How to Select the Right HPLC Column—Compatibility Check
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Column Selection Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical research sectors. The development trajectory of HPLC column technology has progressed from conventional silica-based packings to advanced materials with enhanced selectivity, efficiency, and stability characteristics. This evolution reflects the growing demand for more precise separation capabilities across diverse analytical challenges.
The current landscape of HPLC column technology encompasses a wide spectrum of stationary phases, including reversed-phase, normal-phase, ion-exchange, size-exclusion, and mixed-mode variants. Each category serves specific separation mechanisms, addressing particular analytical requirements. The proliferation of column options has created both opportunities and complexities for analysts seeking optimal separation solutions.
Column compatibility represents a critical consideration in HPLC method development, directly impacting separation efficiency, resolution, and analytical reliability. The compatibility assessment encompasses multiple dimensions: chemical compatibility with mobile phases, physical compatibility with operating conditions, and functional compatibility with target analytes. Failure to properly evaluate these compatibility factors can result in column degradation, reduced performance, and compromised analytical outcomes.
Recent technological advancements have introduced novel stationary phase chemistries, including hybrid organic-inorganic materials, superficially porous particles, and monolithic structures. These innovations have expanded the application scope of HPLC while simultaneously increasing the complexity of column selection decisions. The emergence of ultra-high-performance liquid chromatography (UHPLC) has further accelerated this trend, introducing additional considerations related to pressure tolerance and particle size optimization.
The primary objective of this technical research report is to establish a systematic framework for HPLC column selection with particular emphasis on compatibility assessment. This framework aims to guide analysts through the multifaceted decision-making process, considering factors such as stationary phase chemistry, particle characteristics, column dimensions, and operational parameters. By developing a structured approach to column selection, we seek to enhance analytical efficiency, reduce method development time, and improve separation outcomes.
Additionally, this report intends to identify emerging trends in column technology that may influence future compatibility considerations. By anticipating technological developments and their implications for column selection strategies, we aim to provide forward-looking insights that support long-term analytical planning and investment decisions.
The current landscape of HPLC column technology encompasses a wide spectrum of stationary phases, including reversed-phase, normal-phase, ion-exchange, size-exclusion, and mixed-mode variants. Each category serves specific separation mechanisms, addressing particular analytical requirements. The proliferation of column options has created both opportunities and complexities for analysts seeking optimal separation solutions.
Column compatibility represents a critical consideration in HPLC method development, directly impacting separation efficiency, resolution, and analytical reliability. The compatibility assessment encompasses multiple dimensions: chemical compatibility with mobile phases, physical compatibility with operating conditions, and functional compatibility with target analytes. Failure to properly evaluate these compatibility factors can result in column degradation, reduced performance, and compromised analytical outcomes.
Recent technological advancements have introduced novel stationary phase chemistries, including hybrid organic-inorganic materials, superficially porous particles, and monolithic structures. These innovations have expanded the application scope of HPLC while simultaneously increasing the complexity of column selection decisions. The emergence of ultra-high-performance liquid chromatography (UHPLC) has further accelerated this trend, introducing additional considerations related to pressure tolerance and particle size optimization.
The primary objective of this technical research report is to establish a systematic framework for HPLC column selection with particular emphasis on compatibility assessment. This framework aims to guide analysts through the multifaceted decision-making process, considering factors such as stationary phase chemistry, particle characteristics, column dimensions, and operational parameters. By developing a structured approach to column selection, we seek to enhance analytical efficiency, reduce method development time, and improve separation outcomes.
Additionally, this report intends to identify emerging trends in column technology that may influence future compatibility considerations. By anticipating technological developments and their implications for column selection strategies, we aim to provide forward-looking insights that support long-term analytical planning and investment decisions.
Market Analysis of HPLC Column Technologies
The global HPLC column market continues to demonstrate robust growth, valued at approximately $2.5 billion in 2022 and projected to reach $3.8 billion by 2028, representing a compound annual growth rate (CAGR) of 7.2%. This growth is primarily driven by increasing applications in pharmaceutical research, biotechnology, food safety testing, and environmental monitoring sectors.
The pharmaceutical and biopharmaceutical industries remain the largest consumers of HPLC columns, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where precise analytical methods are essential. The biotechnology sector follows closely, with a growing demand for specialized columns capable of handling complex biomolecules and protein analysis.
Regional analysis reveals North America as the leading market for HPLC columns, holding approximately 38% of the global market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 9.5% annually, primarily due to expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing investments in research infrastructure.
The market is characterized by significant technological segmentation. Reversed-phase columns continue to dominate with approximately 60% market share due to their versatility and broad application range. However, specialty columns such as HILIC (Hydrophilic Interaction Liquid Chromatography), chiral columns, and bio-compatible columns are witnessing accelerated growth rates as analytical requirements become more sophisticated.
Key market trends include the rising demand for ultra-high-performance liquid chromatography (UHPLC) columns, which offer enhanced resolution and faster analysis times. These columns now represent approximately 35% of new column purchases, reflecting the industry's shift toward more efficient analytical processes. Additionally, columns with improved pH stability, temperature resistance, and compatibility with diverse mobile phases are gaining traction as laboratories seek more versatile and durable solutions.
The competitive landscape features both established players and innovative newcomers. Major manufacturers like Waters Corporation, Agilent Technologies, Thermo Fisher Scientific, and Shimadzu Corporation collectively hold approximately 65% of the market share. These companies maintain their positions through continuous innovation and comprehensive product portfolios. Meanwhile, specialized manufacturers focusing on niche applications and novel stationary phases are capturing growing market segments through technological differentiation.
The pharmaceutical and biopharmaceutical industries remain the largest consumers of HPLC columns, accounting for nearly 45% of the total market share. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where precise analytical methods are essential. The biotechnology sector follows closely, with a growing demand for specialized columns capable of handling complex biomolecules and protein analysis.
Regional analysis reveals North America as the leading market for HPLC columns, holding approximately 38% of the global market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 9.5% annually, primarily due to expanding pharmaceutical manufacturing capabilities in China and India, coupled with increasing investments in research infrastructure.
The market is characterized by significant technological segmentation. Reversed-phase columns continue to dominate with approximately 60% market share due to their versatility and broad application range. However, specialty columns such as HILIC (Hydrophilic Interaction Liquid Chromatography), chiral columns, and bio-compatible columns are witnessing accelerated growth rates as analytical requirements become more sophisticated.
Key market trends include the rising demand for ultra-high-performance liquid chromatography (UHPLC) columns, which offer enhanced resolution and faster analysis times. These columns now represent approximately 35% of new column purchases, reflecting the industry's shift toward more efficient analytical processes. Additionally, columns with improved pH stability, temperature resistance, and compatibility with diverse mobile phases are gaining traction as laboratories seek more versatile and durable solutions.
The competitive landscape features both established players and innovative newcomers. Major manufacturers like Waters Corporation, Agilent Technologies, Thermo Fisher Scientific, and Shimadzu Corporation collectively hold approximately 65% of the market share. These companies maintain their positions through continuous innovation and comprehensive product portfolios. Meanwhile, specialized manufacturers focusing on niche applications and novel stationary phases are capturing growing market segments through technological differentiation.
Current Challenges in HPLC Column Compatibility
Despite significant advancements in HPLC technology, column compatibility remains a persistent challenge for analytical chemists and researchers. The primary issue stems from the complex interplay between mobile phases, analytes, and stationary phases. Many laboratories report inconsistent results when transferring methods between different column types or even between columns from different manufacturers that are nominally the same. This variability undermines reproducibility, a cornerstone of scientific methodology.
Column degradation presents another significant challenge. Even with careful handling, HPLC columns gradually lose efficiency over time due to chemical interactions with sample components and mobile phases. This degradation is often unpredictable and can vary significantly between batches of nominally identical columns, making long-term method stability difficult to maintain.
pH compatibility continues to be problematic, particularly with silica-based columns that typically operate optimally between pH 2-8. Outside this range, silica dissolution occurs, leading to column deterioration. While polymer-based and hybrid columns offer extended pH ranges, they often present different selectivity profiles, complicating method transfer.
Temperature effects on column compatibility are frequently underestimated. Column selectivity can change dramatically with temperature variations, and different column chemistries respond differently to temperature changes. This becomes particularly challenging when developing methods intended for transfer between laboratories with different ambient conditions.
The increasing complexity of sample matrices in pharmaceutical, environmental, and biological applications exacerbates compatibility issues. Modern samples often contain components that can irreversibly bind to stationary phases or cause unexpected secondary interactions, leading to column fouling and altered selectivity.
Emerging challenges include compatibility with new mobile phase additives and modifiers used in specialized applications like chiral separations or biomolecule analysis. These additives can interact unpredictably with different column chemistries, creating method development hurdles.
Cross-platform compatibility presents additional difficulties as laboratories increasingly employ multiple HPLC and UHPLC systems. Differences in system volumes, pressure capabilities, and detector configurations can significantly impact separation performance even when using identical columns.
The lack of standardized testing protocols for column compatibility assessment further complicates the situation. Without universally accepted benchmarks, laboratories must develop their own compatibility testing regimes, leading to inconsistent approaches across the industry and hindering knowledge sharing.
Column degradation presents another significant challenge. Even with careful handling, HPLC columns gradually lose efficiency over time due to chemical interactions with sample components and mobile phases. This degradation is often unpredictable and can vary significantly between batches of nominally identical columns, making long-term method stability difficult to maintain.
pH compatibility continues to be problematic, particularly with silica-based columns that typically operate optimally between pH 2-8. Outside this range, silica dissolution occurs, leading to column deterioration. While polymer-based and hybrid columns offer extended pH ranges, they often present different selectivity profiles, complicating method transfer.
Temperature effects on column compatibility are frequently underestimated. Column selectivity can change dramatically with temperature variations, and different column chemistries respond differently to temperature changes. This becomes particularly challenging when developing methods intended for transfer between laboratories with different ambient conditions.
The increasing complexity of sample matrices in pharmaceutical, environmental, and biological applications exacerbates compatibility issues. Modern samples often contain components that can irreversibly bind to stationary phases or cause unexpected secondary interactions, leading to column fouling and altered selectivity.
Emerging challenges include compatibility with new mobile phase additives and modifiers used in specialized applications like chiral separations or biomolecule analysis. These additives can interact unpredictably with different column chemistries, creating method development hurdles.
Cross-platform compatibility presents additional difficulties as laboratories increasingly employ multiple HPLC and UHPLC systems. Differences in system volumes, pressure capabilities, and detector configurations can significantly impact separation performance even when using identical columns.
The lack of standardized testing protocols for column compatibility assessment further complicates the situation. Without universally accepted benchmarks, laboratories must develop their own compatibility testing regimes, leading to inconsistent approaches across the industry and hindering knowledge sharing.
Contemporary HPLC Column Selection Methodologies
01 Column material compatibility for HPLC applications
Different stationary phase materials can be used in HPLC columns to ensure compatibility with various analytes and mobile phases. These materials include silica-based, polymer-based, and hybrid materials that offer different selectivity and stability characteristics. The choice of column material affects separation efficiency, peak resolution, and column lifetime, particularly when working with challenging samples or extreme pH conditions.- Column material compatibility for HPLC applications: Different column materials offer varying compatibility with mobile phases and analytes in HPLC. Materials such as silica, polymeric resins, and hybrid materials each have specific chemical resistance properties that determine their suitability for particular applications. The selection of appropriate column material ensures optimal separation performance and column longevity when working with different pH ranges, organic solvents, and sample types.
- Mobile phase compatibility considerations: The compatibility between HPLC columns and mobile phases is critical for successful chromatographic separations. Factors such as pH range tolerance, organic solvent compatibility, and buffer salt concentration limits must be considered when selecting column-mobile phase combinations. Improper mobile phase selection can lead to column degradation, reduced efficiency, and shortened column lifetime. Understanding these compatibility factors helps optimize separation conditions while preserving column integrity.
- Connection and hardware compatibility systems: HPLC column compatibility extends to physical connections and hardware interfaces. Standardized fittings, connectors, and adapters enable columns from different manufacturers to be used interchangeably within HPLC systems. These components must maintain proper sealing under high pressure while minimizing dead volume to prevent peak broadening. Advanced connection systems may feature tool-free operation, adjustable depth settings, and materials resistant to chemical degradation.
- Temperature and pressure compatibility ranges: HPLC columns have specific temperature and pressure operating ranges that affect their compatibility with various analytical methods. High-temperature compatibility allows for faster analysis and improved peak shapes for certain compounds, while pressure tolerance determines suitability for ultra-high-performance applications. Column manufacturers specify these parameters to guide proper usage and prevent damage from exceeding operational limits. Understanding these specifications is essential when developing methods across different instrument platforms.
- Cross-platform column transferability: The ability to transfer methods between different HPLC systems while maintaining separation performance is an important aspect of column compatibility. Factors affecting transferability include column dimensions, particle technology, and stationary phase chemistry. Geometric scaling calculations and adjustment of operational parameters may be necessary when transferring methods between conventional HPLC and UHPLC systems. Specialized column designs may offer enhanced cross-platform compatibility to facilitate method transfer across different laboratory environments.
02 Mobile phase compatibility considerations
The compatibility between HPLC columns and mobile phases is critical for successful chromatographic separations. Factors such as pH range, buffer composition, organic solvent content, and ionic strength must be considered when selecting column-mobile phase combinations. Improper combinations can lead to column degradation, reduced efficiency, or complete separation failure. Optimizing these parameters ensures column longevity and reproducible analytical results.Expand Specific Solutions03 Column connection systems and hardware compatibility
HPLC column connection systems must be compatible with the instrument hardware to prevent leaks, dead volumes, and system failures. This includes considerations for fitting types, tubing dimensions, pressure ratings, and connection materials. Standardized connection systems allow for interchangeability between different manufacturers' columns and instruments, while specialized connections may offer advantages for high-pressure or micro-flow applications.Expand Specific Solutions04 Temperature and pressure compatibility
HPLC columns must be compatible with the operating temperature and pressure conditions of the analysis. Temperature affects selectivity, efficiency, and column lifetime, while pressure limitations are determined by the column hardware and packing material. Modern columns are designed to withstand specific ranges of temperatures and pressures, with some specialized columns capable of handling extreme conditions for advanced applications like ultra-high-pressure liquid chromatography (UHPLC).Expand Specific Solutions05 Sample matrix compatibility and column protection
The compatibility between HPLC columns and sample matrices is essential for maintaining column performance and extending column life. Complex samples may contain components that irreversibly bind to or damage the column. Strategies to ensure compatibility include sample preparation techniques, the use of guard columns, in-line filters, and selecting appropriate column chemistries that resist fouling or degradation from the sample components.Expand Specific Solutions
Major Manufacturers and Competitive Landscape
The HPLC column selection market is in a mature growth phase, characterized by established technologies and steady innovation. The global analytical chromatography market exceeds $10 billion, with HPLC systems representing a significant segment. Technologically, column compatibility assessment has reached high maturity, with leading players like Agilent Technologies, Waters Technology, and Thermo Fisher Scientific (through Dionex Softron) offering comprehensive solutions. These companies have developed sophisticated column selection tools integrating chemical compatibility databases, mobile phase considerations, and separation requirements. Pharmaceutical companies such as Janssen Pharmaceutica and research institutions like Shanghai Institute of Pharmaceutical Industry are driving demand through increasingly complex analytical requirements, while instrument manufacturers continue to enhance column selection technologies through software integration and automated compatibility checking systems.
IDEX Health & Science LLC
Technical Solution: IDEX Health & Science approaches HPLC column selection through their comprehensive compatibility assessment framework that evaluates multiple factors affecting column performance. Their methodology focuses on systematic evaluation of chemical compatibility between column materials and mobile phase components, including detailed solvent miscibility charts to prevent phase separation issues. IDEX has developed specialized column hardware designed to withstand high pressures and aggressive mobile phases, with particular attention to the compatibility of frits, end-fittings, and tubing materials with various solvents. Their column selection process emphasizes the importance of material compatibility throughout the entire flow path, not just the column itself, to prevent system contamination or component degradation. IDEX provides detailed guidelines for column storage and regeneration procedures based on specific stationary phase chemistries, helping to maintain column performance and extend column lifetime across different application environments. Their approach includes evaluation of potential leachables from column materials that might interfere with sensitive detection methods, particularly for mass spectrometry applications.
Strengths: Holistic system compatibility approach that considers the entire flow path; specialized hardware solutions for challenging mobile phases; extensive technical support for method troubleshooting. Weaknesses: More focused on hardware compatibility than stationary phase chemistry development; relies more on partnerships with column manufacturers rather than proprietary column technologies.
Agilent Technologies, Inc.
Technical Solution: Agilent's approach to HPLC column selection involves a comprehensive compatibility assessment framework that considers multiple factors. Their methodology includes evaluating chemical compatibility between mobile phases and stationary phases, pH range tolerance, temperature stability, and pressure limitations. Agilent has developed specialized software tools that help analysts predict column performance based on analyte properties and separation goals. Their InfinityLab Poroshell 120 columns feature superficially porous particles that offer enhanced efficiency while maintaining lower backpressure compared to fully porous sub-2μm particles, allowing compatibility with both UHPLC and conventional HPLC systems. Agilent's column selection guides incorporate detailed selectivity maps that visualize how different columns perform across various compound classes, enabling scientists to make data-driven decisions about column compatibility for specific applications.
Strengths: Comprehensive selection tools that incorporate multiple compatibility parameters; extensive column characterization data; columns designed for backward compatibility with conventional HPLC systems. Weaknesses: Premium pricing structure; some proprietary column technologies may limit transferability to other manufacturers' instruments.
Key Technical Parameters for Column Compatibility
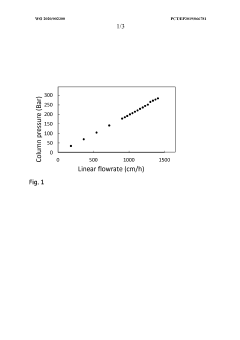
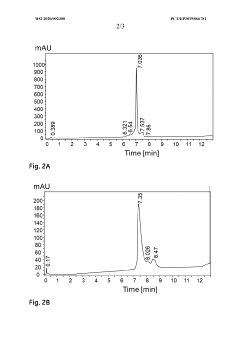
Chromatography beads, production and use thereof
PatentWO2020002300A1
Innovation
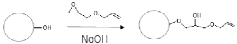
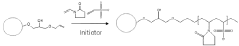
- Development of small, rigid, and non-permeable agarose beads with diameters of 1-25 μm, produced through a method involving emulsification, cross-linking, and surface grafting to create polymer tentacles for functionalization, which can withstand high pressures and exclude compounds as small as 100 g/mol, enabling efficient mass transfer and sharper peaks.
HPLC column holder apparatus
PatentInactiveUS20060008390A1
Innovation
- A column holding apparatus, referred to as a clip, with a clip-device interface and a clip-column interface, allowing for secure attachment of HPLC columns to various securing devices such as bars, wires, or chromatography hardware, using configurations like square orifices, protruding leg elements, and suction-cup devices for versatile mounting options.
Regulatory Considerations for HPLC Method Development
Regulatory considerations play a crucial role in HPLC method development, particularly when selecting appropriate columns for pharmaceutical analysis. The FDA, EMA, ICH, and USP have established specific guidelines that must be adhered to during method development and validation processes. These regulatory bodies require comprehensive documentation of column selection rationale, which must demonstrate scientific soundness and reproducibility.
Column compatibility verification is a mandatory requirement under GMP regulations, ensuring that analytical methods remain robust across different manufacturing and testing environments. Regulatory agencies expect thorough evaluation of column chemistry interactions with mobile phases, sample matrices, and buffer systems to prevent unexpected chromatographic behavior that could compromise data integrity.
The ICH Q2(R1) guideline specifically addresses validation parameters that are directly impacted by column selection, including specificity, accuracy, precision, and robustness. When selecting HPLC columns, analysts must consider how the column characteristics will affect these validation parameters and document their assessment accordingly.
Method transfer considerations are particularly important from a regulatory perspective. The selected column must perform consistently across different laboratories and geographic locations, especially for global pharmaceutical companies operating under multiple regulatory jurisdictions. This requires detailed specifications of column parameters and acceptable alternatives.
Regulatory bodies increasingly emphasize lifecycle management of analytical methods, including column selection strategies. The FDA's process validation guidance recommends ongoing monitoring of critical method parameters, including column performance over time. This necessitates establishing column equivalency protocols and defining acceptance criteria for column replacement.
For regulated industries, column selection must also address data integrity concerns. The column must enable sufficient resolution of critical pairs and impurities at levels required by pharmacopeial standards. Documentation should include verification that the selected column can reliably detect and quantify impurities at the required reporting thresholds.
Pharmacopeial methods often specify column parameters, but when developing new methods, justification for column selection becomes part of the regulatory submission. This includes demonstrating that the chosen column provides adequate separation of the active pharmaceutical ingredient from related substances, degradation products, and excipients under various stress conditions as required by stability-indicating method requirements.
Column compatibility verification is a mandatory requirement under GMP regulations, ensuring that analytical methods remain robust across different manufacturing and testing environments. Regulatory agencies expect thorough evaluation of column chemistry interactions with mobile phases, sample matrices, and buffer systems to prevent unexpected chromatographic behavior that could compromise data integrity.
The ICH Q2(R1) guideline specifically addresses validation parameters that are directly impacted by column selection, including specificity, accuracy, precision, and robustness. When selecting HPLC columns, analysts must consider how the column characteristics will affect these validation parameters and document their assessment accordingly.
Method transfer considerations are particularly important from a regulatory perspective. The selected column must perform consistently across different laboratories and geographic locations, especially for global pharmaceutical companies operating under multiple regulatory jurisdictions. This requires detailed specifications of column parameters and acceptable alternatives.
Regulatory bodies increasingly emphasize lifecycle management of analytical methods, including column selection strategies. The FDA's process validation guidance recommends ongoing monitoring of critical method parameters, including column performance over time. This necessitates establishing column equivalency protocols and defining acceptance criteria for column replacement.
For regulated industries, column selection must also address data integrity concerns. The column must enable sufficient resolution of critical pairs and impurities at levels required by pharmacopeial standards. Documentation should include verification that the selected column can reliably detect and quantify impurities at the required reporting thresholds.
Pharmacopeial methods often specify column parameters, but when developing new methods, justification for column selection becomes part of the regulatory submission. This includes demonstrating that the chosen column provides adequate separation of the active pharmaceutical ingredient from related substances, degradation products, and excipients under various stress conditions as required by stability-indicating method requirements.
Cost-Benefit Analysis of Column Selection Strategies
The selection of HPLC columns represents a significant investment decision for analytical laboratories that directly impacts both operational efficiency and financial outcomes. When evaluating column selection strategies from a cost-benefit perspective, laboratories must consider both immediate acquisition costs and long-term operational implications.
Initial column purchase prices vary considerably, ranging from $300 for basic silica-based columns to over $1,500 for specialized chiral or UHPLC columns. However, focusing solely on purchase price often leads to suboptimal decisions. A comprehensive cost analysis must include solvent consumption, which can exceed $10,000 annually per instrument depending on analysis frequency and mobile phase composition.
Column lifetime represents another critical economic factor. While less expensive columns may require replacement every 500-1000 injections, premium columns with advanced particle technology and end-capping can withstand 2000-3000 injections, significantly reducing the annualized replacement cost. The economic impact becomes particularly evident in high-throughput environments where frequent column replacements disrupt workflow and decrease productivity.
Method development time constitutes a substantial hidden cost often overlooked in column selection calculations. Laboratories employing systematic column screening approaches with compatibility databases can reduce method development time by 40-60% compared to trial-and-error approaches. This efficiency translates to faster time-to-result and better utilization of highly skilled analytical personnel.
Regulatory considerations further complicate the cost-benefit equation. Columns that provide more robust separations may command premium prices but reduce the risk of method failures during regulatory inspections or audits. The cost of a single failed batch or regulatory submission delay can dwarf the incremental investment in superior column technology.
A strategic approach to column selection involves establishing a laboratory-wide column inventory system that balances specialized needs with standardization benefits. Standardizing on compatible column chemistries across multiple applications can yield volume discounts from suppliers (typically 15-25%) while simplifying training requirements and reducing method transfer complications between instruments.
Return on investment calculations should incorporate all these factors, with the optimal strategy typically involving tiered column selection: premium columns for critical, frequently run methods; mid-range columns for routine applications; and economy columns for exploratory work or infrequently performed analyses. This balanced approach optimizes the laboratory's analytical capabilities while maintaining fiscal responsibility.
Initial column purchase prices vary considerably, ranging from $300 for basic silica-based columns to over $1,500 for specialized chiral or UHPLC columns. However, focusing solely on purchase price often leads to suboptimal decisions. A comprehensive cost analysis must include solvent consumption, which can exceed $10,000 annually per instrument depending on analysis frequency and mobile phase composition.
Column lifetime represents another critical economic factor. While less expensive columns may require replacement every 500-1000 injections, premium columns with advanced particle technology and end-capping can withstand 2000-3000 injections, significantly reducing the annualized replacement cost. The economic impact becomes particularly evident in high-throughput environments where frequent column replacements disrupt workflow and decrease productivity.
Method development time constitutes a substantial hidden cost often overlooked in column selection calculations. Laboratories employing systematic column screening approaches with compatibility databases can reduce method development time by 40-60% compared to trial-and-error approaches. This efficiency translates to faster time-to-result and better utilization of highly skilled analytical personnel.
Regulatory considerations further complicate the cost-benefit equation. Columns that provide more robust separations may command premium prices but reduce the risk of method failures during regulatory inspections or audits. The cost of a single failed batch or regulatory submission delay can dwarf the incremental investment in superior column technology.
A strategic approach to column selection involves establishing a laboratory-wide column inventory system that balances specialized needs with standardization benefits. Standardizing on compatible column chemistries across multiple applications can yield volume discounts from suppliers (typically 15-25%) while simplifying training requirements and reducing method transfer complications between instruments.
Return on investment calculations should incorporate all these factors, with the optimal strategy typically involving tiered column selection: premium columns for critical, frequently run methods; mid-range columns for routine applications; and economy columns for exploratory work or infrequently performed analyses. This balanced approach optimizes the laboratory's analytical capabilities while maintaining fiscal responsibility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!