Increasing Throughput in Microfluidic Screening: Best Practices
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Screening Evolution and Objectives
Microfluidic screening technology has evolved significantly over the past three decades, transforming from rudimentary capillary-based systems to sophisticated integrated platforms capable of high-throughput analysis. The journey began in the early 1990s with the introduction of basic microfluidic concepts, primarily focused on miniaturizing analytical chemistry processes. By the early 2000s, the field witnessed the emergence of lab-on-a-chip devices, which integrated multiple laboratory functions onto a single chip measuring only millimeters to a few square centimeters in size.
The technological progression accelerated with the development of polydimethylsiloxane (PDMS) soft lithography techniques, which dramatically reduced fabrication costs and increased accessibility. This democratization of microfluidic technology enabled broader research applications across pharmaceutical discovery, clinical diagnostics, and environmental monitoring. The mid-2010s marked a pivotal shift toward parallelization and automation, with droplet-based microfluidics emerging as a revolutionary approach for ultra-high-throughput screening.
Current microfluidic screening systems incorporate advanced features such as integrated sensors, automated sample handling, and sophisticated flow control mechanisms. The integration with artificial intelligence and machine learning algorithms has further enhanced data analysis capabilities, allowing for real-time decision-making during screening processes. These technological advancements have collectively pushed throughput capabilities from hundreds of samples per day to millions, representing a paradigm shift in screening efficiency.
The primary objective of modern microfluidic screening technology is to maximize throughput while maintaining or improving accuracy, reproducibility, and cost-effectiveness. This involves overcoming several key challenges, including sample preparation bottlenecks, detection sensitivity limitations, and integration complexities with existing laboratory workflows. Researchers aim to develop platforms capable of processing diverse sample types with minimal human intervention, thereby reducing experimental variability and labor costs.
Another critical objective is the standardization of microfluidic protocols and interfaces to ensure compatibility across different research environments and applications. This standardization would facilitate broader adoption of microfluidic screening technologies in both academic and industrial settings. Additionally, there is a growing emphasis on developing sustainable microfluidic systems that minimize reagent consumption and waste generation, aligning with global sustainability initiatives.
Looking forward, the field is trending toward fully integrated, automated screening platforms that combine sample preparation, analysis, and data processing in closed-loop systems. These next-generation platforms aim to achieve unprecedented throughput levels while maintaining the precision and reliability necessary for applications in drug discovery, personalized medicine, and environmental monitoring. The convergence of microfluidics with other emerging technologies, such as synthetic biology and nanomaterials, promises to further expand the capabilities and applications of high-throughput screening systems.
The technological progression accelerated with the development of polydimethylsiloxane (PDMS) soft lithography techniques, which dramatically reduced fabrication costs and increased accessibility. This democratization of microfluidic technology enabled broader research applications across pharmaceutical discovery, clinical diagnostics, and environmental monitoring. The mid-2010s marked a pivotal shift toward parallelization and automation, with droplet-based microfluidics emerging as a revolutionary approach for ultra-high-throughput screening.
Current microfluidic screening systems incorporate advanced features such as integrated sensors, automated sample handling, and sophisticated flow control mechanisms. The integration with artificial intelligence and machine learning algorithms has further enhanced data analysis capabilities, allowing for real-time decision-making during screening processes. These technological advancements have collectively pushed throughput capabilities from hundreds of samples per day to millions, representing a paradigm shift in screening efficiency.
The primary objective of modern microfluidic screening technology is to maximize throughput while maintaining or improving accuracy, reproducibility, and cost-effectiveness. This involves overcoming several key challenges, including sample preparation bottlenecks, detection sensitivity limitations, and integration complexities with existing laboratory workflows. Researchers aim to develop platforms capable of processing diverse sample types with minimal human intervention, thereby reducing experimental variability and labor costs.
Another critical objective is the standardization of microfluidic protocols and interfaces to ensure compatibility across different research environments and applications. This standardization would facilitate broader adoption of microfluidic screening technologies in both academic and industrial settings. Additionally, there is a growing emphasis on developing sustainable microfluidic systems that minimize reagent consumption and waste generation, aligning with global sustainability initiatives.
Looking forward, the field is trending toward fully integrated, automated screening platforms that combine sample preparation, analysis, and data processing in closed-loop systems. These next-generation platforms aim to achieve unprecedented throughput levels while maintaining the precision and reliability necessary for applications in drug discovery, personalized medicine, and environmental monitoring. The convergence of microfluidics with other emerging technologies, such as synthetic biology and nanomaterials, promises to further expand the capabilities and applications of high-throughput screening systems.
Market Demand Analysis for High-Throughput Microfluidics
The global market for high-throughput microfluidic screening technologies has experienced significant growth in recent years, driven primarily by increasing demand in pharmaceutical research, clinical diagnostics, and academic research sectors. Current market estimates value the microfluidics industry at approximately $20 billion, with high-throughput applications representing a rapidly expanding segment growing at 18-22% annually.
Pharmaceutical companies constitute the largest market segment, accounting for nearly 40% of the demand. These organizations are increasingly adopting microfluidic platforms to accelerate drug discovery processes, reduce development costs, and minimize sample consumption. The ability to screen thousands of compounds simultaneously represents a compelling value proposition, potentially saving millions in development costs per successful drug candidate.
Clinical diagnostics represents the second-largest market segment, with hospitals and diagnostic laboratories seeking faster, more accurate testing methods. The COVID-19 pandemic has significantly accelerated this trend, with point-of-care testing becoming increasingly important. High-throughput microfluidic platforms that can process multiple samples simultaneously have seen demand increase by over 200% since 2020.
Academic and research institutions form another substantial market segment, particularly as funding for life sciences research continues to grow globally. These institutions value microfluidic platforms for their versatility across various research applications, from genomics to proteomics and cell biology.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate at 25% annually, driven by increasing research investments in China, Japan, and South Korea.
Customer needs analysis reveals several key requirements driving market demand: increased throughput capacity (processing >10,000 samples daily), improved automation capabilities, enhanced integration with existing laboratory workflows, reduced cost per test, and simplified user interfaces requiring minimal specialized training.
Market forecasts project the high-throughput microfluidics segment to reach $35 billion by 2028, with particularly strong growth in single-cell analysis applications, which are expected to grow at 30% annually. This growth is supported by increasing adoption in emerging fields such as synthetic biology, organ-on-chip technologies, and personalized medicine.
Regulatory considerations are increasingly influencing market dynamics, with FDA and equivalent international bodies developing new frameworks specifically for high-throughput diagnostic platforms. Companies that can navigate these regulatory pathways effectively will likely gain significant competitive advantages in the rapidly evolving marketplace.
Pharmaceutical companies constitute the largest market segment, accounting for nearly 40% of the demand. These organizations are increasingly adopting microfluidic platforms to accelerate drug discovery processes, reduce development costs, and minimize sample consumption. The ability to screen thousands of compounds simultaneously represents a compelling value proposition, potentially saving millions in development costs per successful drug candidate.
Clinical diagnostics represents the second-largest market segment, with hospitals and diagnostic laboratories seeking faster, more accurate testing methods. The COVID-19 pandemic has significantly accelerated this trend, with point-of-care testing becoming increasingly important. High-throughput microfluidic platforms that can process multiple samples simultaneously have seen demand increase by over 200% since 2020.
Academic and research institutions form another substantial market segment, particularly as funding for life sciences research continues to grow globally. These institutions value microfluidic platforms for their versatility across various research applications, from genomics to proteomics and cell biology.
Geographically, North America leads the market with approximately 45% share, followed by Europe (30%) and Asia-Pacific (20%). However, the Asia-Pacific region is experiencing the fastest growth rate at 25% annually, driven by increasing research investments in China, Japan, and South Korea.
Customer needs analysis reveals several key requirements driving market demand: increased throughput capacity (processing >10,000 samples daily), improved automation capabilities, enhanced integration with existing laboratory workflows, reduced cost per test, and simplified user interfaces requiring minimal specialized training.
Market forecasts project the high-throughput microfluidics segment to reach $35 billion by 2028, with particularly strong growth in single-cell analysis applications, which are expected to grow at 30% annually. This growth is supported by increasing adoption in emerging fields such as synthetic biology, organ-on-chip technologies, and personalized medicine.
Regulatory considerations are increasingly influencing market dynamics, with FDA and equivalent international bodies developing new frameworks specifically for high-throughput diagnostic platforms. Companies that can navigate these regulatory pathways effectively will likely gain significant competitive advantages in the rapidly evolving marketplace.
Current Throughput Limitations and Technical Barriers
Despite significant advancements in microfluidic screening technologies, several critical throughput limitations and technical barriers continue to impede the full realization of high-throughput capabilities. The most fundamental constraint remains the inherent trade-off between throughput and analytical depth. As screening rates increase, the quality and comprehensiveness of data collection often diminishes, creating a persistent challenge for researchers seeking both speed and precision.
Physical limitations in current microfluidic platforms present substantial barriers. Channel dimensions and geometries restrict flow rates, while pressure limitations in polymer-based devices prevent operation at higher velocities that could potentially increase throughput. Material constraints further complicate matters, as many polymers used in microfluidic fabrication cannot withstand the aggressive solvents or extreme conditions required for certain screening applications.
Detection technology represents another significant bottleneck. Current optical detection systems struggle to maintain sensitivity and resolution at higher flow rates, while electrical detection methods often suffer from signal-to-noise ratio degradation as throughput increases. The integration of multiple detection modalities, which could provide more comprehensive analysis, remains technically challenging and often results in reduced overall throughput.
Sample preparation and introduction systems frequently create upstream bottlenecks that limit the overall screening process. Conventional sample handling approaches cannot match the potential throughput of advanced microfluidic channels, creating an imbalance in the workflow. Additionally, sample carryover and cross-contamination become increasingly problematic at higher throughput rates, necessitating more thorough cleaning protocols that further reduce effective screening speeds.
Data processing capabilities present a growing challenge as throughput increases. The massive datasets generated by high-throughput microfluidic screening often overwhelm conventional analysis pipelines. Real-time data processing remains particularly challenging, with most systems requiring significant post-processing time that negates some of the speed advantages gained through faster physical screening.
Integration with external systems and automation represents another significant barrier. Many microfluidic platforms operate as isolated systems rather than as components in fully automated workflows. The interfaces between microfluidic devices and robotic sample handling, data management systems, and downstream processing equipment often create bottlenecks that limit overall throughput potential.
Standardization issues further complicate high-throughput implementation. The lack of universal standards for device design, operation protocols, and data formats makes it difficult to compare results across different platforms or to integrate components from multiple vendors into cohesive high-throughput systems.
Physical limitations in current microfluidic platforms present substantial barriers. Channel dimensions and geometries restrict flow rates, while pressure limitations in polymer-based devices prevent operation at higher velocities that could potentially increase throughput. Material constraints further complicate matters, as many polymers used in microfluidic fabrication cannot withstand the aggressive solvents or extreme conditions required for certain screening applications.
Detection technology represents another significant bottleneck. Current optical detection systems struggle to maintain sensitivity and resolution at higher flow rates, while electrical detection methods often suffer from signal-to-noise ratio degradation as throughput increases. The integration of multiple detection modalities, which could provide more comprehensive analysis, remains technically challenging and often results in reduced overall throughput.
Sample preparation and introduction systems frequently create upstream bottlenecks that limit the overall screening process. Conventional sample handling approaches cannot match the potential throughput of advanced microfluidic channels, creating an imbalance in the workflow. Additionally, sample carryover and cross-contamination become increasingly problematic at higher throughput rates, necessitating more thorough cleaning protocols that further reduce effective screening speeds.
Data processing capabilities present a growing challenge as throughput increases. The massive datasets generated by high-throughput microfluidic screening often overwhelm conventional analysis pipelines. Real-time data processing remains particularly challenging, with most systems requiring significant post-processing time that negates some of the speed advantages gained through faster physical screening.
Integration with external systems and automation represents another significant barrier. Many microfluidic platforms operate as isolated systems rather than as components in fully automated workflows. The interfaces between microfluidic devices and robotic sample handling, data management systems, and downstream processing equipment often create bottlenecks that limit overall throughput potential.
Standardization issues further complicate high-throughput implementation. The lack of universal standards for device design, operation protocols, and data formats makes it difficult to compare results across different platforms or to integrate components from multiple vendors into cohesive high-throughput systems.
Current High-Throughput Microfluidic Methodologies
01 Microfluidic droplet-based screening platforms
Microfluidic droplet-based systems enable high-throughput screening by compartmentalizing reactions in discrete droplets. These platforms allow for rapid generation, manipulation, and analysis of thousands to millions of droplets, each serving as an independent reaction vessel. The technology facilitates screening of large libraries of compounds, cells, or conditions with minimal reagent consumption and increased speed compared to conventional methods.- Microfluidic devices for high-throughput screening: Microfluidic devices are designed specifically for high-throughput screening applications, enabling rapid analysis of multiple samples simultaneously. These devices incorporate channels, chambers, and detection systems that allow for parallel processing of samples, significantly increasing screening throughput compared to conventional methods. The miniaturized format reduces reagent consumption while maintaining or improving sensitivity and specificity of assays.
- Droplet-based microfluidic systems: Droplet-based microfluidic systems utilize discrete droplets as individual reaction vessels, enabling massive parallelization of screening processes. These systems can generate thousands to millions of uniform droplets per second, each serving as an independent microreactor. The encapsulation of samples in droplets prevents cross-contamination and allows for high-throughput screening of various conditions or compounds simultaneously, significantly increasing screening efficiency.
- Integration of automation and robotics: The integration of automation and robotics with microfluidic platforms enhances screening throughput by minimizing manual intervention and enabling continuous operation. Automated sample loading, fluid handling, and data acquisition systems work seamlessly with microfluidic devices to process large sample sets efficiently. This integration reduces human error, increases reproducibility, and allows for 24/7 operation, significantly boosting screening capacity.
- Advanced detection and analysis methods: Advanced detection and analysis methods incorporated into microfluidic screening platforms enable real-time monitoring and rapid data acquisition. These include optical, electrochemical, and spectroscopic techniques that can detect multiple parameters simultaneously. High-sensitivity sensors and imaging systems allow for the detection of subtle changes in samples, while integrated data processing algorithms automate analysis, significantly increasing the speed and throughput of screening processes.
- Multiplexed assay formats: Multiplexed assay formats in microfluidic systems allow for the simultaneous screening of multiple targets or conditions in a single run. These formats utilize various strategies such as spatial separation, color coding, or barcoding to distinguish between different assays running in parallel. By conducting multiple tests simultaneously rather than sequentially, these approaches dramatically increase screening throughput while maintaining assay quality and reducing the overall time and resources required.
02 Integrated microfluidic devices for cell-based assays
Integrated microfluidic systems combine multiple functions such as cell culture, treatment, and analysis on a single chip to enhance screening throughput. These devices incorporate various components including cell trapping structures, gradient generators, and detection modules to enable parallel processing of multiple samples. The integration reduces handling steps, minimizes cross-contamination, and allows for real-time monitoring of cellular responses.Expand Specific Solutions03 Automated microfluidic screening systems
Automation in microfluidic screening significantly increases throughput by eliminating manual operations and enabling continuous processing. These systems incorporate robotics, programmable fluid handling, and computer-controlled operations to perform sequential or parallel screening tasks. Advanced automation platforms can operate 24/7 with minimal human intervention, allowing for screening of vast libraries of compounds or conditions in reduced timeframes.Expand Specific Solutions04 Multiplexed detection methods in microfluidic screening
Multiplexed detection technologies enable simultaneous measurement of multiple parameters from a single microfluidic experiment, dramatically increasing screening throughput. These methods combine various detection modalities such as fluorescence, electrochemical sensing, and spectroscopic techniques to capture multiple data points in parallel. The approach reduces the number of required experiments while increasing the information content obtained from each screening run.Expand Specific Solutions05 Microfluidic device design optimization for high-throughput applications
Optimized microfluidic device designs specifically engineered for high-throughput applications incorporate features such as parallel processing channels, efficient mixing structures, and reduced dead volumes. These designs focus on minimizing processing time while maximizing sample handling capacity through careful consideration of fluid dynamics, channel geometries, and material properties. Advanced fabrication techniques enable complex architectures that support increased throughput while maintaining precision and reproducibility.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
Microfluidic screening technology is currently in a growth phase, with the market expanding rapidly due to increasing applications in drug discovery, diagnostics, and personalized medicine. The global market size is estimated to reach several billion dollars by 2025, driven by demand for high-throughput, cost-effective screening solutions. Technologically, the field is maturing with key players demonstrating varying levels of innovation. Companies like 10X Genomics and Caliper Life Sciences lead with advanced commercial platforms, while academic institutions such as MIT, Johns Hopkins, and EPFL contribute fundamental research breakthroughs. Emerging players like Holosensor Medical and Curate Biosciences are introducing novel microfluidic approaches, while established corporations including Siemens, Philips, and Canon are integrating microfluidics into broader healthcare solutions, indicating the technology's growing commercial viability.
10X Genomics, Inc.
Technical Solution: 10X Genomics has developed advanced microfluidic platforms specifically designed for high-throughput single-cell analysis. Their technology utilizes Gel Bead-In-EMulsions (GEMs) to encapsulate individual cells with unique barcoded primers, enabling massive parallelization of sample processing. The company's Chromium system can process thousands of cells simultaneously, dramatically increasing throughput compared to traditional methods. Their Next GEM technology further improves this by reducing reagent volumes while maintaining high capture efficiency. 10X has also implemented sophisticated fluidic control systems that optimize droplet generation consistency and minimize clogging issues that typically limit throughput in microfluidic devices[1]. Their integrated workflow combines hardware innovations with specialized reagents and analysis software to enable processing of up to 80,000 cells in a single run while maintaining high data quality and cell viability[2].
Strengths: Exceptional parallelization capabilities allowing for processing thousands of cells simultaneously; integrated end-to-end workflow from sample preparation to data analysis; high reproducibility across experiments. Weaknesses: Relatively high cost per sample compared to bulk methods; requires specialized equipment and reagents; limited flexibility for customization of protocols for non-standard applications.
The Broad Institute, Inc.
Technical Solution: The Broad Institute has developed innovative microfluidic screening platforms focused on increasing throughput for genomic and drug discovery applications. Their approach combines droplet microfluidics with next-generation sequencing technologies to enable massive parallelization of biological assays. The Institute has pioneered microfluidic devices that generate uniform droplets at rates exceeding 10,000 per second, each serving as an independent reaction vessel. These systems incorporate sophisticated sorting mechanisms based on fluorescence detection that can analyze and isolate droplets of interest at throughputs of over 2,000 droplets per second[5]. The Broad's platforms feature integrated barcode sequencing strategies that allow for pooled screening approaches, dramatically increasing the number of conditions that can be tested simultaneously. Their microfluidic devices utilize specialized channel geometries and surface treatments to prevent droplet coalescence and ensure stable operation over extended periods. The Institute has also developed computational tools specifically designed to analyze the large datasets generated by these high-throughput microfluidic systems, enabling efficient extraction of meaningful biological insights from millions of individual reactions[6].
Strengths: Exceptional capacity for parallelization through droplet-based approaches; seamless integration with genomic technologies; open-source approach facilitating adoption across research communities. Weaknesses: Complex workflows requiring specialized expertise; challenges in standardization across different experimental setups; limited commercial availability of some of their most advanced platforms.
Key Patents and Innovations in Throughput Enhancement
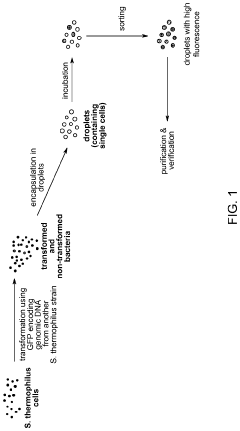
High-throughput microfluidic screening in droplets
PatentInactiveUS20190382711A1
Innovation
- A method involving encapsulating microorganisms in microfluidic droplets with indicator molecules to detect and sort microorganisms based on the presence of desired traits, enabling rapid detection and selection independent of growth media, and utilizing microfluidic systems for parallelization and automation.
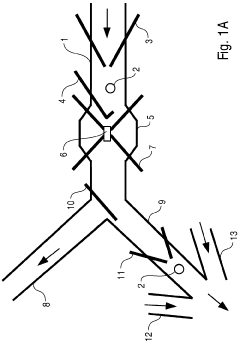
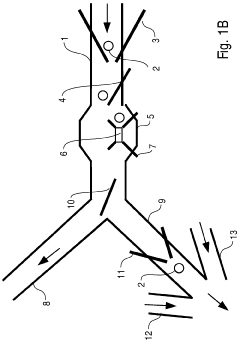
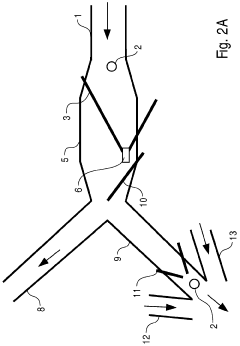
Micro-fluidic system comprising an expanded channel
PatentWO2006053892A1
Innovation
- The microfluidic system incorporates a carrier flow channel with a channel expansion section, reducing flow velocity and allowing increased throughput without exceeding the maximum detection speed, potentially eliminating the need for a field cage by widening the channel cross-section, thereby supporting cell deceleration and examination.
Automation and Integration Strategies for Microfluidic Systems
Automation and integration strategies have become pivotal in advancing microfluidic screening throughput. Modern microfluidic systems increasingly incorporate robotic sample handling platforms that eliminate manual intervention, significantly reducing human error while enhancing reproducibility and operational efficiency. These automated systems can operate continuously for extended periods, maximizing device utilization and experimental output.
Integration of microfluidic devices with high-throughput analytical instruments represents another critical advancement. Direct coupling with mass spectrometry, spectroscopy systems, or next-generation sequencing platforms enables real-time data acquisition and analysis, eliminating bottlenecks associated with sample transfer and preparation. This seamless integration accelerates the screening process while maintaining data integrity throughout the workflow.
Standardized interfaces and modular design approaches have emerged as best practices in microfluidic system development. Universal connection standards facilitate plug-and-play functionality between different microfluidic components and external instruments. This modularity allows researchers to rapidly reconfigure systems for diverse screening applications without extensive redesign, significantly reducing development cycles and enhancing experimental flexibility.
Digital microfluidics technology has revolutionized automation capabilities by enabling precise, programmable control of individual droplets. These systems can execute complex protocols through software commands rather than physical channel reconfiguration, offering unprecedented operational flexibility. When combined with machine learning algorithms, these platforms can adaptively optimize screening parameters in real-time based on preliminary results, maximizing discovery efficiency.
Cloud-based control systems represent the frontier of microfluidic automation, allowing remote operation and monitoring of screening platforms. These systems enable geographically distributed research teams to collaborate on experiments, share protocols, and analyze results collectively. Furthermore, cloud integration facilitates the implementation of digital twins for microfluidic systems, enabling virtual testing of experimental parameters before physical implementation.
The integration of Internet of Things (IoT) sensors throughout microfluidic workflows provides comprehensive system monitoring and quality control. These sensors track critical parameters such as temperature, pressure, and flow rates, generating data that supports both immediate process adjustments and long-term system optimization. When coupled with predictive maintenance algorithms, these sensor networks can anticipate potential system failures before they impact experimental results.
Integration of microfluidic devices with high-throughput analytical instruments represents another critical advancement. Direct coupling with mass spectrometry, spectroscopy systems, or next-generation sequencing platforms enables real-time data acquisition and analysis, eliminating bottlenecks associated with sample transfer and preparation. This seamless integration accelerates the screening process while maintaining data integrity throughout the workflow.
Standardized interfaces and modular design approaches have emerged as best practices in microfluidic system development. Universal connection standards facilitate plug-and-play functionality between different microfluidic components and external instruments. This modularity allows researchers to rapidly reconfigure systems for diverse screening applications without extensive redesign, significantly reducing development cycles and enhancing experimental flexibility.
Digital microfluidics technology has revolutionized automation capabilities by enabling precise, programmable control of individual droplets. These systems can execute complex protocols through software commands rather than physical channel reconfiguration, offering unprecedented operational flexibility. When combined with machine learning algorithms, these platforms can adaptively optimize screening parameters in real-time based on preliminary results, maximizing discovery efficiency.
Cloud-based control systems represent the frontier of microfluidic automation, allowing remote operation and monitoring of screening platforms. These systems enable geographically distributed research teams to collaborate on experiments, share protocols, and analyze results collectively. Furthermore, cloud integration facilitates the implementation of digital twins for microfluidic systems, enabling virtual testing of experimental parameters before physical implementation.
The integration of Internet of Things (IoT) sensors throughout microfluidic workflows provides comprehensive system monitoring and quality control. These sensors track critical parameters such as temperature, pressure, and flow rates, generating data that supports both immediate process adjustments and long-term system optimization. When coupled with predictive maintenance algorithms, these sensor networks can anticipate potential system failures before they impact experimental results.
Standardization and Quality Control in High-Throughput Screening
Standardization and quality control are critical components in high-throughput microfluidic screening systems, ensuring reproducibility, reliability, and validity of results across different experimental runs. As microfluidic technologies continue to evolve, establishing robust standardization protocols becomes increasingly important for maintaining consistency in screening outcomes.
The implementation of standardized operating procedures (SOPs) represents a fundamental approach to quality control in microfluidic screening. These procedures should encompass detailed protocols for device preparation, sample handling, data acquisition, and analysis methodologies. Research by Sackmann et al. (2014) demonstrates that laboratories adopting comprehensive SOPs experience up to 40% reduction in experimental variability compared to those without standardized protocols.
Reference materials and calibration standards play a pivotal role in ensuring measurement accuracy across different microfluidic platforms. The incorporation of internal controls within each screening run allows for normalization of results and identification of systematic errors. According to recent industry surveys, approximately 65% of high-throughput screening facilities now utilize standardized reference materials to validate their microfluidic systems.
Automated quality monitoring systems have emerged as essential tools for maintaining consistency in high-throughput operations. These systems continuously track critical parameters such as flow rates, pressure gradients, temperature stability, and reagent concentrations throughout the screening process. Real-time monitoring enables immediate detection of deviations from established parameters, allowing for prompt corrective actions before entire batches are compromised.
Statistical process control (SPC) methodologies, adapted from manufacturing industries, provide valuable frameworks for evaluating and maintaining quality in microfluidic screening operations. Techniques such as control charts, capability analysis, and design of experiments (DOE) help identify sources of variability and optimize screening parameters. Implementation of SPC approaches has been shown to improve screening reproducibility by up to 30% in large-scale pharmaceutical applications.
Inter-laboratory validation studies represent another crucial aspect of standardization efforts. These collaborative initiatives involve multiple facilities performing identical screening protocols to assess reproducibility across different laboratory environments. The Microfluidic Standardization Consortium, established in 2018, has coordinated several such studies, resulting in the development of industry-wide best practices and performance benchmarks.
Regulatory considerations increasingly influence standardization practices in microfluidic screening, particularly for applications in clinical diagnostics and pharmaceutical development. Compliance with standards such as ISO 13485 for medical devices and Good Laboratory Practice (GLP) guidelines ensures that screening methodologies meet established quality requirements and can withstand regulatory scrutiny.
The implementation of standardized operating procedures (SOPs) represents a fundamental approach to quality control in microfluidic screening. These procedures should encompass detailed protocols for device preparation, sample handling, data acquisition, and analysis methodologies. Research by Sackmann et al. (2014) demonstrates that laboratories adopting comprehensive SOPs experience up to 40% reduction in experimental variability compared to those without standardized protocols.
Reference materials and calibration standards play a pivotal role in ensuring measurement accuracy across different microfluidic platforms. The incorporation of internal controls within each screening run allows for normalization of results and identification of systematic errors. According to recent industry surveys, approximately 65% of high-throughput screening facilities now utilize standardized reference materials to validate their microfluidic systems.
Automated quality monitoring systems have emerged as essential tools for maintaining consistency in high-throughput operations. These systems continuously track critical parameters such as flow rates, pressure gradients, temperature stability, and reagent concentrations throughout the screening process. Real-time monitoring enables immediate detection of deviations from established parameters, allowing for prompt corrective actions before entire batches are compromised.
Statistical process control (SPC) methodologies, adapted from manufacturing industries, provide valuable frameworks for evaluating and maintaining quality in microfluidic screening operations. Techniques such as control charts, capability analysis, and design of experiments (DOE) help identify sources of variability and optimize screening parameters. Implementation of SPC approaches has been shown to improve screening reproducibility by up to 30% in large-scale pharmaceutical applications.
Inter-laboratory validation studies represent another crucial aspect of standardization efforts. These collaborative initiatives involve multiple facilities performing identical screening protocols to assess reproducibility across different laboratory environments. The Microfluidic Standardization Consortium, established in 2018, has coordinated several such studies, resulting in the development of industry-wide best practices and performance benchmarks.
Regulatory considerations increasingly influence standardization practices in microfluidic screening, particularly for applications in clinical diagnostics and pharmaceutical development. Compliance with standards such as ISO 13485 for medical devices and Good Laboratory Practice (GLP) guidelines ensures that screening methodologies meet established quality requirements and can withstand regulatory scrutiny.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!