Isoelectric Focusing vs Capillary Electrophoresis: Sensitivity Comparison
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF and CE Technology Evolution and Objectives
Isoelectric focusing (IEF) and capillary electrophoresis (CE) represent two pivotal analytical techniques in the field of protein separation and characterization. The evolution of these technologies spans several decades, with each method undergoing significant refinements to address the growing demands for higher sensitivity, resolution, and reproducibility in bioanalytical applications.
IEF emerged in the 1960s as a technique based on the principle of protein separation according to their isoelectric points (pI) in a pH gradient. Initially performed in polyacrylamide gels, IEF faced limitations in terms of resolution and detection sensitivity. The 1970s witnessed the introduction of carrier ampholytes, which significantly improved the stability of pH gradients. By the 1980s, immobilized pH gradient (IPG) strips revolutionized IEF by offering enhanced reproducibility and loading capacity.
Concurrently, CE developed as an alternative separation technique in the late 1980s, utilizing the principles of electrophoresis within narrow capillaries. The miniaturization inherent in CE systems offered advantages in terms of heat dissipation, allowing for higher electric fields and consequently faster separations. The 1990s saw the integration of various detection methods with CE, including UV-Vis, fluorescence, and mass spectrometry, substantially enhancing its analytical capabilities.
The technological objectives for both IEF and CE have consistently centered around improving sensitivity, which remains a critical parameter for detecting low-abundance proteins in complex biological samples. For IEF, this has involved developing more sensitive staining methods, optimizing sample loading techniques, and enhancing detection systems. Modern IEF systems aim to achieve detection limits in the nanogram to picogram range, depending on the specific protein and detection method employed.
CE has pursued sensitivity enhancements through innovations in sample stacking techniques, development of specialized capillary coatings to minimize protein adsorption, and implementation of advanced detection systems. Contemporary CE platforms can achieve detection limits in the femtomole to attomole range under optimized conditions, particularly when coupled with laser-induced fluorescence or mass spectrometry.
The ongoing evolution of both technologies reflects a response to the increasing complexity of proteomic research and the need for more sophisticated analytical tools. Current development trajectories focus on miniaturization, automation, and integration with complementary techniques to create comprehensive analytical platforms. The ultimate goal remains the achievement of single-molecule detection capabilities while maintaining high throughput and reproducibility.
As we examine the comparative sensitivity of IEF versus CE, it is essential to consider not only their historical development but also the specific technical innovations that have shaped their current performance characteristics and their potential for future enhancement in increasingly demanding bioanalytical applications.
IEF emerged in the 1960s as a technique based on the principle of protein separation according to their isoelectric points (pI) in a pH gradient. Initially performed in polyacrylamide gels, IEF faced limitations in terms of resolution and detection sensitivity. The 1970s witnessed the introduction of carrier ampholytes, which significantly improved the stability of pH gradients. By the 1980s, immobilized pH gradient (IPG) strips revolutionized IEF by offering enhanced reproducibility and loading capacity.
Concurrently, CE developed as an alternative separation technique in the late 1980s, utilizing the principles of electrophoresis within narrow capillaries. The miniaturization inherent in CE systems offered advantages in terms of heat dissipation, allowing for higher electric fields and consequently faster separations. The 1990s saw the integration of various detection methods with CE, including UV-Vis, fluorescence, and mass spectrometry, substantially enhancing its analytical capabilities.
The technological objectives for both IEF and CE have consistently centered around improving sensitivity, which remains a critical parameter for detecting low-abundance proteins in complex biological samples. For IEF, this has involved developing more sensitive staining methods, optimizing sample loading techniques, and enhancing detection systems. Modern IEF systems aim to achieve detection limits in the nanogram to picogram range, depending on the specific protein and detection method employed.
CE has pursued sensitivity enhancements through innovations in sample stacking techniques, development of specialized capillary coatings to minimize protein adsorption, and implementation of advanced detection systems. Contemporary CE platforms can achieve detection limits in the femtomole to attomole range under optimized conditions, particularly when coupled with laser-induced fluorescence or mass spectrometry.
The ongoing evolution of both technologies reflects a response to the increasing complexity of proteomic research and the need for more sophisticated analytical tools. Current development trajectories focus on miniaturization, automation, and integration with complementary techniques to create comprehensive analytical platforms. The ultimate goal remains the achievement of single-molecule detection capabilities while maintaining high throughput and reproducibility.
As we examine the comparative sensitivity of IEF versus CE, it is essential to consider not only their historical development but also the specific technical innovations that have shaped their current performance characteristics and their potential for future enhancement in increasingly demanding bioanalytical applications.
Market Applications and Demand Analysis for Separation Techniques
The separation techniques market has witnessed substantial growth in recent years, driven by increasing demand across pharmaceutical, biotechnology, and clinical diagnostic sectors. Isoelectric Focusing (IEF) and Capillary Electrophoresis (CE) represent two critical technologies within this market, each serving distinct applications while competing in overlapping domains.
The global market for protein separation techniques, including IEF and CE, was valued at approximately 19.7 billion USD in 2022 and is projected to grow at a compound annual growth rate of 8.3% through 2030. This growth is primarily fueled by expanding biopharmaceutical research, increasing prevalence of chronic diseases, and rising demand for personalized medicine.
Pharmaceutical and biotechnology sectors constitute the largest market segment for these separation techniques, accounting for over 45% of the total market share. Within these industries, there is particular demand for high-sensitivity analytical methods capable of detecting and quantifying proteins at increasingly lower concentrations, especially for biomarker discovery and therapeutic protein characterization.
Clinical diagnostics represents another significant market driver, with hospitals and diagnostic laboratories increasingly adopting advanced separation techniques for disease biomarker detection. The sensitivity comparison between IEF and CE becomes particularly relevant in this context, as diagnostic applications often require detection of low-abundance proteins in complex biological matrices.
Academic research institutions form a stable market segment, contributing approximately 20% to the overall demand. Here, the focus tends to be on versatility and cost-effectiveness rather than ultimate sensitivity, though research applications involving rare proteins or limited sample volumes still benefit from high-sensitivity techniques.
Regional analysis reveals North America as the dominant market for separation techniques, holding approximately 38% of the global market share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, demonstrates the fastest growth rate due to expanding biotechnology sectors and increasing healthcare investments.
Market trends indicate a growing preference for integrated analytical platforms that combine multiple separation techniques to enhance overall sensitivity and specificity. This has led to the development of hybrid systems incorporating both IEF and CE methodologies, addressing the limitations of each individual technique while capitalizing on their respective strengths.
Consumer demand increasingly emphasizes automation, reproducibility, and high-throughput capabilities alongside sensitivity requirements. This trend is reshaping product development strategies among major market players, who are now focusing on creating comprehensive analytical solutions rather than standalone separation technologies.
The global market for protein separation techniques, including IEF and CE, was valued at approximately 19.7 billion USD in 2022 and is projected to grow at a compound annual growth rate of 8.3% through 2030. This growth is primarily fueled by expanding biopharmaceutical research, increasing prevalence of chronic diseases, and rising demand for personalized medicine.
Pharmaceutical and biotechnology sectors constitute the largest market segment for these separation techniques, accounting for over 45% of the total market share. Within these industries, there is particular demand for high-sensitivity analytical methods capable of detecting and quantifying proteins at increasingly lower concentrations, especially for biomarker discovery and therapeutic protein characterization.
Clinical diagnostics represents another significant market driver, with hospitals and diagnostic laboratories increasingly adopting advanced separation techniques for disease biomarker detection. The sensitivity comparison between IEF and CE becomes particularly relevant in this context, as diagnostic applications often require detection of low-abundance proteins in complex biological matrices.
Academic research institutions form a stable market segment, contributing approximately 20% to the overall demand. Here, the focus tends to be on versatility and cost-effectiveness rather than ultimate sensitivity, though research applications involving rare proteins or limited sample volumes still benefit from high-sensitivity techniques.
Regional analysis reveals North America as the dominant market for separation techniques, holding approximately 38% of the global market share, followed by Europe at 30% and Asia-Pacific at 25%. The Asia-Pacific region, particularly China and India, demonstrates the fastest growth rate due to expanding biotechnology sectors and increasing healthcare investments.
Market trends indicate a growing preference for integrated analytical platforms that combine multiple separation techniques to enhance overall sensitivity and specificity. This has led to the development of hybrid systems incorporating both IEF and CE methodologies, addressing the limitations of each individual technique while capitalizing on their respective strengths.
Consumer demand increasingly emphasizes automation, reproducibility, and high-throughput capabilities alongside sensitivity requirements. This trend is reshaping product development strategies among major market players, who are now focusing on creating comprehensive analytical solutions rather than standalone separation technologies.
Current Sensitivity Limitations and Technical Challenges
Despite significant advancements in both Isoelectric Focusing (IEF) and Capillary Electrophoresis (CE) technologies, several sensitivity limitations and technical challenges persist that impact their comparative performance in protein and peptide analysis. Current IEF systems typically demonstrate detection limits in the range of 1-10 ng/mL for proteins, while advanced CE systems can achieve detection limits approaching 0.1-1 ng/mL under optimized conditions.
The primary sensitivity limitation for IEF stems from its two-dimensional nature and the diffusion that occurs during the focusing process. As proteins concentrate at their isoelectric points, band broadening can occur, particularly for low-abundance proteins, resulting in decreased detection sensitivity. Additionally, the ampholyte carrier molecules used in IEF can interfere with detection systems, especially when UV absorbance is employed as the detection method.
For CE, while offering superior theoretical plate numbers (often exceeding 500,000 plates/meter), sensitivity is fundamentally limited by the small sample injection volumes (typically 1-50 nL) and the short optical path length in detection cells. This creates an inherent trade-off between resolution and sensitivity that remains challenging to overcome in conventional CE systems.
Both techniques face challenges with sample matrix effects. Complex biological samples containing high salt concentrations, lipids, or other interfering compounds can significantly reduce sensitivity and reproducibility. This is particularly problematic when analyzing clinical or environmental samples where target analytes may be present at trace levels within complex matrices.
Detection technology limitations represent another significant challenge. While laser-induced fluorescence (LIF) has improved detection limits for both techniques, it requires analyte derivatization, which can introduce variability and potentially alter protein properties. Mass spectrometry coupling has enhanced detection capabilities but introduces additional complexity and cost to the analytical workflow.
Reproducibility remains a persistent challenge, particularly for IEF. Environmental factors such as temperature fluctuations can significantly impact the establishment of pH gradients in IEF, leading to run-to-run variability. CE faces similar challenges with migration time reproducibility, often requiring internal standards to normalize results.
Automation and integration challenges also affect both techniques. While CE has seen significant advances in automation, particularly with commercial systems offering integrated sample preparation and analysis, IEF automation remains more limited, especially for two-dimensional applications combining IEF with other separation techniques.
The dynamic range limitation presents another significant challenge, with both techniques typically offering 2-3 orders of magnitude of linear dynamic range. This restricts their application in samples containing both high-abundance and low-abundance proteins without additional fractionation steps.
The primary sensitivity limitation for IEF stems from its two-dimensional nature and the diffusion that occurs during the focusing process. As proteins concentrate at their isoelectric points, band broadening can occur, particularly for low-abundance proteins, resulting in decreased detection sensitivity. Additionally, the ampholyte carrier molecules used in IEF can interfere with detection systems, especially when UV absorbance is employed as the detection method.
For CE, while offering superior theoretical plate numbers (often exceeding 500,000 plates/meter), sensitivity is fundamentally limited by the small sample injection volumes (typically 1-50 nL) and the short optical path length in detection cells. This creates an inherent trade-off between resolution and sensitivity that remains challenging to overcome in conventional CE systems.
Both techniques face challenges with sample matrix effects. Complex biological samples containing high salt concentrations, lipids, or other interfering compounds can significantly reduce sensitivity and reproducibility. This is particularly problematic when analyzing clinical or environmental samples where target analytes may be present at trace levels within complex matrices.
Detection technology limitations represent another significant challenge. While laser-induced fluorescence (LIF) has improved detection limits for both techniques, it requires analyte derivatization, which can introduce variability and potentially alter protein properties. Mass spectrometry coupling has enhanced detection capabilities but introduces additional complexity and cost to the analytical workflow.
Reproducibility remains a persistent challenge, particularly for IEF. Environmental factors such as temperature fluctuations can significantly impact the establishment of pH gradients in IEF, leading to run-to-run variability. CE faces similar challenges with migration time reproducibility, often requiring internal standards to normalize results.
Automation and integration challenges also affect both techniques. While CE has seen significant advances in automation, particularly with commercial systems offering integrated sample preparation and analysis, IEF automation remains more limited, especially for two-dimensional applications combining IEF with other separation techniques.
The dynamic range limitation presents another significant challenge, with both techniques typically offering 2-3 orders of magnitude of linear dynamic range. This restricts their application in samples containing both high-abundance and low-abundance proteins without additional fractionation steps.
Comparative Analysis of IEF and CE Detection Methodologies
01 Enhanced detection methods for capillary electrophoresis
Various detection methods have been developed to improve the sensitivity of capillary electrophoresis. These include laser-induced fluorescence, UV-visible absorption, and electrochemical detection techniques. These methods can significantly lower detection limits and enhance the ability to analyze trace amounts of analytes. Advanced optical systems and signal processing algorithms further improve detection capabilities in both isoelectric focusing and capillary electrophoresis applications.- Enhanced sensitivity techniques in capillary electrophoresis: Various techniques have been developed to enhance the sensitivity of capillary electrophoresis systems. These include specialized detection methods, sample concentration techniques, and optimized buffer compositions that improve signal-to-noise ratios. These enhancements allow for the detection of lower concentrations of analytes, making capillary electrophoresis more suitable for trace analysis in complex biological and environmental samples.
- Isoelectric focusing apparatus and methodology: Specialized apparatus and methodologies for isoelectric focusing have been developed to improve separation efficiency. These include innovative electrode designs, carrier ampholyte formulations, and temperature control systems that maintain stable pH gradients. Such advancements allow for higher resolution separation of proteins and other amphoteric compounds based on their isoelectric points, enhancing the overall analytical capability.
- Integration of isoelectric focusing with capillary electrophoresis: Combining isoelectric focusing with capillary electrophoresis creates powerful two-dimensional separation systems. These integrated approaches allow for separation based on both isoelectric point and size/charge ratio, significantly increasing resolution for complex sample analysis. The integration can be achieved through various coupling mechanisms, including direct transfer systems and microfluidic platforms that maintain separation integrity between dimensions.
- Microfluidic platforms for electrophoretic techniques: Miniaturized microfluidic platforms have been developed for both isoelectric focusing and capillary electrophoresis, offering advantages in terms of reduced sample volume, faster analysis times, and improved sensitivity. These platforms incorporate innovative channel designs, integrated detection systems, and automated sample handling capabilities, making them suitable for high-throughput applications and point-of-care diagnostics.
- Detection and quantification methods for improved sensitivity: Advanced detection methods have been developed to enhance the sensitivity of both isoelectric focusing and capillary electrophoresis. These include laser-induced fluorescence, mass spectrometry coupling, electrochemical detection, and various spectroscopic techniques. These detection methods, combined with signal amplification strategies and data processing algorithms, significantly lower detection limits and improve quantification accuracy for a wide range of analytes.
02 Microfluidic platforms for isoelectric focusing
Miniaturized microfluidic devices have been developed for isoelectric focusing applications, offering advantages such as reduced sample volume requirements, faster analysis times, and improved sensitivity. These platforms integrate multiple functions including sample preparation, separation, and detection into a single device. The miniaturization allows for more precise control of electric fields and pH gradients, resulting in higher resolution separations and increased sensitivity for protein and peptide analysis.Expand Specific Solutions03 Sample preconcentration techniques
Various sample preconcentration techniques have been developed to enhance the sensitivity of both isoelectric focusing and capillary electrophoresis. These include field-amplified sample stacking, isotachophoresis, and sweeping methods. By concentrating analytes before or during the separation process, these techniques can improve detection limits by several orders of magnitude. This is particularly valuable for the analysis of low-abundance proteins and peptides in complex biological samples.Expand Specific Solutions04 Novel buffer systems and carrier ampholytes
Advanced buffer systems and carrier ampholytes have been developed to improve the stability and reproducibility of pH gradients in isoelectric focusing. These innovations help maintain consistent separation conditions, resulting in better resolution and sensitivity. Specialized buffer compositions can also enhance the solubility of proteins throughout the pH range, preventing precipitation at their isoelectric points and allowing for more sensitive detection. Some formulations are specifically designed to minimize interference with detection methods.Expand Specific Solutions05 Integration of isoelectric focusing with capillary electrophoresis
Two-dimensional separation systems that combine isoelectric focusing with capillary electrophoresis provide enhanced resolving power and sensitivity. In these systems, proteins are first separated based on their isoelectric points and then further resolved based on their size-to-charge ratios. This orthogonal separation approach significantly increases peak capacity and improves the detection of low-abundance components in complex mixtures. Automated systems have been developed to seamlessly transfer samples between the two separation dimensions.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Analytical Instrumentation
The electrophoresis technology market is in a mature growth phase, with isoelectric focusing (IEF) and capillary electrophoresis (CE) representing complementary analytical approaches. The global market for these technologies exceeds $3 billion, growing at 5-7% annually. Regarding sensitivity comparison, CE generally offers higher sensitivity and resolution for small sample volumes, making it preferred for pharmaceutical applications as evidenced by Beckman Coulter and Waters Corporation (Micromass) investments. IEF provides superior protein isoform separation capabilities, with companies like ProteinSimple and Hamamatsu Photonics developing enhanced detection systems. Academic institutions (Tsinghua University, Zhejiang University) are advancing hybrid approaches, while industry players (FUJIFILM Wako, DH Technologies) focus on improving automation and reproducibility to address the growing biopharmaceutical characterization demands.
Beckman Coulter, Inc.
Technical Solution: Beckman Coulter has developed advanced capillary electrophoresis (CE) systems with proprietary ProteomeLab technology that offers high-resolution protein separation. Their PA 800 Plus Pharmaceutical Analysis System utilizes a combination of UV and laser-induced fluorescence detection methods to achieve sensitivity in the nanogram to picogram range for protein analysis. The company has implemented dynamic coating technologies to minimize protein adsorption to capillary walls, significantly improving reproducibility and quantification accuracy. Their systems feature automated sample handling with temperature control (4-60°C) that enhances reproducibility and stability during long analytical runs. Beckman's CE technology employs specialized buffer systems optimized for various pH ranges (2-12) allowing for versatile separation of proteins with different isoelectric points, while maintaining high efficiency and resolution comparable to traditional IEF methods.
Strengths: Superior automation capabilities, high reproducibility, and rapid analysis times (typically 10-30 minutes per sample compared to hours for traditional IEF). Weaknesses: Higher initial equipment investment compared to conventional IEF systems, and potentially lower loading capacity which may limit detection of very low abundance proteins in complex samples.
ProteinSimple
Technical Solution: ProteinSimple has pioneered automated capillary isoelectric focusing (cIEF) technology through their Maurice and Jess systems, which combine the principles of traditional IEF with capillary format for enhanced sensitivity. Their proprietary whole-column imaging detection (WCID) technology allows real-time visualization of protein focusing across the entire capillary length, achieving detection limits in the nanogram range. The company's systems incorporate automated sample preparation, separation, and detection in a closed environment, minimizing sample handling and reducing variability. ProteinSimple's technology utilizes specialized ampholytes and proprietary cartridge designs that create stable pH gradients within capillaries, enabling high-resolution separation of proteins with pI differences as small as 0.01 pH units. Their systems feature built-in UV and fluorescence detection capabilities, with the latter providing up to 100-fold improvement in sensitivity compared to conventional IEF methods, allowing detection of proteins at concentrations as low as 5-10 ng/mL in some applications.
Strengths: Exceptional automation and reproducibility, minimal sample consumption (typically 5-10 μL), and comprehensive software for data analysis and regulatory compliance. Weaknesses: Limited multiplexing capabilities compared to some CE platforms, and specialized consumables that may increase per-sample operational costs.
Key Patents and Innovations in Sensitivity Enhancement
An apparatus for protein separation using capillary isoelectric focusing-hollow fiber flow field flow fractionation and method thereof
PatentInactiveEP1987053A1
Innovation
- A capillary isoelectric focusing-hollow fiber flow field flow fractionation apparatus that separates proteins based on isoelectric point and molecular weight in a two-dimensional, non-gel, and liquid phase manner, using a combination of capillary isoelectric focusing and hollow fiber flow field flow fractionation units to automatically remove ampholytes and prevent protein denaturation.
Method for quantitatively detecting antigen
PatentInactiveUS7153701B1
Innovation
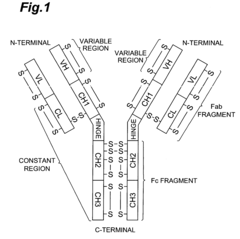
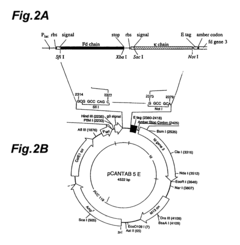
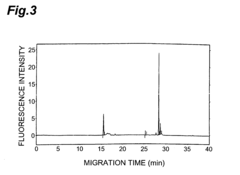
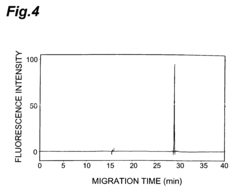
- A method involving the use of an Fab′ antibody with a uniform isoelectric point, modified by adding an amino acid sequence comprising a charged amino acid residue and labeled with a fluorescent dye, which forms an immune complex with the antigen, allowing for precise detection through electrophoresis and fluorescence detection.
Regulatory Considerations for Analytical Method Validation
Regulatory frameworks governing analytical method validation are critical considerations when comparing Isoelectric Focusing (IEF) and Capillary Electrophoresis (CE) for sensitivity analysis. The FDA, EMA, and ICH have established comprehensive guidelines that directly impact the implementation of these techniques in regulated environments.
The ICH Q2(R1) guideline specifically addresses validation parameters including specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit—all of which must be rigorously evaluated when comparing IEF and CE sensitivity. For protein characterization applications, regulatory bodies typically require demonstration of method robustness and reproducibility across different laboratories and equipment.
FDA guidance documents emphasize the importance of method transfer protocols when implementing either IEF or CE techniques. These protocols must include detailed procedures for verifying that sensitivity specifications are consistently met across different testing sites. The higher sensitivity often observed with CE methods may require additional validation steps to ensure results remain consistent across the method's lifecycle.
EMA guidelines place particular emphasis on the qualification of reference standards used in comparative sensitivity studies between IEF and CE. When validating these methods, sponsors must demonstrate that reference materials are suitable for their intended use and that system suitability tests adequately control for variations in sensitivity performance.
Regulatory expectations for method lifecycle management have evolved significantly, with increased focus on continuous verification of method performance. For CE methods, which often demonstrate superior sensitivity but may be more susceptible to environmental factors, regulatory bodies now expect ongoing monitoring programs to ensure consistent performance throughout routine use.
Risk-based approaches to method validation have gained regulatory acceptance, allowing for tailored validation strategies based on the intended application of IEF or CE. When sensitivity is a critical quality attribute, regulators expect more extensive validation studies with statistical justification for acceptance criteria.
Compliance with pharmacopeial standards presents another regulatory consideration, as both United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs related to electrophoretic methods. These compendial methods often serve as reference points against which the sensitivity of new or modified IEF and CE procedures must be evaluated.
The ICH Q2(R1) guideline specifically addresses validation parameters including specificity, linearity, range, accuracy, precision, detection limit, and quantitation limit—all of which must be rigorously evaluated when comparing IEF and CE sensitivity. For protein characterization applications, regulatory bodies typically require demonstration of method robustness and reproducibility across different laboratories and equipment.
FDA guidance documents emphasize the importance of method transfer protocols when implementing either IEF or CE techniques. These protocols must include detailed procedures for verifying that sensitivity specifications are consistently met across different testing sites. The higher sensitivity often observed with CE methods may require additional validation steps to ensure results remain consistent across the method's lifecycle.
EMA guidelines place particular emphasis on the qualification of reference standards used in comparative sensitivity studies between IEF and CE. When validating these methods, sponsors must demonstrate that reference materials are suitable for their intended use and that system suitability tests adequately control for variations in sensitivity performance.
Regulatory expectations for method lifecycle management have evolved significantly, with increased focus on continuous verification of method performance. For CE methods, which often demonstrate superior sensitivity but may be more susceptible to environmental factors, regulatory bodies now expect ongoing monitoring programs to ensure consistent performance throughout routine use.
Risk-based approaches to method validation have gained regulatory acceptance, allowing for tailored validation strategies based on the intended application of IEF or CE. When sensitivity is a critical quality attribute, regulators expect more extensive validation studies with statistical justification for acceptance criteria.
Compliance with pharmacopeial standards presents another regulatory consideration, as both United States Pharmacopeia (USP) and European Pharmacopoeia (Ph. Eur.) contain specific monographs related to electrophoretic methods. These compendial methods often serve as reference points against which the sensitivity of new or modified IEF and CE procedures must be evaluated.
Cost-Benefit Analysis of IEF versus CE Implementation
When evaluating the implementation of Isoelectric Focusing (IEF) versus Capillary Electrophoresis (CE) technologies, cost-benefit analysis reveals significant differences in initial investment, operational expenses, and long-term value proposition.
The initial capital expenditure for IEF equipment typically ranges from $15,000 to $50,000, depending on system sophistication and automation level. In contrast, CE systems generally require higher upfront investment, ranging from $30,000 to $100,000, with fully automated systems reaching the upper end of this spectrum. This substantial difference in initial costs can be a decisive factor for laboratories with limited budgets.
Operational expenses present another important consideration. IEF consumables (ampholytes, gels, buffers) average $20-40 per sample run, while CE operational costs tend to be lower at approximately $10-25 per sample. The difference becomes particularly significant in high-throughput environments where thousands of samples are processed annually.
Labor costs favor CE technology, which typically requires 0.5-1 hour of technician time per sample batch compared to IEF's 1-2 hours. At an average laboratory technician rate of $30-40 per hour, this translates to substantial savings in personnel expenses for facilities implementing CE, especially those processing large sample volumes.
Maintenance requirements and associated costs also differ markedly. IEF systems generally require maintenance every 3-6 months at approximately $1,000-2,000 annually. CE systems, while more complex, often operate with greater reliability, requiring similar maintenance intervals but at higher costs of $2,000-4,000 annually due to specialized components and calibration requirements.
Return on investment calculations indicate that despite higher initial costs, CE systems typically achieve ROI within 2-3 years in high-throughput environments, compared to 1-2 years for IEF systems. However, the superior resolution and automation capabilities of CE often justify the extended payback period through improved data quality and reduced manual intervention.
Space utilization efficiency favors CE technology, which typically occupies 50-60% less laboratory space than comparable IEF setups. This spatial economy translates to significant savings in facilities with premium laboratory space, estimated at $300-500 per square foot annually in research-intensive urban areas.
When considering total cost of ownership over a five-year period, CE systems demonstrate approximately 15-20% higher costs than IEF systems for low-volume applications (<500 samples/year), but become increasingly cost-effective as sample volumes increase, showing 10-15% lower total costs for high-volume applications (>2,000 samples/year).
The initial capital expenditure for IEF equipment typically ranges from $15,000 to $50,000, depending on system sophistication and automation level. In contrast, CE systems generally require higher upfront investment, ranging from $30,000 to $100,000, with fully automated systems reaching the upper end of this spectrum. This substantial difference in initial costs can be a decisive factor for laboratories with limited budgets.
Operational expenses present another important consideration. IEF consumables (ampholytes, gels, buffers) average $20-40 per sample run, while CE operational costs tend to be lower at approximately $10-25 per sample. The difference becomes particularly significant in high-throughput environments where thousands of samples are processed annually.
Labor costs favor CE technology, which typically requires 0.5-1 hour of technician time per sample batch compared to IEF's 1-2 hours. At an average laboratory technician rate of $30-40 per hour, this translates to substantial savings in personnel expenses for facilities implementing CE, especially those processing large sample volumes.
Maintenance requirements and associated costs also differ markedly. IEF systems generally require maintenance every 3-6 months at approximately $1,000-2,000 annually. CE systems, while more complex, often operate with greater reliability, requiring similar maintenance intervals but at higher costs of $2,000-4,000 annually due to specialized components and calibration requirements.
Return on investment calculations indicate that despite higher initial costs, CE systems typically achieve ROI within 2-3 years in high-throughput environments, compared to 1-2 years for IEF systems. However, the superior resolution and automation capabilities of CE often justify the extended payback period through improved data quality and reduced manual intervention.
Space utilization efficiency favors CE technology, which typically occupies 50-60% less laboratory space than comparable IEF setups. This spatial economy translates to significant savings in facilities with premium laboratory space, estimated at $300-500 per square foot annually in research-intensive urban areas.
When considering total cost of ownership over a five-year period, CE systems demonstrate approximately 15-20% higher costs than IEF systems for low-volume applications (<500 samples/year), but become increasingly cost-effective as sample volumes increase, showing 10-15% lower total costs for high-volume applications (>2,000 samples/year).
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!