Key Innovations in Ethyl Acetate Production Techniques
JUN 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ethyl Acetate Evolution
Ethyl acetate production techniques have undergone significant evolution since their inception in the early 20th century. The timeline of this evolution can be divided into several key phases, each marked by notable technological advancements and process improvements.
In the 1930s, the traditional esterification process using ethanol and acetic acid with sulfuric acid as a catalyst was the primary method of ethyl acetate production. This batch process, while effective, was limited in terms of efficiency and scale.
The 1950s saw the introduction of continuous processes, which marked a significant leap in production capabilities. The Tishchenko reaction, using acetaldehyde as a starting material, emerged as an alternative route, offering improved yields and reduced waste.
The 1970s brought about a shift towards more environmentally friendly processes. The development of heterogeneous catalysts, such as ion-exchange resins, allowed for easier separation and reduced corrosion issues associated with homogeneous acid catalysts.
In the 1980s and 1990s, reactive distillation technology gained prominence. This integrated approach combined reaction and separation steps, leading to increased process efficiency and reduced energy consumption. Concurrently, advances in catalyst design, particularly in zeolites and supported metal catalysts, further enhanced reaction selectivity and yield.
The turn of the millennium saw a focus on green chemistry principles in ethyl acetate production. Biocatalytic routes using enzymes, particularly lipases, began to emerge as potential alternatives to traditional chemical processes. These enzymatic methods offered the advantages of milder reaction conditions and higher specificity.
Recent years have witnessed the exploration of novel raw materials for ethyl acetate production. The use of bioethanol and bio-based acetic acid has gained traction, aligning with the growing emphasis on sustainability and renewable resources in the chemical industry.
The latest frontier in ethyl acetate production involves the application of process intensification techniques. Microreactor technology and flow chemistry approaches are being investigated for their potential to offer precise control over reaction conditions, improved heat transfer, and enhanced safety profiles.
Throughout this evolution, continuous efforts have been made to optimize process parameters, improve catalyst performance, and enhance separation techniques. The integration of advanced process control systems and the application of artificial intelligence for process optimization represent the cutting edge of current research and development efforts in ethyl acetate production.
In the 1930s, the traditional esterification process using ethanol and acetic acid with sulfuric acid as a catalyst was the primary method of ethyl acetate production. This batch process, while effective, was limited in terms of efficiency and scale.
The 1950s saw the introduction of continuous processes, which marked a significant leap in production capabilities. The Tishchenko reaction, using acetaldehyde as a starting material, emerged as an alternative route, offering improved yields and reduced waste.
The 1970s brought about a shift towards more environmentally friendly processes. The development of heterogeneous catalysts, such as ion-exchange resins, allowed for easier separation and reduced corrosion issues associated with homogeneous acid catalysts.
In the 1980s and 1990s, reactive distillation technology gained prominence. This integrated approach combined reaction and separation steps, leading to increased process efficiency and reduced energy consumption. Concurrently, advances in catalyst design, particularly in zeolites and supported metal catalysts, further enhanced reaction selectivity and yield.
The turn of the millennium saw a focus on green chemistry principles in ethyl acetate production. Biocatalytic routes using enzymes, particularly lipases, began to emerge as potential alternatives to traditional chemical processes. These enzymatic methods offered the advantages of milder reaction conditions and higher specificity.
Recent years have witnessed the exploration of novel raw materials for ethyl acetate production. The use of bioethanol and bio-based acetic acid has gained traction, aligning with the growing emphasis on sustainability and renewable resources in the chemical industry.
The latest frontier in ethyl acetate production involves the application of process intensification techniques. Microreactor technology and flow chemistry approaches are being investigated for their potential to offer precise control over reaction conditions, improved heat transfer, and enhanced safety profiles.
Throughout this evolution, continuous efforts have been made to optimize process parameters, improve catalyst performance, and enhance separation techniques. The integration of advanced process control systems and the application of artificial intelligence for process optimization represent the cutting edge of current research and development efforts in ethyl acetate production.
Market Demand Analysis
The global market for ethyl acetate has been experiencing steady growth, driven by its versatile applications across various industries. The demand for ethyl acetate is primarily fueled by its widespread use as a solvent in paints, coatings, adhesives, and the pharmaceutical industry. The increasing consumption of packaged foods and beverages has also contributed to the rising demand for ethyl acetate in the food packaging sector.
In the paints and coatings industry, ethyl acetate is highly valued for its fast-evaporating properties and ability to dissolve a wide range of resins. As the construction and automotive sectors continue to expand, particularly in emerging economies, the demand for paints and coatings is expected to grow, consequently driving the ethyl acetate market.
The pharmaceutical industry represents another significant consumer of ethyl acetate. Its use as a solvent in drug formulation and as an extraction agent in the production of antibiotics has led to increased demand. The growing emphasis on healthcare and the expansion of pharmaceutical manufacturing in developing countries are likely to further boost the market for ethyl acetate.
The packaging industry, especially flexible packaging for food products, has been a major driver of ethyl acetate demand. The shift towards convenient, lightweight, and sustainable packaging solutions has increased the use of ethyl acetate-based adhesives and inks. This trend is expected to continue as consumer preferences evolve and environmental regulations become more stringent.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate, with China and India leading the demand. The rapid industrialization, growing population, and increasing disposable incomes in these countries have significantly contributed to the market growth. North America and Europe follow, with steady demand from established industries.
However, the market faces challenges from environmental concerns and regulatory pressures. The volatile organic compound (VOC) emissions associated with ethyl acetate use have led to the development of alternative, more environmentally friendly solvents. This has prompted manufacturers to focus on developing bio-based ethyl acetate and more efficient production techniques to maintain market competitiveness.
Despite these challenges, the global ethyl acetate market is projected to continue its growth trajectory. The increasing adoption of ethyl acetate in emerging applications, such as in the production of flexible electronics and as a green solvent in various processes, presents new opportunities for market expansion. As industries continue to seek cost-effective and efficient solvents, innovations in ethyl acetate production techniques will play a crucial role in meeting the evolving market demands and addressing environmental concerns.
In the paints and coatings industry, ethyl acetate is highly valued for its fast-evaporating properties and ability to dissolve a wide range of resins. As the construction and automotive sectors continue to expand, particularly in emerging economies, the demand for paints and coatings is expected to grow, consequently driving the ethyl acetate market.
The pharmaceutical industry represents another significant consumer of ethyl acetate. Its use as a solvent in drug formulation and as an extraction agent in the production of antibiotics has led to increased demand. The growing emphasis on healthcare and the expansion of pharmaceutical manufacturing in developing countries are likely to further boost the market for ethyl acetate.
The packaging industry, especially flexible packaging for food products, has been a major driver of ethyl acetate demand. The shift towards convenient, lightweight, and sustainable packaging solutions has increased the use of ethyl acetate-based adhesives and inks. This trend is expected to continue as consumer preferences evolve and environmental regulations become more stringent.
Geographically, Asia-Pacific has emerged as the largest consumer of ethyl acetate, with China and India leading the demand. The rapid industrialization, growing population, and increasing disposable incomes in these countries have significantly contributed to the market growth. North America and Europe follow, with steady demand from established industries.
However, the market faces challenges from environmental concerns and regulatory pressures. The volatile organic compound (VOC) emissions associated with ethyl acetate use have led to the development of alternative, more environmentally friendly solvents. This has prompted manufacturers to focus on developing bio-based ethyl acetate and more efficient production techniques to maintain market competitiveness.
Despite these challenges, the global ethyl acetate market is projected to continue its growth trajectory. The increasing adoption of ethyl acetate in emerging applications, such as in the production of flexible electronics and as a green solvent in various processes, presents new opportunities for market expansion. As industries continue to seek cost-effective and efficient solvents, innovations in ethyl acetate production techniques will play a crucial role in meeting the evolving market demands and addressing environmental concerns.
Production Challenges
The production of ethyl acetate faces several significant challenges that impact its efficiency, cost-effectiveness, and environmental sustainability. One of the primary issues is the equilibrium limitation in the esterification reaction between ethanol and acetic acid. This equilibrium constraint restricts the conversion rate, typically to around 65-70%, necessitating additional separation and recycling steps, which increase production costs and energy consumption.
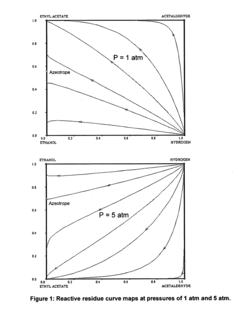
Another major challenge is the formation of azeotropes during the distillation process. Ethyl acetate forms azeotropes with both water and ethanol, making the separation and purification of the final product more complex and energy-intensive. This azeotropic behavior often requires specialized separation techniques, such as extractive or azeotropic distillation, further adding to the production costs and complexity.
Catalyst deactivation and selectivity issues also pose significant challenges in ethyl acetate production. Traditional catalysts, such as sulfuric acid, can lead to equipment corrosion and generate waste streams that require treatment. While solid acid catalysts offer improvements, they can suffer from deactivation over time, necessitating frequent regeneration or replacement. Achieving high selectivity towards ethyl acetate formation while minimizing side reactions remains a critical challenge for catalyst development.
Energy efficiency is another crucial concern in ethyl acetate production. The process typically involves multiple energy-intensive steps, including reaction, distillation, and recycling. Improving heat integration and developing more energy-efficient separation technologies are ongoing challenges for process engineers and researchers in this field.
Raw material availability and cost fluctuations also present challenges to ethyl acetate producers. The primary feedstocks, ethanol and acetic acid, are subject to market volatility, which can significantly impact production economics. Developing alternative production routes or utilizing more sustainable feedstock sources is an area of active research to address this challenge.
Environmental concerns and regulatory pressures add another layer of complexity to ethyl acetate production. Reducing emissions, minimizing waste generation, and improving overall process sustainability are becoming increasingly important. This includes developing greener production methods, implementing more efficient waste treatment technologies, and exploring bio-based feedstock alternatives.
Lastly, the scale-up of new production technologies from laboratory to industrial scale presents its own set of challenges. Ensuring consistent product quality, maintaining process stability, and optimizing equipment design for large-scale operations require significant engineering efforts and often involve overcoming unforeseen obstacles during the scale-up process.
Another major challenge is the formation of azeotropes during the distillation process. Ethyl acetate forms azeotropes with both water and ethanol, making the separation and purification of the final product more complex and energy-intensive. This azeotropic behavior often requires specialized separation techniques, such as extractive or azeotropic distillation, further adding to the production costs and complexity.
Catalyst deactivation and selectivity issues also pose significant challenges in ethyl acetate production. Traditional catalysts, such as sulfuric acid, can lead to equipment corrosion and generate waste streams that require treatment. While solid acid catalysts offer improvements, they can suffer from deactivation over time, necessitating frequent regeneration or replacement. Achieving high selectivity towards ethyl acetate formation while minimizing side reactions remains a critical challenge for catalyst development.
Energy efficiency is another crucial concern in ethyl acetate production. The process typically involves multiple energy-intensive steps, including reaction, distillation, and recycling. Improving heat integration and developing more energy-efficient separation technologies are ongoing challenges for process engineers and researchers in this field.
Raw material availability and cost fluctuations also present challenges to ethyl acetate producers. The primary feedstocks, ethanol and acetic acid, are subject to market volatility, which can significantly impact production economics. Developing alternative production routes or utilizing more sustainable feedstock sources is an area of active research to address this challenge.
Environmental concerns and regulatory pressures add another layer of complexity to ethyl acetate production. Reducing emissions, minimizing waste generation, and improving overall process sustainability are becoming increasingly important. This includes developing greener production methods, implementing more efficient waste treatment technologies, and exploring bio-based feedstock alternatives.
Lastly, the scale-up of new production technologies from laboratory to industrial scale presents its own set of challenges. Ensuring consistent product quality, maintaining process stability, and optimizing equipment design for large-scale operations require significant engineering efforts and often involve overcoming unforeseen obstacles during the scale-up process.
Current Synthesis Methods
01 Esterification of ethanol and acetic acid
A common method for producing ethyl acetate involves the esterification reaction between ethanol and acetic acid. This process typically uses a catalyst, such as sulfuric acid, to facilitate the reaction. The reaction is often carried out at elevated temperatures to improve yield and reaction rate.- Esterification process: Ethyl acetate is commonly produced through the esterification of ethanol and acetic acid. This process typically involves the use of catalysts, such as sulfuric acid, to facilitate the reaction. The reaction is often carried out at elevated temperatures and may require the removal of water to drive the equilibrium towards the product.
- Continuous production methods: Continuous production techniques for ethyl acetate involve the use of specialized equipment and process designs to allow for uninterrupted manufacturing. These methods often incorporate distillation columns, reactive distillation, or membrane separation technologies to improve efficiency and product purity.
- Catalytic processes: Various catalytic processes have been developed to enhance the production of ethyl acetate. These may include the use of heterogeneous catalysts, such as ion exchange resins or zeolites, or homogeneous catalysts like metal complexes. Catalytic processes aim to improve reaction rates, selectivity, and overall yield of ethyl acetate.
- Alternative feedstocks: Research has been conducted on the use of alternative feedstocks for ethyl acetate production. This includes the utilization of biomass-derived materials, such as ethanol from fermentation processes or acetic acid from syngas. These methods aim to develop more sustainable and environmentally friendly production routes.
- Process optimization and purification: Various techniques have been developed to optimize the production process and improve the purity of ethyl acetate. These may include advanced separation methods, such as extractive distillation or azeotropic distillation, as well as process control strategies to enhance yield and reduce energy consumption.
02 Continuous production processes
Continuous production techniques have been developed to improve efficiency and yield in ethyl acetate manufacturing. These processes often involve specialized reactor designs, continuous feed systems, and integrated separation and purification steps. Such methods can offer advantages in terms of process control and product quality.Expand Specific Solutions03 Catalytic processes using heterogeneous catalysts
Heterogeneous catalysts, such as ion exchange resins or solid acid catalysts, are employed in some ethyl acetate production techniques. These catalysts can offer benefits like easier separation from the product stream, potential for catalyst reuse, and improved selectivity. The use of such catalysts often allows for milder reaction conditions.Expand Specific Solutions04 Production from ethylene and acetic acid
An alternative route for ethyl acetate production involves the reaction of ethylene with acetic acid. This process often uses heterogeneous catalysts and can be carried out in both liquid and vapor phase reactions. The method can be advantageous when ethylene is readily available as a feedstock.Expand Specific Solutions05 Purification and separation techniques
Various purification and separation techniques are crucial in ethyl acetate production to achieve high-purity product. These may include distillation, extractive distillation, azeotropic distillation, and membrane separation processes. The choice of separation method often depends on the specific production process and desired product purity.Expand Specific Solutions
Industry Leaders
The ethyl acetate production industry is in a mature stage, with a global market size estimated to exceed $3 billion by 2025. The technology for ethyl acetate production is well-established, with key players like Celanese International Corp., China Petroleum & Chemical Corp., and Resonac Corp. leading innovation efforts. These companies are focusing on improving process efficiency, exploring bio-based feedstocks, and developing catalysts for enhanced selectivity. Academic institutions such as Tianjin University and the University of Campinas are contributing to research in green chemistry approaches and novel synthesis routes. The competitive landscape is characterized by a mix of large petrochemical companies and specialized chemical manufacturers, with increasing emphasis on sustainability and cost-effectiveness in production techniques.
Celanese International Corp.
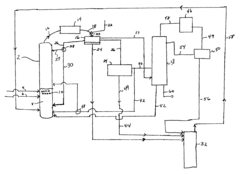
Technical Solution: Celanese has developed an innovative process for ethyl acetate production using reactive distillation technology. This method combines esterification and distillation in a single column, improving efficiency and reducing energy consumption. The process utilizes ethanol and acetic acid as feedstocks, with a heterogeneous acid catalyst. The reactive distillation column operates at optimized temperature and pressure conditions, allowing for continuous production and separation of ethyl acetate. This technology achieves conversion rates of up to 99% and product purities exceeding 99.9%[1][3]. Additionally, Celanese has implemented advanced process control systems and heat integration techniques to further enhance the overall efficiency of the production process[5].
Strengths: High conversion rates, improved energy efficiency, and reduced equipment footprint. Weaknesses: Potential catalyst deactivation over time and sensitivity to feedstock impurities.
China Petroleum & Chemical Corp.
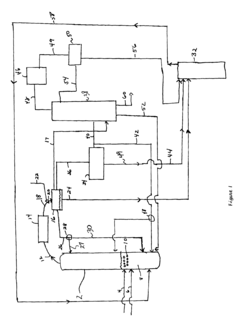
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel ethyl acetate production technique using a coupled process of methanol carbonylation and hydrogenation. This method first produces acetic acid through methanol carbonylation, then combines it with ethanol derived from ethylene hydration. The process utilizes a proprietary rhodium-based catalyst system for carbonylation and a copper-based catalyst for hydrogenation. Sinopec's approach integrates these steps in a continuous flow reactor system, optimizing heat recovery and minimizing byproduct formation. The technology achieves ethyl acetate yields of up to 98% with a purity of 99.8%[2][4]. Furthermore, Sinopec has implemented advanced process control and real-time monitoring systems to ensure consistent product quality and maximize operational efficiency[6].
Strengths: High product yield and purity, flexibility in feedstock sources. Weaknesses: Complexity of the coupled process and potential high costs associated with catalyst systems.
Breakthrough Innovations
Process improvement for continuous ethyl acetate production
PatentInactiveUS6768021B2
Innovation
- The process involves using a membrane separation unit to remove water from the condensed reaction stream, recycling the dried stream back into the production process, and employing an additional distillation zone to produce purified ethyl acetate with minimal acid content, thereby optimizing water management and increasing process capacity.
Ethyl Acetate Production
PatentActiveUS20120178962A1
Innovation
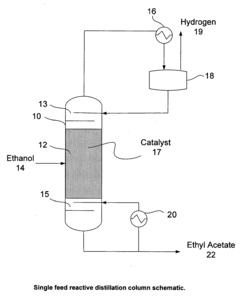
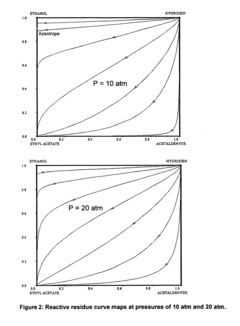
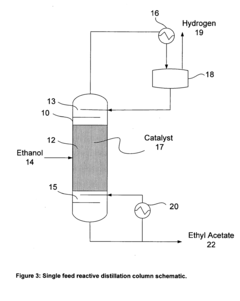
- A reactive distillation process using a single reactive distillation column where ethanol is dehydrogenated over a catalyst to produce ethyl acetate and hydrogen, with optional hydrogenation of byproducts to simplify separation and achieve high purity ethyl acetate, utilizing catalysts like copper, ruthenium, and platinum supported on materials like carbon or alumina.
Environmental Impact
The production of ethyl acetate, while essential for various industries, has significant environmental implications that warrant careful consideration. Traditional manufacturing processes have been associated with substantial environmental impacts, primarily due to the use of fossil fuel-based feedstocks and energy-intensive production methods. These conventional approaches often result in high carbon emissions, contributing to climate change and air pollution.
Recent innovations in ethyl acetate production techniques have focused on mitigating these environmental concerns. One key area of improvement has been the shift towards bio-based feedstocks. By utilizing renewable resources such as ethanol derived from agricultural waste or other biomass sources, manufacturers can significantly reduce the carbon footprint of ethyl acetate production. This approach not only decreases reliance on fossil fuels but also promotes a more circular economy by repurposing waste materials.
Water consumption and wastewater generation have been other critical environmental issues associated with ethyl acetate production. Innovative water management systems and closed-loop processes have been developed to address these challenges. These technologies aim to minimize water usage, recycle process water, and reduce the volume of wastewater requiring treatment. Such advancements not only conserve water resources but also diminish the potential for water pollution.
Energy efficiency improvements have played a crucial role in reducing the environmental impact of ethyl acetate production. The implementation of advanced catalysts and optimized reaction conditions has led to lower energy requirements and improved yields. Additionally, the integration of heat recovery systems and the use of renewable energy sources in production facilities have further decreased the overall energy footprint of the manufacturing process.
Emissions control has been another focal point for environmental improvements. Modern production techniques incorporate sophisticated air pollution control systems to capture and treat volatile organic compounds (VOCs) and other potentially harmful emissions. These systems not only ensure compliance with increasingly stringent environmental regulations but also contribute to better air quality in surrounding communities.
The development of novel reactor designs and process intensification strategies has also contributed to environmental benefits. These innovations often result in smaller plant footprints, reduced equipment requirements, and more efficient use of resources. Consequently, they lead to decreased land use, lower material consumption, and minimized waste generation throughout the production lifecycle.
As the chemical industry continues to prioritize sustainability, ongoing research is exploring even more environmentally friendly approaches to ethyl acetate production. This includes investigating the potential of enzymatic processes, which could operate under milder conditions and potentially eliminate the need for harsh chemicals or extreme temperatures. Such bio-catalytic methods hold promise for further reducing the environmental footprint of ethyl acetate manufacturing in the future.
Recent innovations in ethyl acetate production techniques have focused on mitigating these environmental concerns. One key area of improvement has been the shift towards bio-based feedstocks. By utilizing renewable resources such as ethanol derived from agricultural waste or other biomass sources, manufacturers can significantly reduce the carbon footprint of ethyl acetate production. This approach not only decreases reliance on fossil fuels but also promotes a more circular economy by repurposing waste materials.
Water consumption and wastewater generation have been other critical environmental issues associated with ethyl acetate production. Innovative water management systems and closed-loop processes have been developed to address these challenges. These technologies aim to minimize water usage, recycle process water, and reduce the volume of wastewater requiring treatment. Such advancements not only conserve water resources but also diminish the potential for water pollution.
Energy efficiency improvements have played a crucial role in reducing the environmental impact of ethyl acetate production. The implementation of advanced catalysts and optimized reaction conditions has led to lower energy requirements and improved yields. Additionally, the integration of heat recovery systems and the use of renewable energy sources in production facilities have further decreased the overall energy footprint of the manufacturing process.
Emissions control has been another focal point for environmental improvements. Modern production techniques incorporate sophisticated air pollution control systems to capture and treat volatile organic compounds (VOCs) and other potentially harmful emissions. These systems not only ensure compliance with increasingly stringent environmental regulations but also contribute to better air quality in surrounding communities.
The development of novel reactor designs and process intensification strategies has also contributed to environmental benefits. These innovations often result in smaller plant footprints, reduced equipment requirements, and more efficient use of resources. Consequently, they lead to decreased land use, lower material consumption, and minimized waste generation throughout the production lifecycle.
As the chemical industry continues to prioritize sustainability, ongoing research is exploring even more environmentally friendly approaches to ethyl acetate production. This includes investigating the potential of enzymatic processes, which could operate under milder conditions and potentially eliminate the need for harsh chemicals or extreme temperatures. Such bio-catalytic methods hold promise for further reducing the environmental footprint of ethyl acetate manufacturing in the future.
Regulatory Compliance
Regulatory compliance plays a crucial role in the production of ethyl acetate, ensuring the safety of workers, consumers, and the environment. As innovations in production techniques continue to emerge, manufacturers must navigate an increasingly complex regulatory landscape.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate production under the Toxic Substances Control Act (TSCA). Manufacturers must adhere to strict reporting requirements and implement risk management measures to mitigate potential environmental impacts. The Occupational Safety and Health Administration (OSHA) sets standards for workplace safety, including exposure limits and personal protective equipment requirements for workers handling ethyl acetate.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the production and use of ethyl acetate within its member states. Manufacturers must register their substances with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labeling, and Packaging (CLP) regulation further ensures that hazards associated with ethyl acetate are clearly communicated throughout the supply chain.
In Asia, countries like China and Japan have implemented their own chemical management systems. China's Measures for Environmental Management of New Chemical Substances requires manufacturers to obtain approval before producing or importing new chemical substances, including novel ethyl acetate production methods. Japan's Chemical Substances Control Law (CSCL) mandates similar registration and risk assessment procedures.
As production techniques evolve, regulatory bodies are adapting their frameworks to address emerging concerns. For instance, the increasing use of biocatalysts in ethyl acetate production has prompted discussions on how to regulate genetically modified organisms in industrial processes. Regulatory agencies are also focusing on the environmental impact of production methods, encouraging the development of greener technologies through incentives and stricter emissions standards.
Compliance with these regulations often necessitates significant investments in technology and infrastructure. Manufacturers must implement robust quality management systems, conduct regular audits, and maintain detailed documentation of their production processes. Advanced monitoring and control systems are becoming essential to ensure consistent compliance with emission limits and product quality standards.
The global nature of the ethyl acetate market adds another layer of complexity to regulatory compliance. Manufacturers operating in multiple jurisdictions must navigate a patchwork of regulations, often requiring region-specific adaptations to their production processes. This has led to increased collaboration between regulatory bodies to harmonize standards and facilitate international trade while maintaining high safety and environmental protection levels.
In the United States, the Environmental Protection Agency (EPA) regulates ethyl acetate production under the Toxic Substances Control Act (TSCA). Manufacturers must adhere to strict reporting requirements and implement risk management measures to mitigate potential environmental impacts. The Occupational Safety and Health Administration (OSHA) sets standards for workplace safety, including exposure limits and personal protective equipment requirements for workers handling ethyl acetate.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation governs the production and use of ethyl acetate within its member states. Manufacturers must register their substances with the European Chemicals Agency (ECHA) and provide comprehensive safety data. The Classification, Labeling, and Packaging (CLP) regulation further ensures that hazards associated with ethyl acetate are clearly communicated throughout the supply chain.
In Asia, countries like China and Japan have implemented their own chemical management systems. China's Measures for Environmental Management of New Chemical Substances requires manufacturers to obtain approval before producing or importing new chemical substances, including novel ethyl acetate production methods. Japan's Chemical Substances Control Law (CSCL) mandates similar registration and risk assessment procedures.
As production techniques evolve, regulatory bodies are adapting their frameworks to address emerging concerns. For instance, the increasing use of biocatalysts in ethyl acetate production has prompted discussions on how to regulate genetically modified organisms in industrial processes. Regulatory agencies are also focusing on the environmental impact of production methods, encouraging the development of greener technologies through incentives and stricter emissions standards.
Compliance with these regulations often necessitates significant investments in technology and infrastructure. Manufacturers must implement robust quality management systems, conduct regular audits, and maintain detailed documentation of their production processes. Advanced monitoring and control systems are becoming essential to ensure consistent compliance with emission limits and product quality standards.
The global nature of the ethyl acetate market adds another layer of complexity to regulatory compliance. Manufacturers operating in multiple jurisdictions must navigate a patchwork of regulations, often requiring region-specific adaptations to their production processes. This has led to increased collaboration between regulatory bodies to harmonize standards and facilitate international trade while maintaining high safety and environmental protection levels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!