Ion Pairing in HPLC vs UPLC: Which Is More Effective?
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion Pairing Chromatography Background and Objectives
Ion pairing chromatography emerged in the 1970s as a specialized technique for separating ionic and highly polar compounds that traditionally exhibited poor retention in conventional reversed-phase liquid chromatography. The fundamental principle involves the addition of an ion-pairing reagent to the mobile phase, which forms an ion pair with analytes of opposite charge, effectively increasing their hydrophobicity and improving chromatographic retention.
The evolution of this technique has been closely tied to the broader development of liquid chromatography systems. High-Performance Liquid Chromatography (HPLC) represented the standard analytical platform for decades, while Ultra-Performance Liquid Chromatography (UPLC) emerged in the early 2000s as a significant technological advancement, featuring sub-2μm particles and systems capable of withstanding much higher pressures.
Ion pairing agents have traditionally included alkylsulfonates for basic compounds and tetraalkylammonium salts for acidic compounds. The mechanism involves either the formation of neutral ion pairs in solution or the dynamic modification of the stationary phase through adsorption of the pairing agent, creating a charged surface that interacts with oppositely charged analytes.
The pharmaceutical industry has been a primary driver of ion pairing chromatography development, particularly for the analysis of drug substances, metabolites, and impurities. Environmental analysis represents another significant application area, especially for the determination of ionic pesticides, herbicides, and various water contaminants.
Recent technological trends have focused on developing more environmentally friendly ion pairing agents, reducing mobile phase additives, and enhancing compatibility with mass spectrometric detection. The shift toward green analytical chemistry has prompted research into alternative approaches that maintain separation efficiency while minimizing the use of toxic reagents.
The primary objective of this technical assessment is to comprehensively evaluate the relative effectiveness of ion pairing techniques in HPLC versus UPLC systems. Specifically, we aim to determine which platform offers superior performance in terms of separation efficiency, sensitivity, resolution, analysis time, and robustness when employing ion pairing methodologies.
Additionally, this investigation seeks to identify the optimal conditions for ion pairing in each system, including the most effective ion pairing reagents, concentration ranges, pH considerations, and mobile phase compositions. The assessment will also explore how the fundamental differences in column technology, particle size, and system pressure capabilities between HPLC and UPLC impact ion pairing mechanisms and overall chromatographic performance.
The ultimate goal is to provide evidence-based recommendations for analytical scientists facing the choice between HPLC and UPLC platforms for ion pairing applications across various industries, including pharmaceutical analysis, environmental monitoring, food safety, and clinical diagnostics.
The evolution of this technique has been closely tied to the broader development of liquid chromatography systems. High-Performance Liquid Chromatography (HPLC) represented the standard analytical platform for decades, while Ultra-Performance Liquid Chromatography (UPLC) emerged in the early 2000s as a significant technological advancement, featuring sub-2μm particles and systems capable of withstanding much higher pressures.
Ion pairing agents have traditionally included alkylsulfonates for basic compounds and tetraalkylammonium salts for acidic compounds. The mechanism involves either the formation of neutral ion pairs in solution or the dynamic modification of the stationary phase through adsorption of the pairing agent, creating a charged surface that interacts with oppositely charged analytes.
The pharmaceutical industry has been a primary driver of ion pairing chromatography development, particularly for the analysis of drug substances, metabolites, and impurities. Environmental analysis represents another significant application area, especially for the determination of ionic pesticides, herbicides, and various water contaminants.
Recent technological trends have focused on developing more environmentally friendly ion pairing agents, reducing mobile phase additives, and enhancing compatibility with mass spectrometric detection. The shift toward green analytical chemistry has prompted research into alternative approaches that maintain separation efficiency while minimizing the use of toxic reagents.
The primary objective of this technical assessment is to comprehensively evaluate the relative effectiveness of ion pairing techniques in HPLC versus UPLC systems. Specifically, we aim to determine which platform offers superior performance in terms of separation efficiency, sensitivity, resolution, analysis time, and robustness when employing ion pairing methodologies.
Additionally, this investigation seeks to identify the optimal conditions for ion pairing in each system, including the most effective ion pairing reagents, concentration ranges, pH considerations, and mobile phase compositions. The assessment will also explore how the fundamental differences in column technology, particle size, and system pressure capabilities between HPLC and UPLC impact ion pairing mechanisms and overall chromatographic performance.
The ultimate goal is to provide evidence-based recommendations for analytical scientists facing the choice between HPLC and UPLC platforms for ion pairing applications across various industries, including pharmaceutical analysis, environmental monitoring, food safety, and clinical diagnostics.
Market Applications and Analytical Demands
The pharmaceutical industry represents the largest market segment for ion pairing chromatography techniques, with a significant demand for analyzing complex drug formulations, metabolites, and impurities. The global pharmaceutical analytical testing market, valued at approximately $5.6 billion in 2022, is projected to grow at a CAGR of 8.4% through 2030, driving increased adoption of advanced separation techniques including both HPLC and UPLC with ion pairing capabilities.
Environmental monitoring constitutes another critical application area, where regulatory agencies require increasingly sensitive detection of ionic compounds in water, soil, and air samples. The environmental testing market, particularly for detecting perfluoroalkyl substances (PFAS) and other persistent ionic pollutants, has seen compound annual growth exceeding 7% since 2018, with ion pairing techniques becoming essential analytical tools.
The food and beverage industry presents growing analytical demands for ion pairing techniques, particularly in quality control processes requiring the separation and quantification of additives, preservatives, and natural ionic compounds. This sector values both the robustness of HPLC and the speed advantages of UPLC, depending on specific application requirements and throughput demands.
Clinical diagnostics represents an emerging application area with stringent requirements for both accuracy and turnaround time. The analysis of biological fluids for biomarkers, therapeutic drug monitoring, and metabolomics studies increasingly relies on ion pairing techniques, with UPLC gaining preference due to its compatibility with mass spectrometry and reduced sample volume requirements.
Contract research organizations (CROs) and analytical service providers face unique demands that balance analytical performance with economic considerations. These organizations typically maintain both HPLC and UPLC capabilities with ion pairing functionality to address diverse client requirements, though the trend shows increasing investment in UPLC systems due to their higher sample throughput capabilities and reduced solvent consumption.
Academic and research institutions represent significant users of both techniques, with requirements that often prioritize flexibility and adaptability over throughput. These institutions frequently employ ion pairing in method development for novel compounds and in fundamental studies of separation mechanisms, contributing valuable insights that drive innovation in both HPLC and UPLC applications.
The biopharmaceutical sector presents perhaps the most demanding analytical challenges, requiring separation of complex biomolecules including oligonucleotides, peptides, and proteins. This sector has shown the strongest preference shift toward UPLC with ion pairing, driven by requirements for higher resolution, sensitivity, and compatibility with mass spectrometric detection.
Environmental monitoring constitutes another critical application area, where regulatory agencies require increasingly sensitive detection of ionic compounds in water, soil, and air samples. The environmental testing market, particularly for detecting perfluoroalkyl substances (PFAS) and other persistent ionic pollutants, has seen compound annual growth exceeding 7% since 2018, with ion pairing techniques becoming essential analytical tools.
The food and beverage industry presents growing analytical demands for ion pairing techniques, particularly in quality control processes requiring the separation and quantification of additives, preservatives, and natural ionic compounds. This sector values both the robustness of HPLC and the speed advantages of UPLC, depending on specific application requirements and throughput demands.
Clinical diagnostics represents an emerging application area with stringent requirements for both accuracy and turnaround time. The analysis of biological fluids for biomarkers, therapeutic drug monitoring, and metabolomics studies increasingly relies on ion pairing techniques, with UPLC gaining preference due to its compatibility with mass spectrometry and reduced sample volume requirements.
Contract research organizations (CROs) and analytical service providers face unique demands that balance analytical performance with economic considerations. These organizations typically maintain both HPLC and UPLC capabilities with ion pairing functionality to address diverse client requirements, though the trend shows increasing investment in UPLC systems due to their higher sample throughput capabilities and reduced solvent consumption.
Academic and research institutions represent significant users of both techniques, with requirements that often prioritize flexibility and adaptability over throughput. These institutions frequently employ ion pairing in method development for novel compounds and in fundamental studies of separation mechanisms, contributing valuable insights that drive innovation in both HPLC and UPLC applications.
The biopharmaceutical sector presents perhaps the most demanding analytical challenges, requiring separation of complex biomolecules including oligonucleotides, peptides, and proteins. This sector has shown the strongest preference shift toward UPLC with ion pairing, driven by requirements for higher resolution, sensitivity, and compatibility with mass spectrometric detection.
Current State of Ion Pairing in HPLC and UPLC
Ion pairing techniques have evolved significantly in both HPLC and UPLC systems over the past decade. Currently, ion pairing in HPLC remains widely utilized across pharmaceutical, environmental, and food safety applications, with established methodologies and extensive literature support. The technique employs ion-pairing reagents such as tetrabutylammonium salts for acidic analytes and alkylsulfonates for basic compounds, with trifluoroacetic acid (TFA) being particularly common in peptide and protein analysis.
In contrast, UPLC-based ion pairing represents a more recent development, leveraging sub-2μm particle columns and higher pressure systems (up to 15,000 psi compared to HPLC's typical 6,000 psi). This configuration has demonstrated superior efficiency in ion-pairing separations, with typical run times reduced by 3-5 times while maintaining or improving resolution. Recent studies indicate UPLC ion pairing achieves detection limits approximately 2-3 times lower than comparable HPLC methods.
The current technical landscape shows UPLC ion pairing offering significant advantages in speed and sensitivity, particularly critical for high-throughput environments and trace analysis. However, HPLC ion pairing maintains relevance due to its accessibility, established protocols, and lower equipment costs. The installed base of HPLC systems globally remains approximately 5-7 times larger than UPLC systems, influencing adoption rates of respective ion pairing techniques.
Recent innovations in ion pairing reagents have focused on MS-compatible options for both platforms. Volatile perfluorinated carboxylic acids like pentafluoropropionic acid (PFPA) and heptafluorobutyric acid (HFBA) have gained prominence, particularly in UPLC applications where they complement the platform's inherent sensitivity advantages. These reagents demonstrate reduced ion suppression effects compared to traditional options like TFA.
Geographically, North America and Europe lead in UPLC ion pairing adoption, while HPLC ion pairing maintains stronger presence in developing markets. The pharmaceutical sector has embraced UPLC ion pairing most aggressively, with approximately 65% of new method developments utilizing this approach, compared to continued HPLC dominance in academic and routine testing environments.
Technical challenges persist in both platforms. HPLC ion pairing suffers from longer equilibration times (typically 10-15 column volumes) and potential for irreversible column modification. UPLC ion pairing, while faster, presents challenges in method transfer from existing HPLC protocols and requires more sophisticated instrumentation and maintenance. Both techniques continue to face compatibility issues with mass spectrometry, though recent developments in reagent chemistry have partially addressed these limitations.
In contrast, UPLC-based ion pairing represents a more recent development, leveraging sub-2μm particle columns and higher pressure systems (up to 15,000 psi compared to HPLC's typical 6,000 psi). This configuration has demonstrated superior efficiency in ion-pairing separations, with typical run times reduced by 3-5 times while maintaining or improving resolution. Recent studies indicate UPLC ion pairing achieves detection limits approximately 2-3 times lower than comparable HPLC methods.
The current technical landscape shows UPLC ion pairing offering significant advantages in speed and sensitivity, particularly critical for high-throughput environments and trace analysis. However, HPLC ion pairing maintains relevance due to its accessibility, established protocols, and lower equipment costs. The installed base of HPLC systems globally remains approximately 5-7 times larger than UPLC systems, influencing adoption rates of respective ion pairing techniques.
Recent innovations in ion pairing reagents have focused on MS-compatible options for both platforms. Volatile perfluorinated carboxylic acids like pentafluoropropionic acid (PFPA) and heptafluorobutyric acid (HFBA) have gained prominence, particularly in UPLC applications where they complement the platform's inherent sensitivity advantages. These reagents demonstrate reduced ion suppression effects compared to traditional options like TFA.
Geographically, North America and Europe lead in UPLC ion pairing adoption, while HPLC ion pairing maintains stronger presence in developing markets. The pharmaceutical sector has embraced UPLC ion pairing most aggressively, with approximately 65% of new method developments utilizing this approach, compared to continued HPLC dominance in academic and routine testing environments.
Technical challenges persist in both platforms. HPLC ion pairing suffers from longer equilibration times (typically 10-15 column volumes) and potential for irreversible column modification. UPLC ion pairing, while faster, presents challenges in method transfer from existing HPLC protocols and requires more sophisticated instrumentation and maintenance. Both techniques continue to face compatibility issues with mass spectrometry, though recent developments in reagent chemistry have partially addressed these limitations.
Comparative Analysis of Ion Pairing Methodologies
01 Ion pairing mechanisms in HPLC and UPLC
Ion pairing techniques enhance chromatographic separation of charged analytes by forming neutral complexes with oppositely charged reagents. In both HPLC and UPLC, ion pairing agents modify retention behavior by neutralizing charges on target compounds. However, UPLC's smaller particle size and higher pressure systems allow for more efficient ion pair formation and faster equilibration, resulting in improved peak shapes and resolution compared to traditional HPLC methods.- Ion pairing mechanisms in HPLC vs UPLC: Ion pairing techniques function differently in HPLC compared to UPLC due to the fundamental differences in column technology and pressure systems. In UPLC, the smaller particle size and higher pressure allow for more efficient ion pairing interactions, resulting in improved separation of ionic compounds. The mechanism involves the formation of neutral ion pairs between analytes and reagents, which can be more precisely controlled in UPLC systems due to the enhanced kinetics and reduced diffusion effects.
- Efficiency of ion pairing reagents in different chromatography systems: The effectiveness of ion pairing reagents varies significantly between HPLC and UPLC systems. UPLC typically requires lower concentrations of ion pairing reagents to achieve comparable or superior results compared to HPLC. This is attributed to the enhanced mass transfer and reduced peak broadening in UPLC columns. Common ion pairing reagents such as trifluoroacetic acid, heptafluorobutyric acid, and tetrabutylammonium salts demonstrate different optimal concentration ranges and separation efficiencies between the two chromatographic platforms.
- Resolution and sensitivity improvements in ion pair UPLC: UPLC systems offer significant advantages over HPLC for ion pairing applications, particularly in terms of resolution and sensitivity. The sub-2μm particle size columns used in UPLC provide enhanced peak capacity and improved signal-to-noise ratios when using ion pairing techniques. Studies have demonstrated that ion pair UPLC methods can achieve up to 3-5 times better resolution and 2-10 times higher sensitivity compared to traditional HPLC methods for the same ionic analytes, making UPLC particularly valuable for complex sample matrices and trace analysis.
- Method transfer considerations between ion pair HPLC and UPLC: Transferring ion pairing methods from HPLC to UPLC requires careful optimization of several parameters. The reduced column dimensions and particle size in UPLC necessitate adjustments to flow rates, injection volumes, and gradient profiles. Additionally, the concentration and type of ion pairing reagents often need modification when transitioning between platforms. Temperature control becomes more critical in UPLC ion pairing methods due to the higher sensitivity of the system to thermal fluctuations. Successful method transfer requires systematic evaluation of these parameters to maintain or improve separation efficiency.
- Applications and limitations of ion pairing in advanced chromatography: Ion pairing techniques in both HPLC and UPLC have specific applications and limitations across various analytical fields. UPLC ion pairing shows superior performance for pharmaceutical analysis, particularly for charged drug molecules and their metabolites. However, UPLC ion pairing methods may face challenges with column longevity due to strong adsorption of ion pairing reagents to the stationary phase. HPLC ion pairing remains valuable for routine analysis where extreme resolution is not required, offering greater robustness and lower system pressure requirements. The choice between platforms depends on specific analytical goals, sample complexity, and required throughput.
02 Efficiency comparison between UPLC and HPLC for ion pair chromatography
UPLC demonstrates superior efficiency in ion pair chromatography compared to HPLC due to its sub-2μm particles and higher operating pressures. This results in faster analysis times, improved resolution, and enhanced sensitivity when using ion pairing techniques. UPLC systems can achieve equivalent or better separations with reduced ion pairing reagent concentrations, minimizing system contamination and equilibration times while maintaining separation quality for charged compounds.Expand Specific Solutions03 Optimization of ion pairing reagents for UPLC applications
The selection and optimization of ion pairing reagents is critical for UPLC performance. Lower concentrations of volatile ion pairing agents like trifluoroacetic acid and heptafluorobutyric acid are preferred in UPLC to prevent system contamination while maintaining separation efficiency. The reduced column dimensions in UPLC require careful adjustment of ion pairing agent concentration, pH, and organic modifier content to achieve optimal chromatographic performance while minimizing equilibration times and system backpressure.Expand Specific Solutions04 Method transfer considerations from HPLC to UPLC for ion pair methods
Transferring ion pairing methods from HPLC to UPLC requires systematic adjustment of several parameters. The concentration of ion pairing reagents typically needs reduction in UPLC due to the higher efficiency of smaller particles. Gradient profiles must be scaled appropriately to account for the faster mass transfer and reduced column volume. Additionally, detection parameters require optimization due to narrower peaks and higher sensitivity in UPLC systems. Successful method transfer results in significantly reduced analysis time while maintaining or improving separation quality.Expand Specific Solutions05 Applications benefiting from ion pairing in UPLC versus HPLC
Ion pairing in UPLC offers particular advantages for pharmaceutical analysis, metabolomics, and environmental testing where speed and sensitivity are critical. The enhanced efficiency of UPLC ion pairing methods enables better separation of complex biological samples containing ionic compounds, improved detection of trace contaminants, and faster quality control testing. The combination of UPLC with mass spectrometry is especially powerful when using volatile ion pairing agents, providing superior detection limits compared to traditional HPLC methods while maintaining chromatographic resolution of charged analytes.Expand Specific Solutions
Leading Manufacturers and Research Institutions
Ion pairing chromatography in HPLC and UPLC is evolving rapidly within the analytical chemistry market, currently in a growth phase with increasing adoption across pharmaceutical and biotechnology sectors. The global chromatography market is expanding at approximately 6-8% annually, with ion pairing techniques representing a significant segment. Waters Technology Corp. leads the UPLC technology space with their advanced systems offering superior resolution and speed, while companies like IDEX Health & Science and Micromass UK provide specialized components enhancing ion pairing applications. Established pharmaceutical firms including Biocon, Dr. Reddy's, and Wyeth utilize these techniques extensively in drug development. Academic institutions such as MIT and Oxford University Innovation contribute significant research advancing ion pairing methodologies, particularly in addressing complex separation challenges where UPLC demonstrates superior efficiency for volatile ion-pairing reagents.
IDEX Health & Science LLC
Technical Solution: IDEX Health & Science has developed specialized fluidic components and systems optimized for both HPLC and UPLC ion pairing applications. Their technology focuses on the unique challenges of handling ion pairing reagents, which can be corrosive and require specialized materials. For UPLC applications, IDEX has engineered high-pressure fluidic components capable of withstanding pressures up to 20,000 psi while maintaining chemical compatibility with common ion pairing reagents like perfluorinated carboxylic acids and alkylsulfonates. Their comparative studies on ion pairing effectiveness show that their UPLC-optimized components enable more efficient mixing of ion pairing reagents with mobile phases, resulting in more consistent retention time reproducibility (typically <0.1% RSD compared to 0.3-0.5% in conventional HPLC systems). IDEX's specialized connection systems minimize dead volume, which is particularly critical for maintaining the narrow peak widths achieved in UPLC ion pairing applications.
Strengths: Superior chemical compatibility with corrosive ion pairing reagents; ultra-low dispersion fluidic paths; enhanced pressure capabilities for UPLC applications; improved mixing efficiency for consistent ion pairing performance. Weaknesses: Higher cost components; more stringent installation requirements; limited backward compatibility with older HPLC systems; requires more precise system optimization.
Waters Technology Corp.
Technical Solution: Waters Technology has pioneered significant advancements in both HPLC and UPLC technologies with specific focus on ion pairing applications. Their ACQUITY UPLC system utilizes sub-2μm particle columns operating at higher pressures (up to 15,000 psi) compared to conventional HPLC (typically 6,000 psi), enabling superior separation of ionic compounds. For ion pairing applications, Waters has developed specialized column chemistries like the ACQUITY UPLC HSS T3 that work effectively with ion pairing reagents such as trifluoroacetic acid and heptafluorobutyric acid. Their technology demonstrates that ion pairing in UPLC provides significantly improved peak capacity, resolution, and sensitivity compared to traditional HPLC methods, with analysis times reduced by up to 9 times while maintaining equivalent resolution. Waters' systems incorporate advanced solvent delivery systems specifically designed to handle the precision requirements of ion pairing reagents at UPLC pressures.
Strengths: Superior resolution and sensitivity for ionic compounds; significantly faster analysis times; reduced solvent consumption (up to 95% less); enhanced method transferability from HPLC to UPLC platforms. Weaknesses: Higher system backpressure requirements; more stringent system cleanliness needs; potentially higher initial investment costs; some ion pairing reagents may cause issues with MS detection.
Key Patents and Scientific Literature Review
Packed bed
PatentInactiveEP3435078A3
Innovation
- A packed bed design featuring a cylindrical housing with integral ends that axially retain and secure packed bed components using crimpable fingers, minimizing internal swept volume and allowing for high-pressure sealing with polymer male fittings, such as PEEK, to form a zero-dead-volume connection.
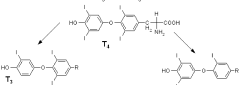
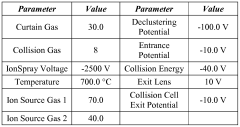
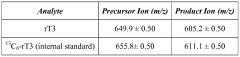
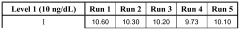
Methods for detecting reverse triiodothyronine by mass spectrometry
PatentWO2013085818A1
Innovation
- The method involves ionizing rT3 from body fluid samples using mass spectrometry, potentially after protein precipitation and liquid chromatography, without prior solid phase extraction, to detect and quantify rT3 using tandem mass spectrometry, with the option of using internal standards and specific ionization modes like electrospray ionization.
Method Transfer Considerations Between HPLC and UPLC
When transferring analytical methods between HPLC and UPLC systems, several critical considerations must be addressed to ensure comparable results. The fundamental differences in column dimensions, particle size, and operating pressures between these technologies necessitate careful adjustment of method parameters.
Column selection represents the primary consideration, as UPLC typically employs sub-2μm particles compared to HPLC's 3-5μm particles. This reduction in particle size significantly impacts separation efficiency and retention characteristics, particularly for ion-pairing applications. When transferring ion-pairing methods, the surface area available for interaction increases substantially in UPLC systems, potentially altering the equilibrium between mobile and stationary phases.
Mobile phase composition requires recalibration during method transfer. Ion-pairing reagent concentrations often need adjustment when moving between platforms, as the increased efficiency of UPLC may require lower concentrations to achieve similar retention. The ratio of organic modifier to aqueous buffer may also require optimization to maintain comparable selectivity while leveraging UPLC's enhanced efficiency.
Gradient profiles demand particular attention during transfer. UPLC systems typically operate with steeper gradients and shorter run times. When transferring ion-pairing methods, gradient slopes must be adjusted proportionally to column dimensions while accounting for system dwell volumes. The reduced extra-column volume in UPLC systems means that gradient delay times differ significantly from HPLC.
Injection volume scaling is essential to prevent column overloading. As UPLC columns have substantially lower loading capacity due to reduced dimensions, injection volumes must be proportionally decreased. For ion-pairing applications, this is particularly important as overloading can disrupt the delicate equilibrium between the ion-pairing reagent, analyte, and stationary phase.
Detection parameters require recalibration during transfer. The narrower peaks produced by UPLC systems necessitate faster detector sampling rates to maintain peak resolution and quantitative accuracy. For ion-paired compounds, this becomes especially important as peak shapes can be significantly affected by the transfer process.
Temperature control represents another critical factor, as UPLC often operates at elevated temperatures to reduce mobile phase viscosity and improve mass transfer. When transferring ion-pairing methods, temperature adjustments must account for potential changes in ionization equilibria and partition coefficients between the phases.
System hardware differences, including mixer volumes, connection tubing dimensions, and detector cell volumes, can significantly impact chromatographic performance during method transfer. These differences become particularly pronounced when working with ion-pairing techniques, where system equilibration times and peak shapes are highly sensitive to extra-column effects.
Column selection represents the primary consideration, as UPLC typically employs sub-2μm particles compared to HPLC's 3-5μm particles. This reduction in particle size significantly impacts separation efficiency and retention characteristics, particularly for ion-pairing applications. When transferring ion-pairing methods, the surface area available for interaction increases substantially in UPLC systems, potentially altering the equilibrium between mobile and stationary phases.
Mobile phase composition requires recalibration during method transfer. Ion-pairing reagent concentrations often need adjustment when moving between platforms, as the increased efficiency of UPLC may require lower concentrations to achieve similar retention. The ratio of organic modifier to aqueous buffer may also require optimization to maintain comparable selectivity while leveraging UPLC's enhanced efficiency.
Gradient profiles demand particular attention during transfer. UPLC systems typically operate with steeper gradients and shorter run times. When transferring ion-pairing methods, gradient slopes must be adjusted proportionally to column dimensions while accounting for system dwell volumes. The reduced extra-column volume in UPLC systems means that gradient delay times differ significantly from HPLC.
Injection volume scaling is essential to prevent column overloading. As UPLC columns have substantially lower loading capacity due to reduced dimensions, injection volumes must be proportionally decreased. For ion-pairing applications, this is particularly important as overloading can disrupt the delicate equilibrium between the ion-pairing reagent, analyte, and stationary phase.
Detection parameters require recalibration during transfer. The narrower peaks produced by UPLC systems necessitate faster detector sampling rates to maintain peak resolution and quantitative accuracy. For ion-paired compounds, this becomes especially important as peak shapes can be significantly affected by the transfer process.
Temperature control represents another critical factor, as UPLC often operates at elevated temperatures to reduce mobile phase viscosity and improve mass transfer. When transferring ion-pairing methods, temperature adjustments must account for potential changes in ionization equilibria and partition coefficients between the phases.
System hardware differences, including mixer volumes, connection tubing dimensions, and detector cell volumes, can significantly impact chromatographic performance during method transfer. These differences become particularly pronounced when working with ion-pairing techniques, where system equilibration times and peak shapes are highly sensitive to extra-column effects.
Environmental and Cost Efficiency Analysis
When comparing the environmental and cost efficiency of ion pairing techniques in HPLC versus UPLC systems, several critical factors emerge that significantly impact laboratory operations and sustainability goals. UPLC demonstrates superior environmental performance through substantially reduced solvent consumption—typically 60-80% less than traditional HPLC methods. This reduction directly translates to decreased hazardous waste generation and lower disposal costs, which can represent significant expenditure for analytical laboratories.
The environmental footprint comparison reveals UPLC's advantage in energy consumption as well. UPLC systems generally operate with shorter run times (often 5-10 times faster than conventional HPLC), resulting in reduced power requirements per analysis. Quantitative assessments indicate that a typical UPLC method consuming 0.3-0.5 mL/min of mobile phase versus 1-2 mL/min for HPLC represents not only resource conservation but also reduced greenhouse gas emissions associated with solvent production and disposal.
From a cost efficiency perspective, ion pairing in UPLC offers compelling advantages despite higher initial capital investment. The total cost of ownership analysis demonstrates that UPLC systems typically recover their price premium within 2-3 years through operational savings. These savings derive from reduced solvent purchases (approximately 70-80% reduction), decreased waste management costs, and significantly improved laboratory throughput enabling more analyses per unit time.
Reagent efficiency also favors UPLC ion pairing techniques. The specialized ion pairing reagents required for both techniques are often expensive, but UPLC methods typically require lower concentrations and volumes. For example, a standard ion pairing HPLC method might consume 100-200 mL of mobile phase containing ion pairing reagents per hour of operation, while comparable UPLC methods require only 20-30 mL for the same analytical output.
Labor cost considerations further enhance UPLC's economic advantage. The faster analysis times translate directly to improved laboratory productivity, with technician time per sample reduced by 50-80% compared to HPLC methods. This efficiency allows laboratories to process more samples with existing staff or reallocate human resources to other value-adding activities, creating significant operational cost benefits that compound over time.
The environmental footprint comparison reveals UPLC's advantage in energy consumption as well. UPLC systems generally operate with shorter run times (often 5-10 times faster than conventional HPLC), resulting in reduced power requirements per analysis. Quantitative assessments indicate that a typical UPLC method consuming 0.3-0.5 mL/min of mobile phase versus 1-2 mL/min for HPLC represents not only resource conservation but also reduced greenhouse gas emissions associated with solvent production and disposal.
From a cost efficiency perspective, ion pairing in UPLC offers compelling advantages despite higher initial capital investment. The total cost of ownership analysis demonstrates that UPLC systems typically recover their price premium within 2-3 years through operational savings. These savings derive from reduced solvent purchases (approximately 70-80% reduction), decreased waste management costs, and significantly improved laboratory throughput enabling more analyses per unit time.
Reagent efficiency also favors UPLC ion pairing techniques. The specialized ion pairing reagents required for both techniques are often expensive, but UPLC methods typically require lower concentrations and volumes. For example, a standard ion pairing HPLC method might consume 100-200 mL of mobile phase containing ion pairing reagents per hour of operation, while comparable UPLC methods require only 20-30 mL for the same analytical output.
Labor cost considerations further enhance UPLC's economic advantage. The faster analysis times translate directly to improved laboratory productivity, with technician time per sample reduced by 50-80% compared to HPLC methods. This efficiency allows laboratories to process more samples with existing staff or reallocate human resources to other value-adding activities, creating significant operational cost benefits that compound over time.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!