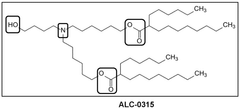
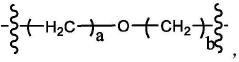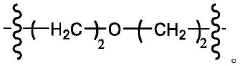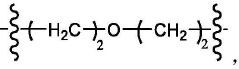Ionizable lipid structure–function relationships for transfection efficacy and manufacturability
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionizable Lipid Evolution and Research Objectives
Ionizable lipids have emerged as critical components in nucleic acid delivery systems, particularly for mRNA therapeutics and vaccines. The evolution of these lipids traces back to the early 1990s when cationic lipids were first utilized for gene delivery. However, these initial formulations faced significant challenges including toxicity and limited efficacy. The breakthrough came with the development of ionizable lipids that maintain a neutral charge at physiological pH but become positively charged in acidic environments, enabling efficient endosomal escape while minimizing systemic toxicity.
The field witnessed transformative advancements with the introduction of DLin-MC3-DMA in 2010, which demonstrated substantially improved potency in hepatic delivery. This milestone catalyzed intensive research into structure-activity relationships, leading to the development of more sophisticated lipids like SM-102 and ALC-0315, which became integral components of the Moderna and Pfizer-BioNTech COVID-19 vaccines, respectively.
Current research objectives focus on elucidating the complex relationship between ionizable lipid structures and their functional performance in nucleic acid delivery systems. Specifically, researchers aim to understand how molecular architecture—including head group composition, linker chemistry, and hydrophobic tail configuration—influences critical parameters such as transfection efficiency, biodistribution, and cellular uptake mechanisms.
Manufacturing considerations represent another crucial research objective. As these technologies transition from laboratory to industrial scale, understanding how structural modifications affect production processes, stability, and batch-to-batch consistency becomes paramount. Researchers are investigating how lipid design can be optimized not only for biological performance but also for scalable, cost-effective manufacturing.
The field is also exploring structure-function relationships that govern immunogenicity profiles, as certain lipid structures may inherently modulate immune responses independent of the payload. This understanding could enable the design of lipids that enhance vaccine efficacy through adjuvant-like properties or, conversely, minimize immune activation for gene therapy applications.
Additionally, research objectives include developing predictive models that correlate molecular structures with functional outcomes, potentially accelerating the discovery process through computational approaches rather than empirical testing alone. Such models would ideally incorporate parameters related to both biological performance and manufacturing feasibility.
The ultimate goal of this research domain is to establish design principles that enable rational engineering of ionizable lipids tailored to specific therapeutic applications, delivery routes, and target tissues, while simultaneously ensuring their commercial viability through robust manufacturing processes.
The field witnessed transformative advancements with the introduction of DLin-MC3-DMA in 2010, which demonstrated substantially improved potency in hepatic delivery. This milestone catalyzed intensive research into structure-activity relationships, leading to the development of more sophisticated lipids like SM-102 and ALC-0315, which became integral components of the Moderna and Pfizer-BioNTech COVID-19 vaccines, respectively.
Current research objectives focus on elucidating the complex relationship between ionizable lipid structures and their functional performance in nucleic acid delivery systems. Specifically, researchers aim to understand how molecular architecture—including head group composition, linker chemistry, and hydrophobic tail configuration—influences critical parameters such as transfection efficiency, biodistribution, and cellular uptake mechanisms.
Manufacturing considerations represent another crucial research objective. As these technologies transition from laboratory to industrial scale, understanding how structural modifications affect production processes, stability, and batch-to-batch consistency becomes paramount. Researchers are investigating how lipid design can be optimized not only for biological performance but also for scalable, cost-effective manufacturing.
The field is also exploring structure-function relationships that govern immunogenicity profiles, as certain lipid structures may inherently modulate immune responses independent of the payload. This understanding could enable the design of lipids that enhance vaccine efficacy through adjuvant-like properties or, conversely, minimize immune activation for gene therapy applications.
Additionally, research objectives include developing predictive models that correlate molecular structures with functional outcomes, potentially accelerating the discovery process through computational approaches rather than empirical testing alone. Such models would ideally incorporate parameters related to both biological performance and manufacturing feasibility.
The ultimate goal of this research domain is to establish design principles that enable rational engineering of ionizable lipids tailored to specific therapeutic applications, delivery routes, and target tissues, while simultaneously ensuring their commercial viability through robust manufacturing processes.
Market Analysis for Lipid-Based Transfection Technologies
The global market for lipid-based transfection technologies has experienced substantial growth in recent years, primarily driven by advancements in gene therapy, vaccine development, and cellular engineering applications. The market value reached approximately $1.2 billion in 2022 and is projected to grow at a CAGR of 14.8% through 2028, potentially reaching $2.7 billion by the end of the forecast period.
The COVID-19 pandemic significantly accelerated market expansion, with lipid nanoparticles (LNPs) becoming the delivery vehicle of choice for mRNA vaccines. This unprecedented global deployment demonstrated the commercial viability and manufacturing scalability of ionizable lipid-based delivery systems, creating a ripple effect across pharmaceutical and biotechnology sectors.
Market segmentation reveals distinct application areas with varying growth trajectories. Research applications currently dominate with approximately 45% market share, followed by therapeutic applications at 35% and vaccine development at 20%. However, therapeutic applications are expected to exhibit the fastest growth rate due to the expanding pipeline of lipid-delivered gene therapies and RNA therapeutics.
Geographically, North America leads the market with 42% share, followed by Europe (28%), Asia-Pacific (22%), and rest of the world (8%). The Asia-Pacific region, particularly China and South Korea, is demonstrating the highest growth rate due to increasing R&D investments and favorable regulatory environments for advanced therapy development.
Key market drivers include the growing pipeline of RNA therapeutics, increasing research funding in genomics and personalized medicine, and technological advancements in lipid chemistry that enhance transfection efficiency and reduce toxicity. The structure-function relationship of ionizable lipids has become a critical focus area as manufacturers seek to optimize both efficacy and manufacturability.
Market restraints include high development costs, complex manufacturing processes, and regulatory uncertainties surrounding novel delivery systems. Additionally, challenges in large-scale production of consistent lipid formulations with precise structure-function properties represent significant barriers to market entry.
Customer demand is increasingly focused on lipid formulations that balance transfection efficacy with manufacturing practicality. End-users are seeking ionizable lipids with predictable structure-function relationships that can be reliably produced at scale while maintaining consistent performance across different therapeutic cargoes and target tissues.
The COVID-19 pandemic significantly accelerated market expansion, with lipid nanoparticles (LNPs) becoming the delivery vehicle of choice for mRNA vaccines. This unprecedented global deployment demonstrated the commercial viability and manufacturing scalability of ionizable lipid-based delivery systems, creating a ripple effect across pharmaceutical and biotechnology sectors.
Market segmentation reveals distinct application areas with varying growth trajectories. Research applications currently dominate with approximately 45% market share, followed by therapeutic applications at 35% and vaccine development at 20%. However, therapeutic applications are expected to exhibit the fastest growth rate due to the expanding pipeline of lipid-delivered gene therapies and RNA therapeutics.
Geographically, North America leads the market with 42% share, followed by Europe (28%), Asia-Pacific (22%), and rest of the world (8%). The Asia-Pacific region, particularly China and South Korea, is demonstrating the highest growth rate due to increasing R&D investments and favorable regulatory environments for advanced therapy development.
Key market drivers include the growing pipeline of RNA therapeutics, increasing research funding in genomics and personalized medicine, and technological advancements in lipid chemistry that enhance transfection efficiency and reduce toxicity. The structure-function relationship of ionizable lipids has become a critical focus area as manufacturers seek to optimize both efficacy and manufacturability.
Market restraints include high development costs, complex manufacturing processes, and regulatory uncertainties surrounding novel delivery systems. Additionally, challenges in large-scale production of consistent lipid formulations with precise structure-function properties represent significant barriers to market entry.
Customer demand is increasingly focused on lipid formulations that balance transfection efficacy with manufacturing practicality. End-users are seeking ionizable lipids with predictable structure-function relationships that can be reliably produced at scale while maintaining consistent performance across different therapeutic cargoes and target tissues.
Current Challenges in Ionizable Lipid Development
Despite significant advancements in ionizable lipid development for nucleic acid delivery, several critical challenges persist that impede optimal transfection efficacy and large-scale manufacturability. The relationship between lipid structure and functional performance remains incompletely understood, creating a substantial barrier to rational design approaches. Current ionizable lipids often exhibit inconsistent transfection efficiency across different cell types and tissues, with particular difficulty penetrating immune cells and crossing the blood-brain barrier.
Manufacturing challenges present equally significant hurdles. The synthesis of complex ionizable lipids frequently involves multi-step processes with low overall yields, making large-scale production economically prohibitive. Quality control issues arise from batch-to-batch variability, affecting the reproducibility of nanoparticle formulations and their subsequent biological performance. The industry lacks standardized analytical methods to comprehensively characterize these lipids and their assembled nanostructures.
Stability concerns further complicate development efforts. Many ionizable lipid formulations demonstrate limited shelf-life under standard storage conditions, with susceptibility to hydrolysis, oxidation, and aggregation. This necessitates cold-chain requirements that increase costs and logistical complexity, particularly problematic for global distribution to resource-limited regions.
Toxicity profiles represent another significant challenge. Current ionizable lipids can trigger immune responses, including cytokine release and complement activation. Accumulation in the liver and potential hepatotoxicity remain concerns for repeated administration scenarios. The molecular mechanisms underlying these adverse effects are not fully elucidated, complicating efforts to design safer alternatives.
Regulatory hurdles add complexity to the development pathway. Novel ionizable lipids face stringent safety assessment requirements, with limited precedent for approval pathways. The lack of established in vitro models that reliably predict in vivo performance necessitates extensive animal testing, increasing development timelines and costs.
The scalability gap between laboratory synthesis and industrial production represents a critical bottleneck. Methods that work effectively at small scale often encounter significant challenges during scale-up, including heat transfer limitations, mixing inefficiencies, and purification complications. This disconnect frequently necessitates complete reformulation of synthetic routes when moving toward commercial production.
Addressing these multifaceted challenges requires interdisciplinary approaches combining medicinal chemistry, pharmaceutical sciences, bioengineering, and manufacturing technology. Systematic structure-activity relationship studies, coupled with advanced computational modeling and high-throughput screening methodologies, offer promising avenues for overcoming these obstacles and realizing the full potential of ionizable lipids in nucleic acid delivery applications.
Manufacturing challenges present equally significant hurdles. The synthesis of complex ionizable lipids frequently involves multi-step processes with low overall yields, making large-scale production economically prohibitive. Quality control issues arise from batch-to-batch variability, affecting the reproducibility of nanoparticle formulations and their subsequent biological performance. The industry lacks standardized analytical methods to comprehensively characterize these lipids and their assembled nanostructures.
Stability concerns further complicate development efforts. Many ionizable lipid formulations demonstrate limited shelf-life under standard storage conditions, with susceptibility to hydrolysis, oxidation, and aggregation. This necessitates cold-chain requirements that increase costs and logistical complexity, particularly problematic for global distribution to resource-limited regions.
Toxicity profiles represent another significant challenge. Current ionizable lipids can trigger immune responses, including cytokine release and complement activation. Accumulation in the liver and potential hepatotoxicity remain concerns for repeated administration scenarios. The molecular mechanisms underlying these adverse effects are not fully elucidated, complicating efforts to design safer alternatives.
Regulatory hurdles add complexity to the development pathway. Novel ionizable lipids face stringent safety assessment requirements, with limited precedent for approval pathways. The lack of established in vitro models that reliably predict in vivo performance necessitates extensive animal testing, increasing development timelines and costs.
The scalability gap between laboratory synthesis and industrial production represents a critical bottleneck. Methods that work effectively at small scale often encounter significant challenges during scale-up, including heat transfer limitations, mixing inefficiencies, and purification complications. This disconnect frequently necessitates complete reformulation of synthetic routes when moving toward commercial production.
Addressing these multifaceted challenges requires interdisciplinary approaches combining medicinal chemistry, pharmaceutical sciences, bioengineering, and manufacturing technology. Systematic structure-activity relationship studies, coupled with advanced computational modeling and high-throughput screening methodologies, offer promising avenues for overcoming these obstacles and realizing the full potential of ionizable lipids in nucleic acid delivery applications.
Structure-Function Relationship Analysis Methods
01 Ionizable lipid structure optimization for transfection efficacy
The molecular structure of ionizable lipids significantly impacts their transfection efficacy. Modifications to the headgroup, linker region, and hydrophobic tails can enhance the ability of lipid nanoparticles to deliver nucleic acids into cells. Specific structural features such as tertiary amines that become protonated at endosomal pH, optimized pKa values, and balanced hydrophobicity contribute to improved cellular uptake, endosomal escape, and overall transfection efficiency.- Ionizable lipid structure optimization for transfection: The molecular structure of ionizable lipids significantly impacts transfection efficacy. Modifications to the lipid head group, hydrophobic tails, and linker regions can enhance nucleic acid encapsulation and cellular uptake. Specific structural features like the pKa value, tail length, and degree of unsaturation affect the lipid's ability to form stable nanoparticles and facilitate endosomal escape, which are critical for successful transfection.
- Lipid nanoparticle formulation techniques: Various formulation techniques can improve the manufacturability and transfection efficacy of ionizable lipid-based delivery systems. Methods such as microfluidic mixing, ethanol injection, and thin-film hydration affect particle size distribution, polydispersity, and encapsulation efficiency. The ratio of ionizable lipids to helper lipids (like cholesterol, phospholipids, and PEG-lipids) plays a crucial role in optimizing the formulation for specific nucleic acid cargo and target tissues.
- pH-responsive mechanisms for enhanced delivery: Ionizable lipids with optimized pH-responsive properties show improved transfection efficacy. These lipids remain neutral at physiological pH to enhance circulation time but become positively charged in the acidic endosomal environment, facilitating membrane disruption and cargo release. Engineering the pKa value of ionizable lipids is critical for balancing serum stability with efficient intracellular delivery, ultimately improving the therapeutic index of nucleic acid-based treatments.
- Scalable manufacturing processes: Developing scalable manufacturing processes for ionizable lipid-based delivery systems is essential for clinical translation. Continuous processing methods, quality-by-design approaches, and process analytical technologies help maintain consistent product quality during scale-up. Critical process parameters such as mixing speed, temperature, and solvent removal techniques significantly impact the physicochemical properties of the final product and its transfection performance.
- Stability enhancement strategies: Various strategies can enhance the stability and shelf-life of ionizable lipid formulations, which directly impacts their manufacturability and transfection efficacy. Lyophilization with appropriate cryoprotectants, buffer optimization, and antioxidant addition can prevent particle aggregation and lipid degradation during storage. Surface modification techniques, such as PEGylation or incorporation of targeting ligands, can improve colloidal stability while maintaining or enhancing transfection efficiency in specific cell types.
02 Formulation strategies for lipid nanoparticle stability and manufacturability
Various formulation approaches can enhance the stability and manufacturability of ionizable lipid-based delivery systems. These include optimizing the ratio of ionizable lipids to helper lipids (such as cholesterol, phospholipids, and PEG-lipids), selecting appropriate buffers and pH conditions, and developing scalable production methods like microfluidic mixing or rapid precipitation. These strategies help maintain consistent particle size, prevent aggregation, and ensure batch-to-batch reproducibility during large-scale manufacturing.Expand Specific Solutions03 Novel ionizable lipid compositions for enhanced delivery
Innovative ionizable lipid compositions have been developed to overcome delivery challenges. These include lipids with biodegradable linkages, pH-responsive elements, and targeting moieties. Some compositions incorporate multiple ionizable groups, branched structures, or cyclic components to improve nucleic acid complexation and release. These novel compositions aim to enhance transfection efficiency while reducing cytotoxicity and improving the therapeutic index of nucleic acid-based treatments.Expand Specific Solutions04 Process optimization for large-scale production of lipid nanoparticles
Manufacturing processes for ionizable lipid nanoparticles can be optimized to improve scalability and consistency. Techniques such as continuous flow manufacturing, controlled mixing parameters, and post-formation purification methods help maintain critical quality attributes during scale-up. Process parameters including temperature, solvent selection, mixing speed, and flow rates significantly impact particle characteristics and transfection efficiency. Advanced analytical methods enable real-time monitoring and control of these parameters during production.Expand Specific Solutions05 Application-specific ionizable lipid designs for targeted delivery
Ionizable lipids can be specifically designed for particular therapeutic applications or delivery challenges. These designs may incorporate tissue-targeting ligands, cell-penetrating peptides, or environmentally responsive elements. For example, some ionizable lipids are optimized for crossing the blood-brain barrier, others for hepatic delivery, and still others for immune cell targeting. These application-specific designs consider the unique biological barriers and cellular uptake mechanisms relevant to each therapeutic context.Expand Specific Solutions
Leading Organizations in Lipid-Based Gene Delivery
The ionizable lipid structure-function relationship for transfection efficacy and manufacturability is currently in a growth phase, with the global lipid nanoparticle (LNP) market expanding rapidly following COVID-19 vaccine successes. Key players include established pharmaceutical companies like Bristol Myers Squibb and BioNTech, alongside specialized RNA therapeutics developers such as Translate Bio, Orna Therapeutics, and Arcturus Therapeutics. Academic institutions (MIT, University of Toronto) contribute significant foundational research, while emerging companies like Rongcan Biomedical and Tessera Therapeutics focus on novel delivery technologies. The technology is approaching commercial maturity for liver-targeted applications, though extrahepatic delivery remains less developed, creating competitive opportunities for companies developing tissue-specific LNP formulations.
Orna Therapeutics, Inc.
Technical Solution: Orna Therapeutics has developed specialized ionizable lipid formulations optimized for their circular RNA (oRNA) technology. Their approach focuses on designing ionizable lipids with structural features that accommodate the unique topological constraints of circular RNA molecules. Orna's lipid systems incorporate ionizable head groups with pKa values carefully tuned to the physiological pH gradient (typically 6.3-6.7) to maximize endosomal escape efficiency. Their manufacturing platform employs proprietary microfluidic mixing techniques that maintain RNA structural integrity while achieving high encapsulation rates (>90%). The company has engineered lipid structures with optimized hydrophobic tail configurations that enhance membrane fusion capabilities specifically for circular RNA cargo, which has different charge density and conformational properties compared to linear mRNA. Orna's lipid formulations also incorporate specialized helper lipids that work synergistically with their ionizable components to enhance cellular uptake and endosomal escape.
Strengths: Specialized formulations optimized for circular RNA delivery; enhanced stability profiles compared to conventional mRNA delivery systems; potential for improved manufacturing efficiency due to the inherent stability of circular RNA. Weaknesses: Relatively new technology with limited clinical validation; may require specialized manufacturing considerations for circular RNA compatibility; potential regulatory complexities as a novel therapeutic modality.
Nitto Denko Corp.
Technical Solution: Nitto Denko has developed a proprietary lipid nanoparticle (LNP) platform featuring novel ionizable lipids specifically engineered for targeted delivery to fibrotic tissues. Their technology incorporates vitamin A-coupled lipids that leverage the retinol binding protein pathway for hepatic stellate cell targeting in liver fibrosis applications. The company's ionizable lipids feature optimized head groups with precisely tuned pKa values (typically 6.2-6.8) that enable efficient endosomal escape while maintaining serum stability. Nitto Denko's manufacturing process employs controlled ethanol injection techniques that produce consistent particle sizes (80-120nm) with narrow size distributions. Their lipid structures incorporate biodegradable ester linkages positioned strategically within the hydrophobic domain to balance stability during circulation with intracellular degradability. The company has demonstrated that modifications to the lipid tail structure, particularly incorporating branched chains with specific stereochemistry, significantly impact transfection efficiency across different cell types.
Strengths: Specialized targeting capabilities for fibrotic tissues; established manufacturing processes with demonstrated scalability; extensive preclinical validation across multiple disease models. Weaknesses: More limited application range compared to some competitors; potential challenges with complex manufacturing of specialized targeting lipids; may require disease-specific formulation optimization.
Critical Patents in Ionizable Lipid Design
Ionizable lipid compound having high transfection efficiency and use thereof
PatentWO2024067639A1
Innovation
- A novel ionizable lipid compound with a nitrogen atom as the charge center, a hydrophilic group as the head, and two hydrophobic groups as the tail, and a -(C=O)O- and carbonate bond introduced between the nitrogen atom and the hydrophobic groups, is used to enhance the ability to disrupt endosomes in acidic environments.
Ionizable lipids and lipid nanoparticles containing thereof
PatentWO2025157953A1
Innovation
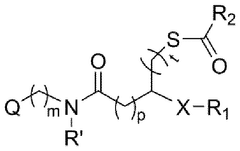
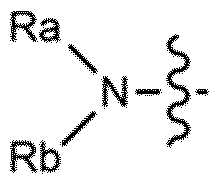
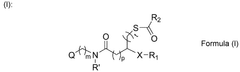
- Development of ionizable lipids with a thioester and amide moiety, such as those in Formula (I), which enhance LNP stability and biodegradability, resulting in improved transfection rates and reduced cytotoxicity.
Scalable Manufacturing Processes for Lipid Nanoparticles
The scalable manufacturing of lipid nanoparticles (LNPs) represents a critical challenge in translating ionizable lipid technologies from laboratory discoveries to commercial therapeutics. Current manufacturing processes must balance the need for consistent particle size distribution, encapsulation efficiency, and stability while maintaining the structure-function relationships that drive transfection efficacy.
Microfluidic mixing has emerged as the gold standard for LNP production, offering superior control over mixing parameters compared to conventional methods. This technology enables precise manipulation of lipid and nucleic acid streams, resulting in reproducible nanoparticle formation through controlled nanoprecipitation. Advanced microfluidic platforms now incorporate in-line monitoring capabilities, allowing real-time adjustment of critical process parameters to maintain quality attributes.
Continuous flow manufacturing represents a significant advancement for large-scale LNP production. Unlike batch processes, continuous systems minimize batch-to-batch variability while increasing throughput. Recent innovations in this area include modular manufacturing platforms that can be scaled from preclinical to commercial production without significant process redesign. These systems maintain critical mixing parameters across different production volumes, preserving the structure-function relationships of ionizable lipids.
Purification and downstream processing present unique challenges for maintaining LNP integrity. Tangential flow filtration has demonstrated advantages over conventional dialysis methods, reducing processing time while effectively removing organic solvents and unencapsulated materials. The development of single-use technologies has further streamlined manufacturing workflows, reducing cross-contamination risks and cleaning validation requirements.
Lyophilization processes have been optimized to enhance LNP stability during storage and distribution. The selection of appropriate cryoprotectants and lyoprotectants is crucial to prevent lipid phase transitions and maintain the ionizable lipid's structural integrity. Recent advances in controlled nucleation technologies have improved batch homogeneity during freeze-drying, resulting in more consistent reconstitution properties.
Quality control strategies have evolved to include advanced analytical techniques for monitoring critical quality attributes throughout the manufacturing process. These include dynamic light scattering for particle size distribution, zeta potential measurements for surface charge characterization, and high-performance liquid chromatography for lipid composition analysis. Novel techniques such as cryo-electron microscopy now enable structural characterization of LNPs at nanometer resolution, providing insights into how manufacturing parameters affect lipid organization and transfection efficiency.
Regulatory considerations increasingly influence manufacturing process design, with quality-by-design approaches becoming standard practice. This systematic approach identifies critical process parameters that maintain the structure-function relationships of ionizable lipids throughout scale-up, ensuring consistent transfection efficacy across manufacturing scales.
Microfluidic mixing has emerged as the gold standard for LNP production, offering superior control over mixing parameters compared to conventional methods. This technology enables precise manipulation of lipid and nucleic acid streams, resulting in reproducible nanoparticle formation through controlled nanoprecipitation. Advanced microfluidic platforms now incorporate in-line monitoring capabilities, allowing real-time adjustment of critical process parameters to maintain quality attributes.
Continuous flow manufacturing represents a significant advancement for large-scale LNP production. Unlike batch processes, continuous systems minimize batch-to-batch variability while increasing throughput. Recent innovations in this area include modular manufacturing platforms that can be scaled from preclinical to commercial production without significant process redesign. These systems maintain critical mixing parameters across different production volumes, preserving the structure-function relationships of ionizable lipids.
Purification and downstream processing present unique challenges for maintaining LNP integrity. Tangential flow filtration has demonstrated advantages over conventional dialysis methods, reducing processing time while effectively removing organic solvents and unencapsulated materials. The development of single-use technologies has further streamlined manufacturing workflows, reducing cross-contamination risks and cleaning validation requirements.
Lyophilization processes have been optimized to enhance LNP stability during storage and distribution. The selection of appropriate cryoprotectants and lyoprotectants is crucial to prevent lipid phase transitions and maintain the ionizable lipid's structural integrity. Recent advances in controlled nucleation technologies have improved batch homogeneity during freeze-drying, resulting in more consistent reconstitution properties.
Quality control strategies have evolved to include advanced analytical techniques for monitoring critical quality attributes throughout the manufacturing process. These include dynamic light scattering for particle size distribution, zeta potential measurements for surface charge characterization, and high-performance liquid chromatography for lipid composition analysis. Novel techniques such as cryo-electron microscopy now enable structural characterization of LNPs at nanometer resolution, providing insights into how manufacturing parameters affect lipid organization and transfection efficiency.
Regulatory considerations increasingly influence manufacturing process design, with quality-by-design approaches becoming standard practice. This systematic approach identifies critical process parameters that maintain the structure-function relationships of ionizable lipids throughout scale-up, ensuring consistent transfection efficacy across manufacturing scales.
Regulatory Considerations for Lipid-Based Therapeutics
The regulatory landscape for lipid-based therapeutics, particularly those utilizing ionizable lipids, has evolved significantly in recent years due to the rapid advancement of lipid nanoparticle (LNP) technologies. Regulatory bodies worldwide, including the FDA, EMA, and PMDA, have established specific guidelines addressing the unique challenges posed by these novel delivery systems.
Key regulatory considerations begin with chemistry, manufacturing, and controls (CMC) requirements. Ionizable lipids must demonstrate consistent quality, purity, and stability profiles. The manufacturing process must be validated to ensure reproducibility of critical quality attributes, including particle size distribution, zeta potential, and encapsulation efficiency. These parameters directly impact transfection efficacy and safety profiles.
Safety assessment frameworks for lipid-based therapeutics require comprehensive toxicological evaluations. Regulatory agencies typically demand studies addressing both acute and chronic toxicity, genotoxicity, carcinogenicity, and reproductive toxicity. For ionizable lipids specifically, their structure-dependent biodistribution and potential for cytotoxicity necessitate specialized safety studies focusing on immunogenicity and complement activation.
Pharmacokinetic and pharmacodynamic (PK/PD) considerations represent another critical regulatory hurdle. Authorities require robust data demonstrating the relationship between lipid structure, in vivo distribution, cellular uptake, and ultimate therapeutic effect. The ionizable nature of these lipids, which transitions between charged and neutral states depending on pH, creates unique regulatory challenges in predicting and controlling in vivo behavior.
Clinical development pathways for lipid-based therapeutics often follow accelerated approval mechanisms, particularly for addressing unmet medical needs. However, this expedited approach typically comes with enhanced post-marketing surveillance requirements. Regulatory agencies increasingly request long-term follow-up studies to monitor potential delayed adverse effects related to lipid accumulation or immune responses.
Global regulatory harmonization efforts are underway to standardize requirements for lipid-based therapeutics. The International Council for Harmonisation (ICH) has initiated working groups specifically addressing nanomedicines, including LNPs. These initiatives aim to establish consistent quality standards, safety assessment protocols, and clinical evaluation frameworks across major regulatory jurisdictions, facilitating more efficient global development programs for novel ionizable lipid formulations.
Key regulatory considerations begin with chemistry, manufacturing, and controls (CMC) requirements. Ionizable lipids must demonstrate consistent quality, purity, and stability profiles. The manufacturing process must be validated to ensure reproducibility of critical quality attributes, including particle size distribution, zeta potential, and encapsulation efficiency. These parameters directly impact transfection efficacy and safety profiles.
Safety assessment frameworks for lipid-based therapeutics require comprehensive toxicological evaluations. Regulatory agencies typically demand studies addressing both acute and chronic toxicity, genotoxicity, carcinogenicity, and reproductive toxicity. For ionizable lipids specifically, their structure-dependent biodistribution and potential for cytotoxicity necessitate specialized safety studies focusing on immunogenicity and complement activation.
Pharmacokinetic and pharmacodynamic (PK/PD) considerations represent another critical regulatory hurdle. Authorities require robust data demonstrating the relationship between lipid structure, in vivo distribution, cellular uptake, and ultimate therapeutic effect. The ionizable nature of these lipids, which transitions between charged and neutral states depending on pH, creates unique regulatory challenges in predicting and controlling in vivo behavior.
Clinical development pathways for lipid-based therapeutics often follow accelerated approval mechanisms, particularly for addressing unmet medical needs. However, this expedited approach typically comes with enhanced post-marketing surveillance requirements. Regulatory agencies increasingly request long-term follow-up studies to monitor potential delayed adverse effects related to lipid accumulation or immune responses.
Global regulatory harmonization efforts are underway to standardize requirements for lipid-based therapeutics. The International Council for Harmonisation (ICH) has initiated working groups specifically addressing nanomedicines, including LNPs. These initiatives aim to establish consistent quality standards, safety assessment protocols, and clinical evaluation frameworks across major regulatory jurisdictions, facilitating more efficient global development programs for novel ionizable lipid formulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!