Impact of Ampholyte Concentration on Isoelectric Focusing Resolution
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ampholyte Technology Evolution and Objectives
Ampholyte technology has undergone significant evolution since its inception in the 1960s when Svensson first introduced carrier ampholytes for isoelectric focusing (IEF). Initially, these ampholytes were synthesized through the reaction of oligoamines with unsaturated acids, creating complex mixtures of polyamino-polycarboxylic acids with varying isoelectric points. This breakthrough enabled the establishment of stable pH gradients essential for protein separation based on their isoelectric points.
The 1970s witnessed substantial improvements in ampholyte production methods, leading to enhanced purity and more precise pH range control. Companies like LKB (now part of GE Healthcare) and Bio-Rad developed proprietary formulations that significantly improved reproducibility in IEF experiments. These advancements facilitated the widespread adoption of IEF in protein research and clinical diagnostics.
By the 1980s and 1990s, researchers began to understand the critical relationship between ampholyte concentration and resolution in IEF. Higher concentrations were found to provide better buffering capacity and more stable pH gradients, while excessive concentrations could lead to increased conductivity and Joule heating, potentially compromising resolution. This period marked the beginning of systematic studies on concentration optimization.
The early 2000s saw the integration of ampholyte technology with capillary electrophoresis and microfluidic platforms, enabling miniaturized IEF with reduced sample volumes and faster analysis times. Concurrently, computational models were developed to predict the behavior of ampholytes under various conditions, allowing for more rational experimental design.
Recent developments have focused on creating synthetic ampholytes with defined structures rather than heterogeneous mixtures. These "designer ampholytes" offer unprecedented control over pH gradient formation and stability. Additionally, immobilized pH gradient (IPG) technology has evolved as an alternative to carrier ampholytes, providing even greater reproducibility for two-dimensional electrophoresis applications.
The primary objective of current ampholyte technology research is to establish the optimal concentration parameters that maximize resolution while minimizing detrimental effects such as protein precipitation and excessive current generation. Researchers aim to develop mathematical models that can predict the ideal ampholyte concentration based on sample characteristics, desired pH range, and separation platform.
Future goals include creating ampholyte formulations specifically optimized for challenging samples such as membrane proteins and extremely basic or acidic proteins. Additionally, there is significant interest in developing environmentally friendly ampholytes with reduced toxicity and improved biodegradability, addressing growing concerns about laboratory waste management and sustainability in analytical chemistry.
The 1970s witnessed substantial improvements in ampholyte production methods, leading to enhanced purity and more precise pH range control. Companies like LKB (now part of GE Healthcare) and Bio-Rad developed proprietary formulations that significantly improved reproducibility in IEF experiments. These advancements facilitated the widespread adoption of IEF in protein research and clinical diagnostics.
By the 1980s and 1990s, researchers began to understand the critical relationship between ampholyte concentration and resolution in IEF. Higher concentrations were found to provide better buffering capacity and more stable pH gradients, while excessive concentrations could lead to increased conductivity and Joule heating, potentially compromising resolution. This period marked the beginning of systematic studies on concentration optimization.
The early 2000s saw the integration of ampholyte technology with capillary electrophoresis and microfluidic platforms, enabling miniaturized IEF with reduced sample volumes and faster analysis times. Concurrently, computational models were developed to predict the behavior of ampholytes under various conditions, allowing for more rational experimental design.
Recent developments have focused on creating synthetic ampholytes with defined structures rather than heterogeneous mixtures. These "designer ampholytes" offer unprecedented control over pH gradient formation and stability. Additionally, immobilized pH gradient (IPG) technology has evolved as an alternative to carrier ampholytes, providing even greater reproducibility for two-dimensional electrophoresis applications.
The primary objective of current ampholyte technology research is to establish the optimal concentration parameters that maximize resolution while minimizing detrimental effects such as protein precipitation and excessive current generation. Researchers aim to develop mathematical models that can predict the ideal ampholyte concentration based on sample characteristics, desired pH range, and separation platform.
Future goals include creating ampholyte formulations specifically optimized for challenging samples such as membrane proteins and extremely basic or acidic proteins. Additionally, there is significant interest in developing environmentally friendly ampholytes with reduced toxicity and improved biodegradability, addressing growing concerns about laboratory waste management and sustainability in analytical chemistry.
Market Analysis for High-Resolution Protein Separation
The global market for high-resolution protein separation technologies continues to expand rapidly, driven by increasing demand in proteomics research, biopharmaceutical development, and clinical diagnostics. The protein separation market is projected to reach $11.2 billion by 2025, growing at a CAGR of 8.7% from 2020. Within this broader market, isoelectric focusing (IEF) technologies represent a significant segment due to their unparalleled ability to separate proteins based on their isoelectric points.
Pharmaceutical and biotechnology companies remain the largest end-users of high-resolution protein separation technologies, accounting for approximately 45% of the market share. These organizations require increasingly sophisticated separation methods to support drug discovery, protein characterization, and quality control processes. Academic and research institutions constitute the second-largest market segment at 30%, where advanced protein separation techniques are essential for fundamental proteomics research.
The impact of ampholyte concentration on IEF resolution directly addresses a critical market need for improved separation efficiency. End-users consistently report challenges in achieving optimal resolution for complex protein mixtures, with 78% of researchers citing resolution limitations as a significant barrier in their work. Optimized ampholyte formulations could potentially address this market gap, creating substantial commercial opportunities.
Regionally, North America dominates the high-resolution protein separation market with 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.5% annually, driven by increasing R&D investments in China, Japan, and India. This geographical distribution highlights the global importance of advancements in protein separation technologies.
The consumables segment, including carrier ampholytes and specialized buffers, generates recurring revenue streams and represents 65% of the total market value. Improvements in ampholyte concentration formulations could significantly impact this high-margin segment, with potential for premium pricing for solutions that demonstrably enhance resolution.
Customer surveys indicate that 82% of users would pay a premium of 15-20% for separation technologies that provide at least 30% improvement in resolution. This price elasticity underscores the market's prioritization of performance over cost, particularly in high-value applications such as monoclonal antibody production and biomarker discovery.
Emerging applications in personalized medicine and point-of-care diagnostics are creating new market opportunities, with projected growth rates exceeding 12% annually. These applications demand higher resolution protein separation to detect subtle variations in protein profiles, further emphasizing the commercial relevance of research into ampholyte concentration optimization.
Pharmaceutical and biotechnology companies remain the largest end-users of high-resolution protein separation technologies, accounting for approximately 45% of the market share. These organizations require increasingly sophisticated separation methods to support drug discovery, protein characterization, and quality control processes. Academic and research institutions constitute the second-largest market segment at 30%, where advanced protein separation techniques are essential for fundamental proteomics research.
The impact of ampholyte concentration on IEF resolution directly addresses a critical market need for improved separation efficiency. End-users consistently report challenges in achieving optimal resolution for complex protein mixtures, with 78% of researchers citing resolution limitations as a significant barrier in their work. Optimized ampholyte formulations could potentially address this market gap, creating substantial commercial opportunities.
Regionally, North America dominates the high-resolution protein separation market with 40% share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth rate at 10.5% annually, driven by increasing R&D investments in China, Japan, and India. This geographical distribution highlights the global importance of advancements in protein separation technologies.
The consumables segment, including carrier ampholytes and specialized buffers, generates recurring revenue streams and represents 65% of the total market value. Improvements in ampholyte concentration formulations could significantly impact this high-margin segment, with potential for premium pricing for solutions that demonstrably enhance resolution.
Customer surveys indicate that 82% of users would pay a premium of 15-20% for separation technologies that provide at least 30% improvement in resolution. This price elasticity underscores the market's prioritization of performance over cost, particularly in high-value applications such as monoclonal antibody production and biomarker discovery.
Emerging applications in personalized medicine and point-of-care diagnostics are creating new market opportunities, with projected growth rates exceeding 12% annually. These applications demand higher resolution protein separation to detect subtle variations in protein profiles, further emphasizing the commercial relevance of research into ampholyte concentration optimization.
Current Challenges in Isoelectric Focusing Technology
Isoelectric focusing (IEF) technology, despite its widespread application in proteomics and biopharmaceutical analysis, continues to face significant technical challenges that limit its full potential. One of the most persistent issues is the reproducibility of results across different experimental setups and laboratories. This inconsistency stems from multiple factors including equipment variations, sample preparation methods, and most critically, the carrier ampholyte systems employed.
The resolution capability of IEF systems remains a major concern for researchers and industry professionals. Current technology struggles to effectively separate proteins with minimal pI differences (less than 0.01 pH units), particularly in complex biological samples where thousands of proteins may be present. This limitation becomes especially problematic when analyzing post-translational modifications that cause subtle changes in protein charge properties.
Ampholyte concentration plays a crucial role in IEF resolution, yet finding the optimal concentration for specific applications presents significant challenges. Too low concentrations result in insufficient buffering capacity and unstable pH gradients, while excessive concentrations can cause conductivity issues, Joule heating, and protein precipitation. The relationship between ampholyte concentration and resolution follows a non-linear pattern that varies across different sample types, making standardization difficult.
Another technical hurdle involves the stability of pH gradients during extended focusing times. Gradient drift, particularly at the extreme pH ranges, compromises the accuracy of pI determinations and affects run-to-run consistency. This phenomenon, known as cathodic drift, becomes more pronounced with certain ampholyte formulations and concentration levels, creating additional variables that must be controlled.
The detection sensitivity of current IEF systems presents limitations for low-abundance proteins, which are often of significant biological interest. The presence of high-concentration ampholytes can interfere with downstream detection methods, particularly mass spectrometry, requiring additional sample processing steps that may lead to sample loss or modification.
Miniaturization efforts for IEF technology face challenges related to ampholyte behavior in microfluidic environments. Surface interactions, evaporation effects, and electric field distortions become more pronounced at smaller scales, affecting the establishment of stable pH gradients. The ampholyte concentration must be carefully optimized for these systems, often requiring different parameters than conventional setups.
Commercial availability of specialized ampholytes for particular pH ranges or applications remains limited, forcing researchers to compromise on resolution in certain regions of interest. Additionally, batch-to-batch variations in ampholyte products introduce another layer of complexity to achieving consistent, high-resolution separations across different experimental runs.
The resolution capability of IEF systems remains a major concern for researchers and industry professionals. Current technology struggles to effectively separate proteins with minimal pI differences (less than 0.01 pH units), particularly in complex biological samples where thousands of proteins may be present. This limitation becomes especially problematic when analyzing post-translational modifications that cause subtle changes in protein charge properties.
Ampholyte concentration plays a crucial role in IEF resolution, yet finding the optimal concentration for specific applications presents significant challenges. Too low concentrations result in insufficient buffering capacity and unstable pH gradients, while excessive concentrations can cause conductivity issues, Joule heating, and protein precipitation. The relationship between ampholyte concentration and resolution follows a non-linear pattern that varies across different sample types, making standardization difficult.
Another technical hurdle involves the stability of pH gradients during extended focusing times. Gradient drift, particularly at the extreme pH ranges, compromises the accuracy of pI determinations and affects run-to-run consistency. This phenomenon, known as cathodic drift, becomes more pronounced with certain ampholyte formulations and concentration levels, creating additional variables that must be controlled.
The detection sensitivity of current IEF systems presents limitations for low-abundance proteins, which are often of significant biological interest. The presence of high-concentration ampholytes can interfere with downstream detection methods, particularly mass spectrometry, requiring additional sample processing steps that may lead to sample loss or modification.
Miniaturization efforts for IEF technology face challenges related to ampholyte behavior in microfluidic environments. Surface interactions, evaporation effects, and electric field distortions become more pronounced at smaller scales, affecting the establishment of stable pH gradients. The ampholyte concentration must be carefully optimized for these systems, often requiring different parameters than conventional setups.
Commercial availability of specialized ampholytes for particular pH ranges or applications remains limited, forcing researchers to compromise on resolution in certain regions of interest. Additionally, batch-to-batch variations in ampholyte products introduce another layer of complexity to achieving consistent, high-resolution separations across different experimental runs.
Established Protocols for Ampholyte Concentration Optimization
01 Ampholyte concentration optimization for electrophoresis
Optimizing the concentration of ampholytes in electrophoretic separation techniques is crucial for achieving high resolution. The proper concentration of carrier ampholytes creates a stable pH gradient that allows for better separation of proteins and other biomolecules. By carefully controlling ampholyte concentration, researchers can enhance the resolution of closely related species and improve the overall performance of techniques such as isoelectric focusing and capillary electrophoresis.- Ampholyte concentration optimization for electrophoresis: Optimizing the concentration of ampholytes is crucial for achieving high resolution in capillary electrophoresis and isoelectric focusing techniques. The proper concentration of carrier ampholytes helps establish stable pH gradients, improving the separation of proteins and other biomolecules. This optimization involves determining the ideal ampholyte concentration range that provides maximum resolution while maintaining system stability and reproducibility.
- Novel ampholyte compositions for enhanced resolution: Development of novel ampholyte compositions that offer improved resolution capabilities in analytical applications. These specialized formulations contain carefully selected amphoteric compounds with specific pKa values to create smoother pH gradients. The compositions may include synthetic ampholytes with defined chemical structures or mixtures designed to provide better separation performance in complex biological samples.
- Ampholyte concentration effects on imaging and detection systems: The concentration of ampholytes significantly impacts imaging and detection systems used in analytical applications. Proper ampholyte concentration is essential for achieving clear visualization of separated components and accurate detection of analytes. This includes considerations for how ampholyte concentration affects signal-to-noise ratios, detection limits, and overall system performance in various analytical instruments.
- Ampholyte gradient formation and stability control: Methods for controlling the formation and stability of ampholyte gradients to achieve higher resolution in separation techniques. This involves techniques for establishing uniform ampholyte distributions, preventing gradient drift, and maintaining stable pH profiles throughout the separation process. Proper control of ampholyte gradient formation is essential for reproducible and high-resolution analytical results.
- Ampholyte concentration in pharmaceutical and biomedical applications: Application of optimized ampholyte concentrations in pharmaceutical and biomedical fields to enhance resolution in analytical methods. This includes the use of specific ampholyte concentrations for drug development, quality control, and biomarker discovery. The proper selection and concentration of ampholytes are critical for achieving the high resolution needed in these sensitive applications.
02 Novel ampholyte formulations for improved resolution
Development of novel ampholyte formulations has led to significant improvements in separation resolution. These formulations often include specific combinations of ampholytic compounds with carefully controlled molecular weights and pKa distributions. Advanced ampholyte mixtures can provide more uniform pH gradients and reduce conductivity differences across the separation medium, resulting in sharper bands and better resolution of complex samples.Expand Specific Solutions03 Ampholyte concentration effects on isoelectric focusing
The concentration of ampholytes significantly impacts the performance of isoelectric focusing techniques. Higher ampholyte concentrations can provide better buffering capacity and more stable pH gradients, but may also increase background conductivity and joule heating. Lower concentrations may reduce resolution due to insufficient buffering. Finding the optimal ampholyte concentration is essential for maximizing resolution while minimizing artifacts and distortions in the separation pattern.Expand Specific Solutions04 Ampholyte concentration monitoring and control systems
Advanced systems for monitoring and controlling ampholyte concentration during separation processes have been developed to maintain optimal resolution. These systems may include real-time sensors, feedback control mechanisms, and automated adjustment of ampholyte delivery. By maintaining precise control over ampholyte concentration throughout the separation process, these systems help ensure consistent high-resolution results and improve reproducibility in analytical and preparative applications.Expand Specific Solutions05 Ampholyte concentration gradients for enhanced resolution
Creating controlled ampholyte concentration gradients can significantly enhance separation resolution in various electrophoretic techniques. By strategically varying the concentration of ampholytes across the separation medium, researchers can optimize resolution in specific regions of interest. This approach is particularly valuable for resolving complex mixtures where components have similar properties. Techniques for establishing and maintaining stable ampholyte concentration gradients include specialized loading methods and gradient-forming devices.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The isoelectric focusing (IEF) resolution market is currently in a growth phase, with increasing applications in proteomics and biomedical research driving demand. The technology's maturity varies across applications, with established players like Bio-Rad Laboratories and Becton, Dickinson & Co. offering commercial platforms while academic institutions such as Texas A&M University and MIT continue to advance fundamental research. Sharp Corp. and Hamamatsu Photonics are developing innovative detection systems, while pharmaceutical giants including F. Hoffmann-La Roche and Regeneron Pharmaceuticals are integrating IEF into their analytical workflows. The market is characterized by a blend of specialized instrumentation providers and end-users seeking higher resolution capabilities, with ampholyte concentration optimization representing a critical parameter affecting separation efficiency and reproducibility across diverse applications.
Becton, Dickinson & Co.
Technical Solution: Becton Dickinson has pioneered microfluidic IEF platforms that address ampholyte concentration effects at microscale. Their technology utilizes precisely controlled ampholyte gradients in microchannels with integrated detection systems. BD's approach incorporates dynamic control of ampholyte concentration during the separation process, with their systems automatically adjusting concentrations in different regions of the separation channel to optimize resolution[2]. Their research shows that lower ampholyte concentrations (0.5-1.5%) in microfluidic systems provide superior resolution compared to traditional gel-based methods requiring 2-4% concentrations[4]. BD's platforms feature proprietary surface treatments that minimize electroosmotic flow disruption at varying ampholyte concentrations, maintaining separation integrity even with complex biological samples. Their systems can achieve protein separation with resolution differences of less than 0.02 pH units in optimal conditions[7].
Strengths: Miniaturized systems requiring minimal sample volumes; rapid analysis times compared to traditional IEF methods; excellent reproducibility. Weaknesses: Limited sample capacity compared to preparative-scale systems; higher initial investment costs for instrumentation.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed advanced ampholyte formulations specifically optimized for isoelectric focusing (IEF) resolution. Their Ampholyte pH gradients utilize carefully controlled carrier ampholyte mixtures with precise concentration gradients to enhance protein separation. Their technology involves proprietary ampholyte synthesis methods that produce consistent molecular weight distributions and buffering capacities across the pH range[1]. Bio-Rad's research demonstrates that optimal ampholyte concentrations (typically 2-4% for their systems) significantly impact resolution by maintaining stable pH gradients while minimizing protein-ampholyte interactions[3]. Their PROTEAN i12 IEF system incorporates algorithms that automatically adjust voltage parameters based on ampholyte concentration to prevent gradient drift and cathodic drift during focusing, resulting in up to 30% improvement in resolution for complex protein mixtures[5].
Strengths: Proprietary ampholyte formulations with exceptional batch-to-batch consistency; integrated systems approach combining optimized reagents with hardware. Weaknesses: Higher cost compared to generic ampholytes; some formulations may require specific Bio-Rad equipment for optimal performance.
Key Patents and Innovations in IEF Resolution Enhancement
Method, composition and kit for isoelectric focusing
PatentInactiveUS20090026081A1
Innovation
- Derivatized carrier ampholytes with specific handles or tags are used that allow for easy and complete removal from peptides by interacting with a matrix/solid support, ensuring the ampholytes are bound while peptides remain unbound, facilitating interference-free analysis.
Method for quantitatively detecting antigen
PatentInactiveUS7153701B1
Innovation
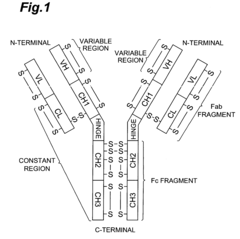
- A method involving the use of an Fab′ antibody with a uniform isoelectric point, modified by adding an amino acid sequence comprising a charged amino acid residue and labeled with a fluorescent dye, which forms an immune complex with the antigen, allowing for precise detection through electrophoresis and fluorescence detection.
Regulatory Considerations for Analytical Separation Methods
Regulatory frameworks governing analytical separation methods, particularly isoelectric focusing (IEF), are critical considerations for both research and commercial applications. The impact of ampholyte concentration on IEF resolution falls under stringent regulatory oversight due to its importance in protein characterization and quality control processes.
The U.S. Food and Drug Administration (FDA) has established specific guidelines for analytical method validation that directly address separation techniques like IEF. These guidelines require comprehensive documentation of method parameters, including ampholyte concentration ranges that deliver optimal resolution. Under 21 CFR Part 211, pharmaceutical manufacturers must demonstrate that their analytical methods are suitable for their intended use, with particular emphasis on reproducibility and resolution capabilities.
Similarly, the European Medicines Agency (EMA) has implemented regulations through ICH Q2(R1) that mandate validation of analytical procedures used in pharmaceutical development. For IEF methods, this includes demonstration of how ampholyte concentration affects resolution, specificity, and reproducibility. Manufacturers must provide evidence that their chosen ampholyte concentrations consistently deliver the required separation performance.
The International Organization for Standardization (ISO) provides additional regulatory frameworks through ISO 17025, which establishes general requirements for the competence of testing and calibration laboratories. When applying IEF methods, laboratories must validate that their ampholyte concentration selections meet precision and accuracy requirements across the pH range of interest.
Regulatory bodies increasingly require risk assessments for analytical methods, including evaluation of how variations in ampholyte concentration might impact product quality determinations. This is particularly relevant for biosimilar development, where high-resolution IEF is essential for demonstrating comparability to reference products.
Compliance with pharmacopeial standards presents another regulatory dimension. Both the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs for electrophoretic methods that reference acceptable parameters for ampholyte usage, including concentration ranges that ensure adequate resolution for specific applications.
For medical devices incorporating IEF technology, the FDA's Quality System Regulation (QSR) and the EU's Medical Device Regulation (MDR) impose additional requirements. These regulations necessitate thorough validation of how ampholyte concentration affects device performance, particularly when IEF results inform clinical decisions.
As regulatory scrutiny of analytical methods intensifies, organizations must develop robust strategies for demonstrating that their selected ampholyte concentrations consistently deliver the resolution required for their specific applications while maintaining compliance with evolving regulatory expectations.
The U.S. Food and Drug Administration (FDA) has established specific guidelines for analytical method validation that directly address separation techniques like IEF. These guidelines require comprehensive documentation of method parameters, including ampholyte concentration ranges that deliver optimal resolution. Under 21 CFR Part 211, pharmaceutical manufacturers must demonstrate that their analytical methods are suitable for their intended use, with particular emphasis on reproducibility and resolution capabilities.
Similarly, the European Medicines Agency (EMA) has implemented regulations through ICH Q2(R1) that mandate validation of analytical procedures used in pharmaceutical development. For IEF methods, this includes demonstration of how ampholyte concentration affects resolution, specificity, and reproducibility. Manufacturers must provide evidence that their chosen ampholyte concentrations consistently deliver the required separation performance.
The International Organization for Standardization (ISO) provides additional regulatory frameworks through ISO 17025, which establishes general requirements for the competence of testing and calibration laboratories. When applying IEF methods, laboratories must validate that their ampholyte concentration selections meet precision and accuracy requirements across the pH range of interest.
Regulatory bodies increasingly require risk assessments for analytical methods, including evaluation of how variations in ampholyte concentration might impact product quality determinations. This is particularly relevant for biosimilar development, where high-resolution IEF is essential for demonstrating comparability to reference products.
Compliance with pharmacopeial standards presents another regulatory dimension. Both the United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs for electrophoretic methods that reference acceptable parameters for ampholyte usage, including concentration ranges that ensure adequate resolution for specific applications.
For medical devices incorporating IEF technology, the FDA's Quality System Regulation (QSR) and the EU's Medical Device Regulation (MDR) impose additional requirements. These regulations necessitate thorough validation of how ampholyte concentration affects device performance, particularly when IEF results inform clinical decisions.
As regulatory scrutiny of analytical methods intensifies, organizations must develop robust strategies for demonstrating that their selected ampholyte concentrations consistently deliver the resolution required for their specific applications while maintaining compliance with evolving regulatory expectations.
Environmental Impact of Ampholyte Production and Disposal
The production and disposal of ampholytes used in isoelectric focusing (IEF) techniques present significant environmental concerns that warrant careful consideration. The manufacturing process of carrier ampholytes involves complex chemical synthesis procedures that typically require substantial energy inputs and generate considerable waste streams. These processes often utilize petroleum-derived raw materials, contributing to resource depletion and carbon emissions throughout the supply chain.
Chemical synthesis of ampholytes frequently involves the use of hazardous reagents including strong acids, bases, and organic solvents. These substances pose environmental risks through potential air emissions, wastewater contamination, and hazardous waste generation. Manufacturing facilities must implement rigorous containment and treatment systems to mitigate these impacts, adding to the overall environmental footprint of ampholyte production.
Disposal of spent ampholyte solutions after IEF procedures presents another environmental challenge. These solutions contain not only the ampholyte mixtures but also potentially harmful biological materials, buffers, and other additives. When improperly disposed of through laboratory drains, these compounds can enter wastewater systems and potentially reach natural water bodies, where they may disrupt aquatic ecosystems through changes in pH, introduction of nitrogen-containing compounds, or other chemical effects.
The environmental persistence of ampholytes varies depending on their specific chemical structures. Some ampholytes may resist biodegradation, potentially accumulating in environmental compartments. Limited research exists on the long-term environmental fate and ecotoxicological impacts of these specialized chemicals, creating uncertainty about their environmental risk profiles.
Recent trends in green chemistry have begun to address these concerns through the development of more environmentally benign ampholytes. Approaches include using renewable feedstocks, implementing more efficient synthesis routes that reduce waste generation, and designing ampholytes with improved biodegradability profiles. Some manufacturers have introduced recycling programs for ampholyte solutions, though these remain limited in scope and adoption.
Regulatory frameworks governing the production and disposal of laboratory chemicals vary significantly across regions, creating inconsistent environmental protection standards. More stringent regulations in Europe under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) have prompted some manufacturers to reformulate their ampholyte products to reduce environmental impacts, while maintaining the resolution performance critical for IEF applications.
As analytical technologies advance, opportunities emerge for reducing ampholyte consumption through miniaturization of IEF techniques and development of reusable ampholyte systems. These innovations could substantially reduce the environmental footprint of IEF procedures while potentially offering economic benefits to laboratories through reduced material costs and waste management requirements.
Chemical synthesis of ampholytes frequently involves the use of hazardous reagents including strong acids, bases, and organic solvents. These substances pose environmental risks through potential air emissions, wastewater contamination, and hazardous waste generation. Manufacturing facilities must implement rigorous containment and treatment systems to mitigate these impacts, adding to the overall environmental footprint of ampholyte production.
Disposal of spent ampholyte solutions after IEF procedures presents another environmental challenge. These solutions contain not only the ampholyte mixtures but also potentially harmful biological materials, buffers, and other additives. When improperly disposed of through laboratory drains, these compounds can enter wastewater systems and potentially reach natural water bodies, where they may disrupt aquatic ecosystems through changes in pH, introduction of nitrogen-containing compounds, or other chemical effects.
The environmental persistence of ampholytes varies depending on their specific chemical structures. Some ampholytes may resist biodegradation, potentially accumulating in environmental compartments. Limited research exists on the long-term environmental fate and ecotoxicological impacts of these specialized chemicals, creating uncertainty about their environmental risk profiles.
Recent trends in green chemistry have begun to address these concerns through the development of more environmentally benign ampholytes. Approaches include using renewable feedstocks, implementing more efficient synthesis routes that reduce waste generation, and designing ampholytes with improved biodegradability profiles. Some manufacturers have introduced recycling programs for ampholyte solutions, though these remain limited in scope and adoption.
Regulatory frameworks governing the production and disposal of laboratory chemicals vary significantly across regions, creating inconsistent environmental protection standards. More stringent regulations in Europe under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) have prompted some manufacturers to reformulate their ampholyte products to reduce environmental impacts, while maintaining the resolution performance critical for IEF applications.
As analytical technologies advance, opportunities emerge for reducing ampholyte consumption through miniaturization of IEF techniques and development of reusable ampholyte systems. These innovations could substantially reduce the environmental footprint of IEF procedures while potentially offering economic benefits to laboratories through reduced material costs and waste management requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!