new generation of intelligent microfluidic systems that not only perform analytical tasks but also learn from each experiment, adapt to changing conditions, and integrate seamlessly with broader healthcare and research ecosystems. This integration pathway represents a significant opportunity to overcome current efficiency limitations in microfluidic LOC platforms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!