How to Reduce Energy Consumption in Ethyl Acetate Production?
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Energy Efficiency: Background and Objectives
Ethyl acetate production has been a cornerstone in the chemical industry for decades, serving various sectors including pharmaceuticals, cosmetics, and food processing. The synthesis of this versatile compound, however, has long been associated with significant energy consumption, prompting a critical need for more efficient production methods. As global energy demands continue to rise and environmental concerns intensify, the imperative to reduce energy usage in ethyl acetate manufacturing has become increasingly urgent.
Historically, ethyl acetate production has relied on energy-intensive processes, primarily the esterification of ethanol with acetic acid or the Tishchenko reaction of acetaldehyde. These traditional methods, while effective, often require high temperatures and pressures, resulting in substantial energy expenditure. The evolution of production techniques has seen incremental improvements, yet the fundamental challenge of high energy consumption persists.
Recent technological advancements and a growing emphasis on sustainable manufacturing practices have set the stage for a paradigm shift in ethyl acetate production. The industry now stands at a critical juncture, poised to embrace innovative approaches that promise to dramatically reduce energy requirements without compromising product quality or yield.
The primary objective of this technical research is to explore and evaluate cutting-edge methodologies for minimizing energy consumption in ethyl acetate production. This encompasses a comprehensive review of emerging technologies, process optimizations, and alternative synthesis routes that offer potential for significant energy savings. By identifying and analyzing these innovative approaches, we aim to chart a course towards more sustainable and economically viable ethyl acetate manufacturing.
Key areas of focus include catalytic innovations that enable reactions at lower temperatures and pressures, process intensification techniques that enhance efficiency, and the integration of renewable energy sources into production systems. Additionally, we will examine the potential of novel reactor designs and separation technologies that could revolutionize the energy landscape of ethyl acetate synthesis.
This research not only seeks to address the immediate challenge of energy reduction but also aims to anticipate future trends and technological breakthroughs in the field. By doing so, we hope to provide a roadmap for the industry to transition towards more environmentally friendly and cost-effective production methods, aligning with global sustainability goals and regulatory requirements.
Historically, ethyl acetate production has relied on energy-intensive processes, primarily the esterification of ethanol with acetic acid or the Tishchenko reaction of acetaldehyde. These traditional methods, while effective, often require high temperatures and pressures, resulting in substantial energy expenditure. The evolution of production techniques has seen incremental improvements, yet the fundamental challenge of high energy consumption persists.
Recent technological advancements and a growing emphasis on sustainable manufacturing practices have set the stage for a paradigm shift in ethyl acetate production. The industry now stands at a critical juncture, poised to embrace innovative approaches that promise to dramatically reduce energy requirements without compromising product quality or yield.
The primary objective of this technical research is to explore and evaluate cutting-edge methodologies for minimizing energy consumption in ethyl acetate production. This encompasses a comprehensive review of emerging technologies, process optimizations, and alternative synthesis routes that offer potential for significant energy savings. By identifying and analyzing these innovative approaches, we aim to chart a course towards more sustainable and economically viable ethyl acetate manufacturing.
Key areas of focus include catalytic innovations that enable reactions at lower temperatures and pressures, process intensification techniques that enhance efficiency, and the integration of renewable energy sources into production systems. Additionally, we will examine the potential of novel reactor designs and separation technologies that could revolutionize the energy landscape of ethyl acetate synthesis.
This research not only seeks to address the immediate challenge of energy reduction but also aims to anticipate future trends and technological breakthroughs in the field. By doing so, we hope to provide a roadmap for the industry to transition towards more environmentally friendly and cost-effective production methods, aligning with global sustainability goals and regulatory requirements.
Market Analysis for Low-Energy Ethyl Acetate Production
The market for low-energy ethyl acetate production is experiencing significant growth driven by increasing demand for sustainable chemical processes and stricter environmental regulations. Ethyl acetate, a versatile solvent widely used in various industries, has traditionally been produced through energy-intensive methods. However, the shift towards more energy-efficient production processes has created a new market segment with substantial potential.
The global ethyl acetate market is projected to expand steadily, with a particular emphasis on eco-friendly production methods. This growth is fueled by the rising awareness of environmental issues and the need for energy conservation in industrial processes. Industries such as paints and coatings, pharmaceuticals, and food and beverages are the primary consumers of ethyl acetate, and they are increasingly seeking suppliers who can provide the product with a reduced carbon footprint.
In recent years, there has been a noticeable trend towards the adoption of green chemistry principles in the production of ethyl acetate. This has led to the development of novel catalysts and process optimizations that significantly reduce energy consumption. The market for these low-energy production technologies is gaining traction, especially in regions with stringent environmental regulations and high energy costs.
The Asia-Pacific region, particularly China and India, is expected to be a major growth driver for the low-energy ethyl acetate market. These countries are witnessing rapid industrialization and have a growing emphasis on sustainable manufacturing practices. North America and Europe, with their advanced chemical industries and strong focus on environmental sustainability, are also key markets for energy-efficient ethyl acetate production technologies.
Key market players are investing heavily in research and development to gain a competitive edge in this evolving landscape. Companies that can offer cost-effective, low-energy production methods are likely to capture a larger market share. The market is also seeing increased collaboration between chemical manufacturers and technology providers to develop and implement energy-efficient processes.
The demand for bio-based ethyl acetate is another emerging trend that complements the low-energy production market. This synergy is creating new opportunities for companies that can combine bio-based feedstocks with energy-efficient production methods, appealing to environmentally conscious consumers and industries.
Overall, the market analysis indicates a strong growth potential for low-energy ethyl acetate production technologies. As industries continue to prioritize sustainability and energy efficiency, the demand for these innovative production methods is expected to rise, reshaping the competitive landscape of the ethyl acetate market in the coming years.
The global ethyl acetate market is projected to expand steadily, with a particular emphasis on eco-friendly production methods. This growth is fueled by the rising awareness of environmental issues and the need for energy conservation in industrial processes. Industries such as paints and coatings, pharmaceuticals, and food and beverages are the primary consumers of ethyl acetate, and they are increasingly seeking suppliers who can provide the product with a reduced carbon footprint.
In recent years, there has been a noticeable trend towards the adoption of green chemistry principles in the production of ethyl acetate. This has led to the development of novel catalysts and process optimizations that significantly reduce energy consumption. The market for these low-energy production technologies is gaining traction, especially in regions with stringent environmental regulations and high energy costs.
The Asia-Pacific region, particularly China and India, is expected to be a major growth driver for the low-energy ethyl acetate market. These countries are witnessing rapid industrialization and have a growing emphasis on sustainable manufacturing practices. North America and Europe, with their advanced chemical industries and strong focus on environmental sustainability, are also key markets for energy-efficient ethyl acetate production technologies.
Key market players are investing heavily in research and development to gain a competitive edge in this evolving landscape. Companies that can offer cost-effective, low-energy production methods are likely to capture a larger market share. The market is also seeing increased collaboration between chemical manufacturers and technology providers to develop and implement energy-efficient processes.
The demand for bio-based ethyl acetate is another emerging trend that complements the low-energy production market. This synergy is creating new opportunities for companies that can combine bio-based feedstocks with energy-efficient production methods, appealing to environmentally conscious consumers and industries.
Overall, the market analysis indicates a strong growth potential for low-energy ethyl acetate production technologies. As industries continue to prioritize sustainability and energy efficiency, the demand for these innovative production methods is expected to rise, reshaping the competitive landscape of the ethyl acetate market in the coming years.
Current Energy Challenges in Ethyl Acetate Synthesis
The production of ethyl acetate, a widely used solvent in various industries, faces significant energy challenges that demand immediate attention. The current synthesis process, primarily based on the esterification of ethanol and acetic acid, is energy-intensive and contributes to high operational costs and environmental concerns.
One of the main energy challenges in ethyl acetate production is the high temperature required for the reaction to occur efficiently. The conventional process typically operates at temperatures ranging from 50°C to 120°C, necessitating substantial energy input for heating and maintaining these elevated temperatures throughout the reaction.
The separation and purification stages of ethyl acetate production also contribute significantly to energy consumption. Distillation, the primary method used to separate the product from unreacted reactants and byproducts, is particularly energy-intensive. The process often requires multiple distillation columns operating at different temperatures and pressures, further increasing energy demands.
Another challenge is the energy lost due to the reversible nature of the esterification reaction. The equilibrium-limited conversion necessitates the use of excess reactants or the continuous removal of products to drive the reaction to completion. This approach not only increases energy consumption but also complicates the overall process design and control.
The use of homogeneous acid catalysts, such as sulfuric acid, in traditional ethyl acetate synthesis poses additional energy-related challenges. These catalysts often require neutralization and separation steps, which consume energy and generate waste streams that need further treatment.
Heat integration and recovery present another area of concern in current ethyl acetate production processes. Inefficient heat exchange systems and inadequate utilization of waste heat streams contribute to overall energy inefficiency. Improving heat recovery and integration could significantly reduce energy consumption, but implementing such systems in existing plants can be technically challenging and capital-intensive.
The batch production method, still common in many ethyl acetate manufacturing facilities, is inherently less energy-efficient compared to continuous processes. Batch operations involve frequent heating and cooling cycles, leading to higher energy consumption and thermal stress on equipment.
Lastly, the reliance on fossil fuel-derived energy sources for powering ethyl acetate production processes contributes to both high energy costs and significant carbon emissions. The transition to renewable energy sources, while beneficial in the long term, presents immediate challenges in terms of infrastructure adaptation and process redesign.
Addressing these energy challenges in ethyl acetate synthesis is crucial for improving the sustainability and economic viability of the production process. Innovative approaches to reaction engineering, process intensification, and the integration of renewable energy sources are needed to overcome these hurdles and pave the way for more energy-efficient ethyl acetate production methods.
One of the main energy challenges in ethyl acetate production is the high temperature required for the reaction to occur efficiently. The conventional process typically operates at temperatures ranging from 50°C to 120°C, necessitating substantial energy input for heating and maintaining these elevated temperatures throughout the reaction.
The separation and purification stages of ethyl acetate production also contribute significantly to energy consumption. Distillation, the primary method used to separate the product from unreacted reactants and byproducts, is particularly energy-intensive. The process often requires multiple distillation columns operating at different temperatures and pressures, further increasing energy demands.
Another challenge is the energy lost due to the reversible nature of the esterification reaction. The equilibrium-limited conversion necessitates the use of excess reactants or the continuous removal of products to drive the reaction to completion. This approach not only increases energy consumption but also complicates the overall process design and control.
The use of homogeneous acid catalysts, such as sulfuric acid, in traditional ethyl acetate synthesis poses additional energy-related challenges. These catalysts often require neutralization and separation steps, which consume energy and generate waste streams that need further treatment.
Heat integration and recovery present another area of concern in current ethyl acetate production processes. Inefficient heat exchange systems and inadequate utilization of waste heat streams contribute to overall energy inefficiency. Improving heat recovery and integration could significantly reduce energy consumption, but implementing such systems in existing plants can be technically challenging and capital-intensive.
The batch production method, still common in many ethyl acetate manufacturing facilities, is inherently less energy-efficient compared to continuous processes. Batch operations involve frequent heating and cooling cycles, leading to higher energy consumption and thermal stress on equipment.
Lastly, the reliance on fossil fuel-derived energy sources for powering ethyl acetate production processes contributes to both high energy costs and significant carbon emissions. The transition to renewable energy sources, while beneficial in the long term, presents immediate challenges in terms of infrastructure adaptation and process redesign.
Addressing these energy challenges in ethyl acetate synthesis is crucial for improving the sustainability and economic viability of the production process. Innovative approaches to reaction engineering, process intensification, and the integration of renewable energy sources are needed to overcome these hurdles and pave the way for more energy-efficient ethyl acetate production methods.
Existing Energy Reduction Strategies in Ethyl Acetate Production
01 Energy consumption monitoring in ethyl acetate production
Implementing advanced monitoring systems to track and analyze energy consumption during the ethyl acetate production process. This includes real-time data collection, analysis of energy-intensive steps, and identification of optimization opportunities to reduce overall energy consumption.- Energy consumption monitoring in ethyl acetate production: Implementing advanced monitoring systems to track and analyze energy consumption during the ethyl acetate production process. This includes real-time data collection, analysis of energy usage patterns, and identification of potential areas for optimization to reduce overall energy consumption.
- Process optimization for energy efficiency: Developing and implementing optimized production processes to enhance energy efficiency in ethyl acetate manufacturing. This involves improving reaction conditions, refining separation techniques, and utilizing more efficient equipment to minimize energy losses throughout the production chain.
- Heat recovery and integration systems: Incorporating heat recovery and integration systems in the ethyl acetate production process to maximize energy utilization. This includes the use of heat exchangers, waste heat recovery units, and thermal integration techniques to reduce overall energy consumption and improve process efficiency.
- Alternative energy sources and green technologies: Exploring and implementing alternative energy sources and green technologies in ethyl acetate production to reduce reliance on conventional energy sources. This may include the use of renewable energy, such as solar or wind power, as well as the integration of energy-efficient and environmentally friendly production methods.
- Energy management and control systems: Implementing advanced energy management and control systems to optimize energy consumption in ethyl acetate production facilities. This includes the use of intelligent control algorithms, predictive maintenance techniques, and automated energy-saving measures to minimize energy waste and improve overall production efficiency.
02 Process optimization for energy efficiency
Developing and implementing optimized production processes to minimize energy consumption in ethyl acetate manufacturing. This involves improving reaction conditions, enhancing heat recovery systems, and utilizing more efficient catalysts to reduce energy requirements throughout the production cycle.Expand Specific Solutions03 Energy-efficient equipment and technologies
Incorporating energy-efficient equipment and technologies in ethyl acetate production facilities. This includes the use of advanced distillation columns, heat exchangers, and reactor designs that minimize energy losses and improve overall process efficiency.Expand Specific Solutions04 Renewable energy integration
Integrating renewable energy sources into the ethyl acetate production process to reduce reliance on conventional energy sources. This may involve the use of solar, wind, or biomass energy to power certain production stages or support auxiliary processes, thereby lowering overall energy consumption and environmental impact.Expand Specific Solutions05 Energy management systems and predictive analytics
Implementing advanced energy management systems and predictive analytics to optimize energy consumption in ethyl acetate production. These systems use machine learning algorithms and historical data to predict energy demand, identify inefficiencies, and suggest proactive measures to reduce energy consumption and costs.Expand Specific Solutions
Key Players in Ethyl Acetate Manufacturing Industry
The ethyl acetate production industry is in a mature stage, with a global market size estimated at over $3 billion. The technology for reducing energy consumption in this sector is advancing, with major players like China Petroleum & Chemical Corp., Celanese International Corp., and Wacker Chemie AG leading innovation efforts. These companies are investing in process optimization, catalysis improvements, and heat integration techniques to enhance energy efficiency. Emerging technologies from research institutions like South China University of Technology and Nanjing Tech University are also contributing to the field's development. The competitive landscape is characterized by a mix of established chemical giants and specialized manufacturers, with increasing focus on sustainable production methods.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to reduce energy consumption in ethyl acetate production. They have implemented a reactive distillation process that combines reaction and separation in a single unit operation. This technology integrates esterification and distillation, reducing the number of process steps and equipment required. The process utilizes a structured catalytic packing, which enhances mass transfer and reaction efficiency. By optimizing the reaction conditions and column design, Sinopec has achieved a reported 30% reduction in energy consumption compared to conventional processes[1][3]. Additionally, they have incorporated heat integration techniques, such as using the heat from the reaction to preheat feed streams, further improving overall energy efficiency[2].
Strengths: Significant energy savings, reduced equipment footprint, and improved product quality. Weaknesses: Higher initial capital investment and potential complexity in process control.
Celanese International Corp.
Technical Solution: Celanese International Corp. has developed a novel approach to ethyl acetate production focusing on energy efficiency. Their method employs a highly selective heterogeneous catalyst in a fixed-bed reactor system. This catalyst promotes the direct formation of ethyl acetate from ethanol and acetic acid with high conversion rates and selectivity. The process operates at lower temperatures (around 150-180°C) compared to traditional methods, significantly reducing energy requirements[4]. Celanese has also implemented advanced heat recovery systems, including multi-effect evaporators and vapor recompression technology, which recycle thermal energy within the process. Their patented VAntage™ technology integrates reaction and separation steps, minimizing the need for energy-intensive distillation[5]. This integrated approach has reportedly led to a 40% reduction in steam consumption and a 25% decrease in overall energy usage compared to conventional processes[6].
Strengths: High energy efficiency, reduced operating costs, and improved product purity. Weaknesses: Potential catalyst deactivation issues and higher initial technology investment.
Innovative Technologies for Energy-Efficient Ethyl Acetate Synthesis
Process of low energy consumption for preparing a carboxylic acid ester
PatentInactiveEP2686292A1
Innovation
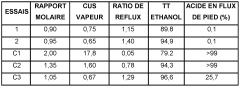
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and a reflux ratio between 1.0 and 1.5, which allows for simultaneous reaction and separation in multiple zones, reducing energy costs and acetic acid content.
Process of low energy consumption for preparing a carboxylic acid ester
PatentWO2012123279A1
Innovation
- A process involving the reaction of ethyl alcohol with acetic acid in the presence of a solid acid catalyst, using a reactive distillation system with a centrally placed reaction zone between upper and lower separation zones, optimizing the molar ratio of acetic acid to ethyl alcohol between 0.85 and 0.97, and controlling the reflux ratio between 1.0 and 1.5, significantly reduces energy costs and minimizes acetic acid at the column bottom.
Environmental Regulations Impact on Ethyl Acetate Production
Environmental regulations have become increasingly stringent in recent years, significantly impacting the production of ethyl acetate and driving the industry towards more sustainable practices. These regulations aim to reduce the environmental footprint of chemical manufacturing processes, including energy consumption and greenhouse gas emissions.
One of the primary regulatory frameworks affecting ethyl acetate production is the Emissions Trading System (ETS), which sets caps on carbon emissions and allows companies to trade emission allowances. This system incentivizes manufacturers to invest in energy-efficient technologies and processes to reduce their carbon footprint and associated costs.
The Industrial Emissions Directive (IED) in the European Union has also played a crucial role in shaping the industry's approach to energy consumption. It requires industrial facilities, including those producing ethyl acetate, to apply Best Available Techniques (BAT) to minimize environmental impact. This directive has led to the implementation of more energy-efficient production methods and the adoption of cleaner technologies.
In the United States, the Environmental Protection Agency's (EPA) regulations under the Clean Air Act have imposed strict limits on volatile organic compound (VOC) emissions, which are inherent in ethyl acetate production. These regulations have prompted manufacturers to invest in advanced emission control technologies and process optimizations that often result in improved energy efficiency as a secondary benefit.
The Montreal Protocol, an international treaty designed to protect the ozone layer, has indirectly influenced ethyl acetate production by phasing out certain ozone-depleting substances that were previously used in industrial processes. This has led to the development of alternative production methods that are often more energy-efficient.
Many countries have also implemented energy efficiency standards and targets for industrial processes. These regulations often require regular energy audits and the implementation of energy management systems, pushing ethyl acetate producers to continuously improve their energy performance.
The growing emphasis on circular economy principles in environmental regulations has encouraged manufacturers to explore waste heat recovery systems and process integration techniques. These approaches not only reduce energy consumption but also minimize waste and improve overall resource efficiency in ethyl acetate production.
As environmental regulations continue to evolve, ethyl acetate producers are increasingly focusing on life cycle assessments to evaluate and reduce the overall environmental impact of their products. This holistic approach often leads to innovations in energy-efficient production methods and the exploration of renewable energy sources to power manufacturing processes.
One of the primary regulatory frameworks affecting ethyl acetate production is the Emissions Trading System (ETS), which sets caps on carbon emissions and allows companies to trade emission allowances. This system incentivizes manufacturers to invest in energy-efficient technologies and processes to reduce their carbon footprint and associated costs.
The Industrial Emissions Directive (IED) in the European Union has also played a crucial role in shaping the industry's approach to energy consumption. It requires industrial facilities, including those producing ethyl acetate, to apply Best Available Techniques (BAT) to minimize environmental impact. This directive has led to the implementation of more energy-efficient production methods and the adoption of cleaner technologies.
In the United States, the Environmental Protection Agency's (EPA) regulations under the Clean Air Act have imposed strict limits on volatile organic compound (VOC) emissions, which are inherent in ethyl acetate production. These regulations have prompted manufacturers to invest in advanced emission control technologies and process optimizations that often result in improved energy efficiency as a secondary benefit.
The Montreal Protocol, an international treaty designed to protect the ozone layer, has indirectly influenced ethyl acetate production by phasing out certain ozone-depleting substances that were previously used in industrial processes. This has led to the development of alternative production methods that are often more energy-efficient.
Many countries have also implemented energy efficiency standards and targets for industrial processes. These regulations often require regular energy audits and the implementation of energy management systems, pushing ethyl acetate producers to continuously improve their energy performance.
The growing emphasis on circular economy principles in environmental regulations has encouraged manufacturers to explore waste heat recovery systems and process integration techniques. These approaches not only reduce energy consumption but also minimize waste and improve overall resource efficiency in ethyl acetate production.
As environmental regulations continue to evolve, ethyl acetate producers are increasingly focusing on life cycle assessments to evaluate and reduce the overall environmental impact of their products. This holistic approach often leads to innovations in energy-efficient production methods and the exploration of renewable energy sources to power manufacturing processes.
Life Cycle Assessment of Ethyl Acetate Production Processes
Life Cycle Assessment (LCA) is a crucial tool for evaluating the environmental impacts of ethyl acetate production processes. This comprehensive approach considers all stages of the product's life cycle, from raw material extraction to disposal, providing valuable insights into energy consumption and potential areas for improvement.
The LCA of ethyl acetate production typically begins with the sourcing of raw materials, primarily ethanol and acetic acid. The production of these precursors involves significant energy inputs, particularly in the case of ethanol derived from agricultural sources. The cultivation, harvesting, and processing of crops for ethanol production contribute substantially to the overall energy footprint.
The manufacturing process itself is energy-intensive, with the most common method being the esterification of ethanol and acetic acid. This reaction requires elevated temperatures and the presence of a catalyst, typically sulfuric acid. The energy demands of maintaining reaction conditions and subsequent purification steps, such as distillation, account for a significant portion of the total energy consumption.
Transportation and distribution of both raw materials and finished products also contribute to the energy profile. The distances involved and the modes of transport used can significantly impact the overall energy efficiency of the production chain.
End-of-life considerations, including recycling or disposal of ethyl acetate and its containers, further add to the energy tally. While ethyl acetate can be recovered and reused in some applications, the energy required for these processes must be factored into the overall assessment.
By conducting a thorough LCA, manufacturers can identify hotspots of energy consumption throughout the production chain. This analysis often reveals unexpected areas of high energy use, such as in auxiliary processes or supply chain logistics, which might otherwise be overlooked in more focused assessments.
The results of an LCA can guide strategic decisions aimed at reducing energy consumption. These may include optimizing reaction conditions, improving heat recovery systems, or exploring alternative raw material sources. Additionally, the assessment can highlight opportunities for process integration, where waste heat or byproducts from one stage can be utilized in another, thereby improving overall energy efficiency.
Furthermore, LCA results can inform the development of more sustainable production methods, such as the use of renewable energy sources or the implementation of bio-based feedstocks. These alternatives can potentially reduce the reliance on fossil fuels and decrease the overall environmental impact of ethyl acetate production.
The LCA of ethyl acetate production typically begins with the sourcing of raw materials, primarily ethanol and acetic acid. The production of these precursors involves significant energy inputs, particularly in the case of ethanol derived from agricultural sources. The cultivation, harvesting, and processing of crops for ethanol production contribute substantially to the overall energy footprint.
The manufacturing process itself is energy-intensive, with the most common method being the esterification of ethanol and acetic acid. This reaction requires elevated temperatures and the presence of a catalyst, typically sulfuric acid. The energy demands of maintaining reaction conditions and subsequent purification steps, such as distillation, account for a significant portion of the total energy consumption.
Transportation and distribution of both raw materials and finished products also contribute to the energy profile. The distances involved and the modes of transport used can significantly impact the overall energy efficiency of the production chain.
End-of-life considerations, including recycling or disposal of ethyl acetate and its containers, further add to the energy tally. While ethyl acetate can be recovered and reused in some applications, the energy required for these processes must be factored into the overall assessment.
By conducting a thorough LCA, manufacturers can identify hotspots of energy consumption throughout the production chain. This analysis often reveals unexpected areas of high energy use, such as in auxiliary processes or supply chain logistics, which might otherwise be overlooked in more focused assessments.
The results of an LCA can guide strategic decisions aimed at reducing energy consumption. These may include optimizing reaction conditions, improving heat recovery systems, or exploring alternative raw material sources. Additionally, the assessment can highlight opportunities for process integration, where waste heat or byproducts from one stage can be utilized in another, thereby improving overall energy efficiency.
Furthermore, LCA results can inform the development of more sustainable production methods, such as the use of renewable energy sources or the implementation of bio-based feedstocks. These alternatives can potentially reduce the reliance on fossil fuels and decrease the overall environmental impact of ethyl acetate production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!