Hydrochloric Acid: Catalyzing Green Chemistry Advancements
JUL 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HCl in Green Chemistry
Hydrochloric acid (HCl) has emerged as a pivotal catalyst in advancing green chemistry principles and practices. Its role in promoting sustainable chemical processes has gained significant attention in recent years, as industries and researchers seek to minimize environmental impact while maintaining or improving efficiency.
HCl's importance in green chemistry stems from its versatility and effectiveness as a catalyst in various reactions. It facilitates numerous organic transformations, including esterification, hydrolysis, and dehydration reactions, which are fundamental in the production of pharmaceuticals, polymers, and fine chemicals. The acid's ability to catalyze these reactions under milder conditions often results in reduced energy consumption and improved atom economy, two key tenets of green chemistry.
One of the most notable applications of HCl in green chemistry is its use in biomass conversion. As the world shifts towards renewable resources, HCl plays a crucial role in the hydrolysis of cellulose and hemicellulose, converting plant-based materials into valuable platform chemicals and biofuels. This process not only utilizes sustainable feedstocks but also reduces reliance on fossil fuel-derived chemicals.
In the realm of waste valorization, HCl catalyzes the conversion of industrial by-products and waste streams into useful chemicals. For instance, it aids in the transformation of glycerol, a biodiesel production by-product, into value-added compounds like epichlorohydrin. This approach aligns with the circular economy concept, turning waste into resources and minimizing overall environmental impact.
HCl's role extends to the development of greener synthetic routes for pharmaceuticals. It catalyzes key steps in the synthesis of active pharmaceutical ingredients (APIs), often enabling more efficient and environmentally benign processes. This is particularly important in the context of continuous flow chemistry, where HCl can facilitate rapid and selective transformations with minimal waste generation.
Furthermore, HCl contributes to the advancement of green solvents. It is instrumental in the production of ionic liquids, which are increasingly recognized as environmentally friendly alternatives to traditional organic solvents. These ionic liquids offer unique properties such as low volatility and high recyclability, aligning well with green chemistry principles.
Despite its corrosive nature, HCl's use in green chemistry is often justified by its efficiency and the ability to recover and recycle it in many processes. Innovations in reactor design and materials have also mitigated some of the handling challenges associated with HCl, further enhancing its green credentials.
As research in green chemistry progresses, the role of HCl continues to evolve. Scientists are exploring novel applications, such as its use in CO2 capture and conversion technologies, which could have significant implications for climate change mitigation strategies. The ongoing development of HCl-based catalytic systems promises to unlock new pathways for sustainable chemical manufacturing and environmental remediation.
HCl's importance in green chemistry stems from its versatility and effectiveness as a catalyst in various reactions. It facilitates numerous organic transformations, including esterification, hydrolysis, and dehydration reactions, which are fundamental in the production of pharmaceuticals, polymers, and fine chemicals. The acid's ability to catalyze these reactions under milder conditions often results in reduced energy consumption and improved atom economy, two key tenets of green chemistry.
One of the most notable applications of HCl in green chemistry is its use in biomass conversion. As the world shifts towards renewable resources, HCl plays a crucial role in the hydrolysis of cellulose and hemicellulose, converting plant-based materials into valuable platform chemicals and biofuels. This process not only utilizes sustainable feedstocks but also reduces reliance on fossil fuel-derived chemicals.
In the realm of waste valorization, HCl catalyzes the conversion of industrial by-products and waste streams into useful chemicals. For instance, it aids in the transformation of glycerol, a biodiesel production by-product, into value-added compounds like epichlorohydrin. This approach aligns with the circular economy concept, turning waste into resources and minimizing overall environmental impact.
HCl's role extends to the development of greener synthetic routes for pharmaceuticals. It catalyzes key steps in the synthesis of active pharmaceutical ingredients (APIs), often enabling more efficient and environmentally benign processes. This is particularly important in the context of continuous flow chemistry, where HCl can facilitate rapid and selective transformations with minimal waste generation.
Furthermore, HCl contributes to the advancement of green solvents. It is instrumental in the production of ionic liquids, which are increasingly recognized as environmentally friendly alternatives to traditional organic solvents. These ionic liquids offer unique properties such as low volatility and high recyclability, aligning well with green chemistry principles.
Despite its corrosive nature, HCl's use in green chemistry is often justified by its efficiency and the ability to recover and recycle it in many processes. Innovations in reactor design and materials have also mitigated some of the handling challenges associated with HCl, further enhancing its green credentials.
As research in green chemistry progresses, the role of HCl continues to evolve. Scientists are exploring novel applications, such as its use in CO2 capture and conversion technologies, which could have significant implications for climate change mitigation strategies. The ongoing development of HCl-based catalytic systems promises to unlock new pathways for sustainable chemical manufacturing and environmental remediation.
Market Demand Analysis
The market demand for hydrochloric acid in green chemistry applications has been steadily increasing, driven by the growing emphasis on sustainable and environmentally friendly chemical processes. As industries worldwide shift towards more eco-conscious practices, the role of hydrochloric acid as a catalyst in various green chemistry advancements has become increasingly significant.
One of the primary drivers of market demand is the use of hydrochloric acid in the production of biodegradable plastics. With the global push to reduce plastic waste, manufacturers are turning to hydrochloric acid-catalyzed processes to create more sustainable packaging materials. This trend is particularly evident in the food and beverage industry, where consumer demand for eco-friendly packaging solutions has surged in recent years.
In the pharmaceutical sector, hydrochloric acid plays a crucial role in the synthesis of active pharmaceutical ingredients (APIs) through greener routes. The industry's focus on reducing environmental impact and improving process efficiency has led to increased adoption of hydrochloric acid-based catalytic systems. This shift not only addresses environmental concerns but also offers potential cost savings and improved product quality.
The renewable energy sector has also contributed to the rising demand for hydrochloric acid in green chemistry applications. In particular, the production of solar panels and wind turbine components often involves hydrochloric acid-catalyzed processes that aim to minimize waste and energy consumption. As governments worldwide invest in clean energy infrastructure, this demand is expected to grow further.
Agricultural industries have shown increasing interest in hydrochloric acid for its potential in developing more sustainable fertilizers and pesticides. Green chemistry approaches using hydrochloric acid as a catalyst have demonstrated promise in creating products with reduced environmental impact and improved efficacy. This trend aligns with the global push for more sustainable farming practices and food security.
The textile industry has also emerged as a significant market for hydrochloric acid in green chemistry applications. Innovative dyeing and finishing processes that utilize hydrochloric acid as a catalyst have shown potential in reducing water consumption and minimizing the release of harmful chemicals. As consumers become more conscious of the environmental impact of their clothing choices, demand for such eco-friendly textile production methods is likely to increase.
Overall, the market demand for hydrochloric acid in green chemistry advancements is expected to continue its upward trajectory. The chemical's versatility as a catalyst, combined with its potential to improve process efficiency and reduce environmental impact across various industries, positions it as a key player in the ongoing transition towards more sustainable chemical processes.
One of the primary drivers of market demand is the use of hydrochloric acid in the production of biodegradable plastics. With the global push to reduce plastic waste, manufacturers are turning to hydrochloric acid-catalyzed processes to create more sustainable packaging materials. This trend is particularly evident in the food and beverage industry, where consumer demand for eco-friendly packaging solutions has surged in recent years.
In the pharmaceutical sector, hydrochloric acid plays a crucial role in the synthesis of active pharmaceutical ingredients (APIs) through greener routes. The industry's focus on reducing environmental impact and improving process efficiency has led to increased adoption of hydrochloric acid-based catalytic systems. This shift not only addresses environmental concerns but also offers potential cost savings and improved product quality.
The renewable energy sector has also contributed to the rising demand for hydrochloric acid in green chemistry applications. In particular, the production of solar panels and wind turbine components often involves hydrochloric acid-catalyzed processes that aim to minimize waste and energy consumption. As governments worldwide invest in clean energy infrastructure, this demand is expected to grow further.
Agricultural industries have shown increasing interest in hydrochloric acid for its potential in developing more sustainable fertilizers and pesticides. Green chemistry approaches using hydrochloric acid as a catalyst have demonstrated promise in creating products with reduced environmental impact and improved efficacy. This trend aligns with the global push for more sustainable farming practices and food security.
The textile industry has also emerged as a significant market for hydrochloric acid in green chemistry applications. Innovative dyeing and finishing processes that utilize hydrochloric acid as a catalyst have shown potential in reducing water consumption and minimizing the release of harmful chemicals. As consumers become more conscious of the environmental impact of their clothing choices, demand for such eco-friendly textile production methods is likely to increase.
Overall, the market demand for hydrochloric acid in green chemistry advancements is expected to continue its upward trajectory. The chemical's versatility as a catalyst, combined with its potential to improve process efficiency and reduce environmental impact across various industries, positions it as a key player in the ongoing transition towards more sustainable chemical processes.
Current Challenges
Despite the significant advancements in green chemistry, the use of hydrochloric acid (HCl) in various industrial processes still presents several challenges. One of the primary concerns is the corrosive nature of HCl, which poses risks to both equipment and personnel safety. This necessitates the use of specialized materials and protective measures, increasing operational costs and complexity.
Environmental impact remains a critical issue, as the production and disposal of HCl can lead to air and water pollution if not properly managed. The chlorine content in HCl contributes to the formation of harmful byproducts, such as dioxins and furans, during certain industrial processes. This necessitates stringent emission control measures and waste treatment protocols.
The energy-intensive nature of HCl production is another significant challenge. Traditional methods of HCl synthesis, such as the chlor-alkali process, consume substantial amounts of electricity, contributing to the overall carbon footprint of industries relying on this acid. This contradicts the principles of green chemistry, which emphasize energy efficiency and reduced environmental impact.
Recycling and recovery of HCl from waste streams present technical difficulties. While some industries have implemented recovery systems, the process is often complex and energy-intensive. The presence of impurities in recovered HCl can limit its reusability in certain high-purity applications, necessitating further purification steps.
The transportation and storage of HCl also pose significant logistical and safety challenges. The acid's corrosive nature requires specialized containment and handling procedures, increasing the risk of accidents and environmental contamination during transit and storage. This aspect not only raises safety concerns but also adds to the overall cost of using HCl in industrial processes.
In the context of green chemistry, finding suitable alternatives to HCl that maintain process efficiency while reducing environmental impact is an ongoing challenge. Many potential substitutes lack the versatility and effectiveness of HCl across various applications, making complete replacement difficult in certain industries.
Regulatory compliance is becoming increasingly stringent, with environmental agencies worldwide imposing stricter limits on HCl emissions and waste disposal. This regulatory landscape necessitates continuous innovation in process design and pollution control technologies, adding to the complexity and cost of industrial operations involving HCl.
Addressing these challenges requires a multifaceted approach, combining technological innovation, process optimization, and policy measures. The development of more efficient catalysts, improved recycling technologies, and the exploration of bio-based alternatives are some of the avenues being pursued to mitigate the environmental impact of HCl use while maintaining its catalytic efficacy in green chemistry applications.
Environmental impact remains a critical issue, as the production and disposal of HCl can lead to air and water pollution if not properly managed. The chlorine content in HCl contributes to the formation of harmful byproducts, such as dioxins and furans, during certain industrial processes. This necessitates stringent emission control measures and waste treatment protocols.
The energy-intensive nature of HCl production is another significant challenge. Traditional methods of HCl synthesis, such as the chlor-alkali process, consume substantial amounts of electricity, contributing to the overall carbon footprint of industries relying on this acid. This contradicts the principles of green chemistry, which emphasize energy efficiency and reduced environmental impact.
Recycling and recovery of HCl from waste streams present technical difficulties. While some industries have implemented recovery systems, the process is often complex and energy-intensive. The presence of impurities in recovered HCl can limit its reusability in certain high-purity applications, necessitating further purification steps.
The transportation and storage of HCl also pose significant logistical and safety challenges. The acid's corrosive nature requires specialized containment and handling procedures, increasing the risk of accidents and environmental contamination during transit and storage. This aspect not only raises safety concerns but also adds to the overall cost of using HCl in industrial processes.
In the context of green chemistry, finding suitable alternatives to HCl that maintain process efficiency while reducing environmental impact is an ongoing challenge. Many potential substitutes lack the versatility and effectiveness of HCl across various applications, making complete replacement difficult in certain industries.
Regulatory compliance is becoming increasingly stringent, with environmental agencies worldwide imposing stricter limits on HCl emissions and waste disposal. This regulatory landscape necessitates continuous innovation in process design and pollution control technologies, adding to the complexity and cost of industrial operations involving HCl.
Addressing these challenges requires a multifaceted approach, combining technological innovation, process optimization, and policy measures. The development of more efficient catalysts, improved recycling technologies, and the exploration of bio-based alternatives are some of the avenues being pursued to mitigate the environmental impact of HCl use while maintaining its catalytic efficacy in green chemistry applications.
Existing Green HCl
01 Production methods of hydrochloric acid
Various methods are employed for the production of hydrochloric acid, including direct synthesis from hydrogen and chlorine, as a byproduct in chlorination processes, and through the reaction of sulfuric acid with sodium chloride. These methods are optimized for efficiency and purity in industrial settings.- Production and purification of hydrochloric acid: Various methods and processes for producing and purifying hydrochloric acid are described. These include techniques for improving the efficiency of production, reducing impurities, and optimizing the concentration of the acid. The processes may involve specialized equipment or chemical reactions to achieve high-quality hydrochloric acid for industrial or laboratory use.
- Applications of hydrochloric acid in chemical processes: Hydrochloric acid is widely used in various chemical processes and industrial applications. It serves as a key reagent in reactions, pH adjustment, metal treatment, and as a catalyst in organic synthesis. The acid's properties make it valuable in sectors such as petrochemicals, pharmaceuticals, and water treatment.
- Storage and handling systems for hydrochloric acid: Specialized equipment and systems are developed for the safe storage, handling, and transportation of hydrochloric acid. These include corrosion-resistant containers, safety valves, and monitoring systems to prevent leaks and ensure worker safety. The designs take into account the highly corrosive nature of the acid and environmental protection measures.
- Recovery and recycling of hydrochloric acid: Methods for recovering and recycling hydrochloric acid from industrial processes are developed to improve efficiency and reduce waste. These techniques may involve separation processes, chemical treatments, or membrane technologies to purify and concentrate the acid for reuse in various applications.
- Environmental and safety considerations in hydrochloric acid use: Innovations focus on minimizing the environmental impact and improving safety in the production, use, and disposal of hydrochloric acid. This includes developing cleaner production methods, implementing advanced emission control systems, and creating safer handling protocols to protect workers and the environment from the hazards associated with this strong acid.
02 Purification and concentration techniques
Techniques for purifying and concentrating hydrochloric acid involve distillation, membrane separation, and adsorption processes. These methods aim to remove impurities and achieve desired acid concentrations for specific industrial applications.Expand Specific Solutions03 Applications in chemical processing
Hydrochloric acid is widely used in various chemical processes, including metal treatment, pH regulation, and as a reagent in organic synthesis. Its versatility makes it a crucial component in industries such as petrochemicals, pharmaceuticals, and food processing.Expand Specific Solutions04 Safety and handling considerations
Proper safety measures and handling procedures are essential when working with hydrochloric acid due to its corrosive nature. This includes the use of specialized storage containers, protective equipment, and neutralization techniques for spills or disposal.Expand Specific Solutions05 Environmental impact and recycling
Efforts to mitigate the environmental impact of hydrochloric acid include developing recycling processes and finding alternative applications for waste acid. These approaches aim to reduce emissions and promote sustainable use in industrial settings.Expand Specific Solutions
Key Industry Players
The competitive landscape for hydrochloric acid in green chemistry advancements is characterized by a mature industry with established players and emerging opportunities. The market is experiencing steady growth due to increasing demand for sustainable chemical processes. Major companies like Mitsui Chemicals, China Petroleum & Chemical Corp., and Sumitomo Chemical are investing in research and development to improve hydrochloric acid production and utilization in green chemistry applications. The technology is relatively mature, but ongoing innovations focus on enhancing efficiency, reducing environmental impact, and exploring new catalytic applications. Smaller players and research institutions, such as Zhejiang University of Technology and Council of Scientific & Industrial Research, are contributing to technological advancements through collaborative research efforts.
Mitsui Chemicals, Inc.
Technical Solution: Mitsui Chemicals has developed a novel green chemistry approach utilizing hydrochloric acid as a catalyst in the production of bio-based materials. Their innovative process involves the acid-catalyzed conversion of plant-derived cellulose into high-value chemical intermediates[1]. The company has optimized the reaction conditions to achieve high yields and selectivity, while minimizing waste generation. Mitsui's technology employs a specially designed reactor system that allows for precise control of temperature and pressure, enabling efficient hydrochloric acid-catalyzed transformations[2]. Furthermore, they have implemented an advanced acid recovery and recycling system, significantly reducing the environmental impact of their processes[3]. This green chemistry approach has been successfully applied to the production of bio-based polyols and other sustainable chemical products.
Strengths: Efficient conversion of renewable resources, reduced reliance on fossil fuels, and improved sustainability. Weaknesses: Potential challenges in scaling up the process and managing the corrosive nature of hydrochloric acid.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed innovative green chemistry processes using hydrochloric acid as a catalyst. Their approach focuses on the production of biodegradable plastics from renewable resources. The company utilizes a hydrochloric acid-catalyzed depolymerization process to break down biomass into monomers, which are then used to synthesize eco-friendly polymers[1]. This method significantly reduces the carbon footprint of plastic production. Additionally, Sinopec has implemented a closed-loop hydrochloric acid recovery system in their chemical plants, minimizing waste and improving resource efficiency[2]. The recovered acid is purified and reused in various catalytic processes, including the production of high-value chemicals from petrochemical by-products[3].
Strengths: Efficient use of renewable resources, reduced environmental impact, and improved resource utilization. Weaknesses: Potential corrosion issues with hydrochloric acid, and the need for specialized equipment and safety measures.
Innovative HCl Tech
A novel green acid catalyst manufactured from waste of organic chemical industries
PatentWO2016193993A1
Innovation
- A novel green acid catalyst is produced from carbonaceous powder, a hazardous waste, through carbonization and water drowning, eliminating the need for purification and serving as a multifunctional alternative to traditional activated carbon.
Hydrochloric acid oxidation catalyst and method for producing chlorine
PatentPendingUS20240359165A1
Innovation
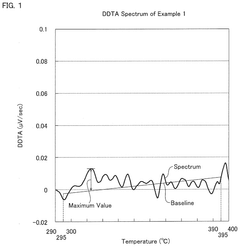
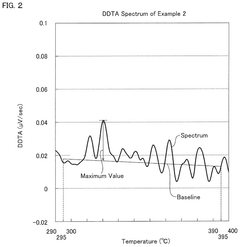
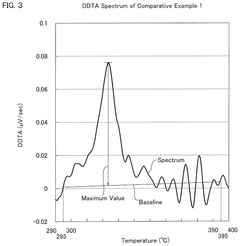
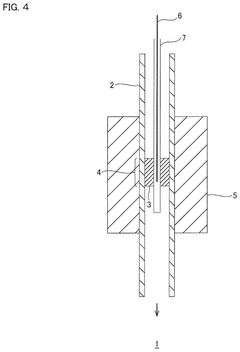
- A hydrochloric acid oxidation catalyst comprising a carrier, copper, an alkali metal, and a rare earth element, with specific properties such as an average pore diameter of 5 nm to 30 nm and a differential thermal analysis spectrum differential value of 0.035 μV/sec or less, which suppresses the dissipation of active components and maintains catalytic activity.
Environmental Impact
Hydrochloric acid (HCl) plays a crucial role in various industrial processes, but its production and use have significant environmental implications. The environmental impact of HCl encompasses both positive and negative aspects, necessitating a comprehensive evaluation of its role in green chemistry advancements.
One of the primary environmental concerns associated with HCl is its potential for air pollution. When released into the atmosphere, HCl can contribute to acid rain formation, which has detrimental effects on ecosystems, water bodies, and infrastructure. However, advancements in green chemistry have led to improved production methods and containment strategies, significantly reducing atmospheric emissions.
Water pollution is another critical environmental issue related to HCl. Improper disposal or accidental spills can lead to the acidification of water bodies, harming aquatic life and disrupting ecosystems. Green chemistry initiatives have focused on developing more efficient recycling and neutralization techniques, minimizing the risk of water contamination.
On the positive side, HCl's role in catalyzing green chemistry advancements has led to more environmentally friendly industrial processes. Its use as a catalyst in various chemical reactions has enabled the development of cleaner production methods, reducing the overall environmental footprint of many industries. For instance, HCl catalysts have been instrumental in improving the efficiency of biodiesel production, contributing to the growth of renewable energy sources.
The lifecycle assessment of HCl production has been a key focus area for environmental scientists. Green chemistry approaches have led to the development of more sustainable production methods, such as the recovery of HCl from waste streams and the use of renewable feedstocks. These innovations have significantly reduced the carbon footprint associated with HCl manufacturing.
In the context of waste management, HCl plays a dual role. While its improper disposal can pose environmental risks, it is also utilized in waste treatment processes. HCl is employed in the neutralization of alkaline waste and in the treatment of industrial effluents, contributing to overall waste reduction and environmental protection efforts.
The impact of HCl on soil quality is another important consideration. Accidental spills or long-term exposure can lead to soil acidification, affecting plant growth and soil microbial communities. However, green chemistry advancements have led to the development of more effective containment and remediation techniques, mitigating these risks.
In conclusion, while HCl poses certain environmental challenges, its role in catalyzing green chemistry advancements has led to significant improvements in industrial processes and environmental protection strategies. The ongoing research and development in this field continue to enhance the environmental profile of HCl, balancing its industrial utility with ecological sustainability.
One of the primary environmental concerns associated with HCl is its potential for air pollution. When released into the atmosphere, HCl can contribute to acid rain formation, which has detrimental effects on ecosystems, water bodies, and infrastructure. However, advancements in green chemistry have led to improved production methods and containment strategies, significantly reducing atmospheric emissions.
Water pollution is another critical environmental issue related to HCl. Improper disposal or accidental spills can lead to the acidification of water bodies, harming aquatic life and disrupting ecosystems. Green chemistry initiatives have focused on developing more efficient recycling and neutralization techniques, minimizing the risk of water contamination.
On the positive side, HCl's role in catalyzing green chemistry advancements has led to more environmentally friendly industrial processes. Its use as a catalyst in various chemical reactions has enabled the development of cleaner production methods, reducing the overall environmental footprint of many industries. For instance, HCl catalysts have been instrumental in improving the efficiency of biodiesel production, contributing to the growth of renewable energy sources.
The lifecycle assessment of HCl production has been a key focus area for environmental scientists. Green chemistry approaches have led to the development of more sustainable production methods, such as the recovery of HCl from waste streams and the use of renewable feedstocks. These innovations have significantly reduced the carbon footprint associated with HCl manufacturing.
In the context of waste management, HCl plays a dual role. While its improper disposal can pose environmental risks, it is also utilized in waste treatment processes. HCl is employed in the neutralization of alkaline waste and in the treatment of industrial effluents, contributing to overall waste reduction and environmental protection efforts.
The impact of HCl on soil quality is another important consideration. Accidental spills or long-term exposure can lead to soil acidification, affecting plant growth and soil microbial communities. However, green chemistry advancements have led to the development of more effective containment and remediation techniques, mitigating these risks.
In conclusion, while HCl poses certain environmental challenges, its role in catalyzing green chemistry advancements has led to significant improvements in industrial processes and environmental protection strategies. The ongoing research and development in this field continue to enhance the environmental profile of HCl, balancing its industrial utility with ecological sustainability.
Regulatory Framework
The regulatory framework surrounding hydrochloric acid and its applications in green chemistry is complex and multifaceted, reflecting the dual nature of this compound as both a hazardous substance and a crucial industrial chemical. At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to communicating chemical hazards, including those associated with hydrochloric acid.
In the United States, the Environmental Protection Agency (EPA) regulates hydrochloric acid under various statutes, including the Clean Air Act and the Toxic Substances Control Act. The Occupational Safety and Health Administration (OSHA) sets workplace exposure limits and safety standards for handling hydrochloric acid. The Department of Transportation (DOT) regulates its transport as a corrosive material.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation governs the production, import, and use of hydrochloric acid within the EU. This comprehensive framework requires manufacturers and importers to register chemicals and provide safety data, promoting the safe use of substances throughout their lifecycle.
In the context of green chemistry, regulatory bodies are increasingly focusing on promoting sustainable practices. The EPA's Green Chemistry Program encourages the design of chemical products and processes that reduce or eliminate the generation of hazardous substances. This initiative has led to the development of guidelines and incentives for industries to adopt greener alternatives or more efficient processes involving hydrochloric acid.
Many countries have implemented specific regulations to control emissions of hydrogen chloride, a precursor to hydrochloric acid, from industrial processes. These regulations often set emission limits and require the use of best available technologies for pollution control. In some jurisdictions, there are also requirements for recycling or neutralizing waste hydrochloric acid to minimize environmental impact.
The regulatory landscape is continually evolving, with a trend towards stricter controls on hazardous substances balanced against the need to support innovation in green chemistry. Recent developments include increased focus on lifecycle assessments, circular economy principles, and the promotion of alternative, less hazardous substances where possible.
Compliance with these diverse regulatory requirements presents challenges for industries using hydrochloric acid. However, it also drives innovation in green chemistry, encouraging the development of novel catalysts, more efficient processes, and safer handling methods. As the field of green chemistry advances, it is likely that regulatory frameworks will continue to adapt, potentially offering more flexibility for proven sustainable technologies while maintaining rigorous safety and environmental standards.
In the United States, the Environmental Protection Agency (EPA) regulates hydrochloric acid under various statutes, including the Clean Air Act and the Toxic Substances Control Act. The Occupational Safety and Health Administration (OSHA) sets workplace exposure limits and safety standards for handling hydrochloric acid. The Department of Transportation (DOT) regulates its transport as a corrosive material.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation governs the production, import, and use of hydrochloric acid within the EU. This comprehensive framework requires manufacturers and importers to register chemicals and provide safety data, promoting the safe use of substances throughout their lifecycle.
In the context of green chemistry, regulatory bodies are increasingly focusing on promoting sustainable practices. The EPA's Green Chemistry Program encourages the design of chemical products and processes that reduce or eliminate the generation of hazardous substances. This initiative has led to the development of guidelines and incentives for industries to adopt greener alternatives or more efficient processes involving hydrochloric acid.
Many countries have implemented specific regulations to control emissions of hydrogen chloride, a precursor to hydrochloric acid, from industrial processes. These regulations often set emission limits and require the use of best available technologies for pollution control. In some jurisdictions, there are also requirements for recycling or neutralizing waste hydrochloric acid to minimize environmental impact.
The regulatory landscape is continually evolving, with a trend towards stricter controls on hazardous substances balanced against the need to support innovation in green chemistry. Recent developments include increased focus on lifecycle assessments, circular economy principles, and the promotion of alternative, less hazardous substances where possible.
Compliance with these diverse regulatory requirements presents challenges for industries using hydrochloric acid. However, it also drives innovation in green chemistry, encouraging the development of novel catalysts, more efficient processes, and safer handling methods. As the field of green chemistry advances, it is likely that regulatory frameworks will continue to adapt, potentially offering more flexibility for proven sustainable technologies while maintaining rigorous safety and environmental standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!