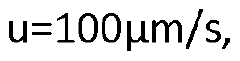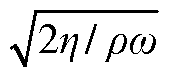Measure Analyte Carryover in HPLC—Actionable Solutions
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Carryover Background and Objectives
High-performance liquid chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical laboratories. The phenomenon of analyte carryover—where residual analytes from previous injections contaminate subsequent analyses—has been a persistent challenge throughout HPLC's development history. This technical challenge has gained increasing attention as analytical sensitivity requirements have become more stringent, with detection limits pushing into the parts-per-trillion range.
The evolution of HPLC technology has seen remarkable improvements in column technology, detector sensitivity, and system automation. However, these advancements have paradoxically made carryover issues more apparent and problematic. As detection capabilities have improved, even minute traces of carryover can now be detected and quantified, potentially compromising data integrity and analytical reliability.
Current industry trends indicate a growing emphasis on ultra-high-performance liquid chromatography (UHPLC) systems, which operate at higher pressures and with smaller particle size columns. While these systems offer enhanced resolution and faster analysis times, they often present unique carryover challenges due to their complex flow paths and higher operating pressures.
Regulatory bodies worldwide, including the FDA, EMA, and ICH, have established increasingly stringent guidelines for analytical method validation, with specific attention to carryover as a critical performance parameter. These regulatory demands have further intensified the need for effective carryover mitigation strategies in modern analytical laboratories.
The primary technical objectives of addressing analyte carryover in HPLC systems include: identifying the root causes and mechanisms of carryover in various system components; developing systematic approaches to measure and quantify carryover accurately; establishing standardized protocols for carryover assessment across different instrument platforms; and implementing effective preventive and corrective measures to minimize carryover to acceptable levels.
Additionally, there is a growing need to understand how different analyte classes interact with system components, as hydrophobic compounds, proteins, and metal-chelating agents each present unique carryover challenges. The ultimate goal is to develop comprehensive solutions that address carryover at its source rather than merely implementing post-analysis corrections or workarounds.
As analytical requirements continue to become more demanding, particularly in fields such as bioanalysis and trace contaminant detection, the importance of controlling carryover cannot be overstated. Future HPLC technology development will likely incorporate innovative materials and design approaches specifically engineered to minimize analyte adsorption and subsequent release, thereby reducing carryover to negligible levels.
The evolution of HPLC technology has seen remarkable improvements in column technology, detector sensitivity, and system automation. However, these advancements have paradoxically made carryover issues more apparent and problematic. As detection capabilities have improved, even minute traces of carryover can now be detected and quantified, potentially compromising data integrity and analytical reliability.
Current industry trends indicate a growing emphasis on ultra-high-performance liquid chromatography (UHPLC) systems, which operate at higher pressures and with smaller particle size columns. While these systems offer enhanced resolution and faster analysis times, they often present unique carryover challenges due to their complex flow paths and higher operating pressures.
Regulatory bodies worldwide, including the FDA, EMA, and ICH, have established increasingly stringent guidelines for analytical method validation, with specific attention to carryover as a critical performance parameter. These regulatory demands have further intensified the need for effective carryover mitigation strategies in modern analytical laboratories.
The primary technical objectives of addressing analyte carryover in HPLC systems include: identifying the root causes and mechanisms of carryover in various system components; developing systematic approaches to measure and quantify carryover accurately; establishing standardized protocols for carryover assessment across different instrument platforms; and implementing effective preventive and corrective measures to minimize carryover to acceptable levels.
Additionally, there is a growing need to understand how different analyte classes interact with system components, as hydrophobic compounds, proteins, and metal-chelating agents each present unique carryover challenges. The ultimate goal is to develop comprehensive solutions that address carryover at its source rather than merely implementing post-analysis corrections or workarounds.
As analytical requirements continue to become more demanding, particularly in fields such as bioanalysis and trace contaminant detection, the importance of controlling carryover cannot be overstated. Future HPLC technology development will likely incorporate innovative materials and design approaches specifically engineered to minimize analyte adsorption and subsequent release, thereby reducing carryover to negligible levels.
Market Demand for Improved HPLC Carryover Control
The global HPLC (High-Performance Liquid Chromatography) market continues to expand rapidly, with a current valuation exceeding $4.5 billion and projected growth rates of 6-8% annually through 2028. Within this expanding market, the demand for improved carryover control solutions has emerged as a critical focus area for laboratories across pharmaceutical, clinical, environmental, and food safety sectors.
Pharmaceutical companies, representing the largest segment of HPLC users at approximately 40% of the market, face increasingly stringent regulatory requirements for analytical precision. FDA and EMA guidelines have progressively lowered acceptable carryover thresholds from 1% to as low as 0.1% for certain applications, creating substantial demand for advanced carryover mitigation technologies.
Clinical laboratories constitute another significant market segment driving demand for improved carryover solutions. With the rise of precision medicine and therapeutic drug monitoring, these facilities routinely analyze samples with concentration differences spanning several orders of magnitude. Market research indicates that approximately 65% of clinical laboratory directors identify carryover as a persistent challenge affecting workflow efficiency and result reliability.
Contract Research Organizations (CROs) represent a rapidly growing market segment with particularly acute carryover concerns. The CRO market, expanding at nearly 10% annually, handles diverse sample types with widely varying analyte concentrations. Industry surveys reveal that 72% of CROs have implemented specialized carryover prevention protocols, indicating strong market demand for more efficient solutions.
From an economic perspective, laboratories are increasingly recognizing the hidden costs associated with carryover issues. A recent industry analysis estimated that carryover-related retesting costs the average high-throughput laboratory between $50,000-$150,000 annually in wasted reagents, staff time, and delayed reporting. This economic impact has transformed carryover control from a technical consideration to a business imperative.
The market is also witnessing a shift toward automated carryover prevention solutions. Approximately 58% of laboratory managers express preference for integrated carryover control features in new HPLC systems rather than manual intervention protocols. This trend is particularly pronounced in high-throughput environments where operational efficiency is paramount.
Geographically, North America represents the largest market for advanced carryover solutions (38% market share), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 9.5% annually, driven by expanding pharmaceutical manufacturing and contract research operations in China and India.
Pharmaceutical companies, representing the largest segment of HPLC users at approximately 40% of the market, face increasingly stringent regulatory requirements for analytical precision. FDA and EMA guidelines have progressively lowered acceptable carryover thresholds from 1% to as low as 0.1% for certain applications, creating substantial demand for advanced carryover mitigation technologies.
Clinical laboratories constitute another significant market segment driving demand for improved carryover solutions. With the rise of precision medicine and therapeutic drug monitoring, these facilities routinely analyze samples with concentration differences spanning several orders of magnitude. Market research indicates that approximately 65% of clinical laboratory directors identify carryover as a persistent challenge affecting workflow efficiency and result reliability.
Contract Research Organizations (CROs) represent a rapidly growing market segment with particularly acute carryover concerns. The CRO market, expanding at nearly 10% annually, handles diverse sample types with widely varying analyte concentrations. Industry surveys reveal that 72% of CROs have implemented specialized carryover prevention protocols, indicating strong market demand for more efficient solutions.
From an economic perspective, laboratories are increasingly recognizing the hidden costs associated with carryover issues. A recent industry analysis estimated that carryover-related retesting costs the average high-throughput laboratory between $50,000-$150,000 annually in wasted reagents, staff time, and delayed reporting. This economic impact has transformed carryover control from a technical consideration to a business imperative.
The market is also witnessing a shift toward automated carryover prevention solutions. Approximately 58% of laboratory managers express preference for integrated carryover control features in new HPLC systems rather than manual intervention protocols. This trend is particularly pronounced in high-throughput environments where operational efficiency is paramount.
Geographically, North America represents the largest market for advanced carryover solutions (38% market share), followed by Europe (29%) and Asia-Pacific (24%). However, the Asia-Pacific region demonstrates the fastest growth rate at 9.5% annually, driven by expanding pharmaceutical manufacturing and contract research operations in China and India.
Current Challenges in HPLC Carryover Reduction
Despite significant advancements in HPLC technology, analyte carryover remains a persistent challenge that compromises data integrity and analytical reliability. Current HPLC systems face several critical obstacles in effectively reducing carryover. The most prevalent issue involves sample residue adhesion to various system components, particularly injection needles, valve surfaces, and connection tubing where analytes can adsorb through hydrophobic or ionic interactions.
Material compatibility presents another significant challenge, as certain compounds demonstrate strong affinity for stainless steel components, creating persistent carryover issues that standard cleaning procedures cannot adequately address. This is especially problematic with basic compounds that interact with metal surfaces through silanol interactions.
Autosampler design limitations contribute substantially to carryover problems. Many current systems utilize fixed needle designs that are more susceptible to contamination compared to newer movable needle technologies. The needle-seat interface in particular represents a critical area where residual sample can accumulate and subsequently contaminate following injections.
Inadequate washing protocols represent another major hurdle in carryover reduction. Standard flush procedures often employ insufficient solvent volumes or inappropriate solvent compositions that fail to effectively remove strongly retained analytes. The timing and sequence of washing steps frequently prove suboptimal for complex sample matrices.
Temperature control deficiencies further exacerbate carryover issues. Many systems lack precise temperature regulation throughout the sample path, leading to inconsistent dissolution and potential precipitation of analytes in cooler regions of the flow path. This creates "cold spots" where analytes can accumulate and gradually release into subsequent samples.
Sample complexity introduces additional complications, particularly with biological matrices containing proteins and lipids that can adhere to surfaces and trap analytes. These matrix effects often require specialized cleaning approaches beyond standard wash procedures.
Method development constraints also impede effective carryover reduction. Analysts frequently face trade-offs between thorough system cleaning and analytical throughput, leading to compromised washing procedures that prioritize speed over complete carryover elimination.
The detection and quantification of carryover itself presents challenges, as low-level carryover may fall below detection limits in standard validation protocols yet still impact sensitive analyses. This creates a false sense of system cleanliness when carryover remains present at analytically significant levels.
Material compatibility presents another significant challenge, as certain compounds demonstrate strong affinity for stainless steel components, creating persistent carryover issues that standard cleaning procedures cannot adequately address. This is especially problematic with basic compounds that interact with metal surfaces through silanol interactions.
Autosampler design limitations contribute substantially to carryover problems. Many current systems utilize fixed needle designs that are more susceptible to contamination compared to newer movable needle technologies. The needle-seat interface in particular represents a critical area where residual sample can accumulate and subsequently contaminate following injections.
Inadequate washing protocols represent another major hurdle in carryover reduction. Standard flush procedures often employ insufficient solvent volumes or inappropriate solvent compositions that fail to effectively remove strongly retained analytes. The timing and sequence of washing steps frequently prove suboptimal for complex sample matrices.
Temperature control deficiencies further exacerbate carryover issues. Many systems lack precise temperature regulation throughout the sample path, leading to inconsistent dissolution and potential precipitation of analytes in cooler regions of the flow path. This creates "cold spots" where analytes can accumulate and gradually release into subsequent samples.
Sample complexity introduces additional complications, particularly with biological matrices containing proteins and lipids that can adhere to surfaces and trap analytes. These matrix effects often require specialized cleaning approaches beyond standard wash procedures.
Method development constraints also impede effective carryover reduction. Analysts frequently face trade-offs between thorough system cleaning and analytical throughput, leading to compromised washing procedures that prioritize speed over complete carryover elimination.
The detection and quantification of carryover itself presents challenges, as low-level carryover may fall below detection limits in standard validation protocols yet still impact sensitive analyses. This creates a false sense of system cleanliness when carryover remains present at analytically significant levels.
Current Methodologies for Carryover Measurement and Reduction
01 Washing and cleaning methods to reduce carryover
Various washing and cleaning methods can be employed in HPLC systems to reduce analyte carryover. These include using specific washing solutions, implementing automated cleaning cycles between sample injections, and optimizing rinse procedures. Effective washing protocols can significantly reduce the amount of residual analyte that might contaminate subsequent samples, thereby improving the accuracy and reliability of HPLC analyses.- Washing and cleaning methods to reduce carryover: Various washing and cleaning methods can be employed in HPLC systems to reduce analyte carryover between sample injections. These methods include using specific washing solutions, implementing multi-step cleaning procedures, and optimizing washing parameters such as duration and pressure. Effective cleaning protocols can significantly reduce residual analytes in the system, particularly in the injection port, needle, and flow path components.
- System design modifications to minimize carryover: Modifications to HPLC system design can help minimize analyte carryover. These include specialized injection port designs, needle geometry improvements, flow path optimizations, and materials selection for components that contact samples. Design innovations focus on reducing dead volumes, eliminating areas where analytes can adhere, and creating more efficient flow paths that prevent sample retention in the system.
- Sample preparation techniques to address carryover issues: Specific sample preparation techniques can be employed to address carryover issues in HPLC analysis. These include sample dilution, filtration methods, addition of specific additives to prevent analyte adsorption, and optimization of sample matrices. Proper sample preparation can reduce the concentration of problematic analytes and modify their chemical properties to decrease their tendency to adhere to system components.
- Mobile phase composition and gradient optimization: The composition of mobile phases and gradient profiles can significantly impact analyte carryover in HPLC systems. Optimization strategies include adjusting pH levels, incorporating organic modifiers, adding ion-pairing agents, and designing specific gradient profiles that help flush residual analytes from the system. Careful selection of mobile phase components can reduce analyte-surface interactions and improve system cleanliness between injections.
- Automated carryover prevention and detection systems: Automated systems for carryover prevention and detection have been developed for HPLC applications. These include intelligent washing sequences, real-time carryover monitoring, automated system suitability testing, and software algorithms that can detect and correct for carryover effects. These automated approaches can adapt cleaning protocols based on sample characteristics and provide more consistent results across different analytes and matrices.
02 System design modifications to minimize carryover
Modifications to HPLC system design can help minimize analyte carryover. These include using inert materials for sample flow paths, optimizing injector designs, implementing specialized valves, and creating dedicated flow paths that reduce dead volumes where analytes might accumulate. Such design improvements can significantly reduce the potential for sample-to-sample contamination in high-sensitivity analytical applications.Expand Specific Solutions03 Mobile phase and solvent selection strategies
The selection of appropriate mobile phases and solvents plays a crucial role in reducing HPLC carryover. Using stronger solvents for washing steps, optimizing mobile phase composition, and implementing gradient elution techniques can help remove strongly adsorbed analytes from the system. Strategic solvent selection based on analyte properties can effectively minimize carryover issues in challenging analytical applications.Expand Specific Solutions04 Sample preparation techniques to prevent carryover
Proper sample preparation techniques can prevent or reduce carryover in HPLC analyses. These include sample dilution, filtration, protein precipitation, and the use of appropriate additives to reduce analyte adsorption. By minimizing the concentration of problematic analytes and removing components that might contribute to carryover, these techniques help maintain the integrity of sequential analyses.Expand Specific Solutions05 Detection and quantification methods for carryover assessment
Various detection and quantification methods can be employed to assess and monitor HPLC carryover. These include running blank injections between samples, using internal standards, implementing statistical approaches to quantify carryover, and developing specialized detection protocols. These methods allow analysts to identify carryover issues, establish acceptable limits, and validate that carryover reduction strategies are effective for specific analytical methods.Expand Specific Solutions
Leading Manufacturers and Research Groups in HPLC Technology
The HPLC analyte carryover market is currently in a growth phase, driven by increasing demands for precision in pharmaceutical and clinical analyses. The global market size for HPLC technologies is expanding steadily, with major players developing innovative solutions to address carryover challenges. Leading companies like Agilent Technologies, Shimadzu, and Roche Diagnostics demonstrate varying levels of technical maturity in their approaches. Agilent and Shimadzu offer advanced washing protocols and system designs specifically targeting carryover reduction, while pharmaceutical giants like F. Hoffmann-La Roche and diagnostic specialists like Beckman Coulter are integrating carryover mitigation into their comprehensive analytical platforms. GERSTEL and other specialized manufacturers are focusing on automated sample preparation systems that minimize contamination risks, indicating a trend toward integrated solutions rather than standalone fixes.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed proprietary carryover reduction technologies for their analytical platforms, focusing particularly on bioanalytical applications where carryover is most problematic. Their approach includes specialized sample preparation protocols integrated with their HPLC systems that minimize analyte adsorption before injection. The company has implemented advanced materials science in their flow path components, utilizing proprietary surface treatments that significantly reduce protein and peptide binding. Roche's systems feature intelligent sample scheduling algorithms that automatically implement application-specific washing protocols based on sample type and concentration. Their technology incorporates dedicated carryover evaluation tools during method development that systematically identify potential sources of carryover and implement targeted mitigation strategies. Additionally, Roche has developed specialized cleaning solutions and procedures specifically formulated for different analyte classes, allowing for optimized carryover reduction based on the chemical properties of the target compounds. Their integrated approach combines hardware innovations with methodological solutions to address carryover comprehensively across their analytical platforms.
Strengths: Specialized expertise in bioanalytical applications where carryover is particularly challenging; comprehensive approach that addresses both hardware and methodological aspects; extensive validation protocols for carryover assessment. Weaknesses: Solutions often optimized for pharmaceutical and clinical applications rather than general-purpose analysis; some technologies may be proprietary and not fully disclosed.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed comprehensive carryover mitigation strategies for HPLC systems through their InfinityLab series. Their approach includes advanced needle wash systems with programmable internal and external needle washing capabilities, utilizing both weak and strong solvents to remove residual analytes. The company has implemented dual-mode needle wash technology that combines both pre-injection and post-injection washing protocols to minimize sample-to-sample contamination. Agilent's systems feature specialized flow path designs with minimized dead volumes and biocompatible materials that reduce adsorption of analytes to surfaces. Their InfinityLab Poroshell columns incorporate particle technology specifically designed to reduce carryover through optimized surface chemistry and reduced secondary interactions. Additionally, Agilent has developed intelligent software algorithms that can detect potential carryover issues and automatically implement corrective actions during method development and routine analysis.
Strengths: Comprehensive integrated hardware and software solutions specifically designed to address carryover; industry-leading column technology with specialized surface treatments; extensive application support and method development resources. Weaknesses: Premium pricing structure may be prohibitive for smaller laboratories; some solutions require complete Agilent ecosystem adoption for optimal performance.
Key Innovations in Carryover Prevention Technologies
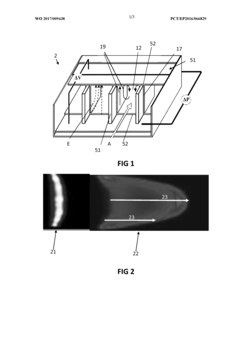
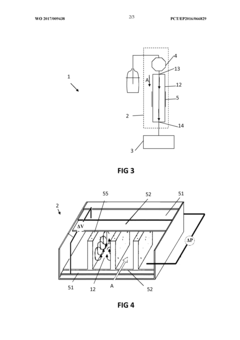
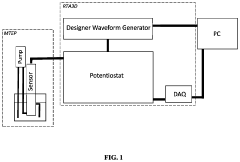
High-performance liquid chromatography with a controllable transverse flow inducer
PatentWO2017009438A1
Innovation
- The use of a controllable transverse flow inducer, such as an array of electrodes generating an alternating current electrokinetic field, to create micro-scale vortices that reduce dispersion and enhance mass transfer between support structures in the chromatography column, allowing for efficient separation without permanent surface charges and minimizing direct contact with electrodes.
System for the Simultaneous Monitoring of Constituents of an Electroplating Bath
PatentPendingUS20240133074A1
Innovation
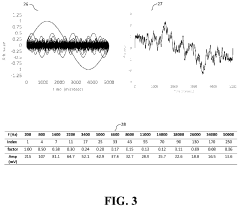
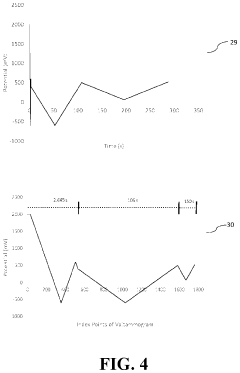
- The development of novel second-order, consolidated voltammetric waveforms combined with chemometric analysis and data compression techniques allows for the simultaneous measurement and analysis of all electroplating bath constituents without pretreatment, using a multi-frequency, variable amplitude waveform to generate a diagnostic voltammetric output that captures the synergistic interactions and maintains process control within the electroplating process.
Regulatory Compliance for HPLC Analysis in Pharmaceutical Applications
Regulatory compliance represents a critical framework governing HPLC analysis in pharmaceutical applications, particularly when addressing analyte carryover issues. The pharmaceutical industry operates under stringent regulatory requirements established by authorities such as the FDA, EMA, and ICH, which mandate specific validation parameters for analytical methods including carryover assessment.
The FDA's Guidance for Industry on Analytical Procedures and Methods Validation specifically addresses carryover as a critical performance characteristic that must be evaluated during method validation. According to 21 CFR Part 211, pharmaceutical manufacturers must establish and follow appropriate written procedures designed to prevent cross-contamination, which directly relates to carryover management in HPLC systems.
Similarly, the European Medicines Agency (EMA) guideline on bioanalytical method validation explicitly requires carryover evaluation, stating that carryover should not exceed 20% of the lower limit of quantification (LLOQ). This threshold has become an industry standard benchmark for acceptable carryover levels in many applications.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1) on Validation of Analytical Procedures, while not explicitly mentioning carryover, establish principles for method specificity that inherently require carryover control. These guidelines emphasize the importance of demonstrating that the analytical procedure is unaffected by interfering substances, which includes residual analytes from previous injections.
Compliance with USP <621> Chromatography standards requires system suitability testing, which may include carryover assessment depending on the specific application. For certain pharmaceutical analyses, especially those involving highly potent compounds or compounds with significant clinical implications, regulatory bodies may require more stringent carryover limits than the general 0.1-0.5% typically considered acceptable in routine analyses.
Documentation requirements for carryover studies are substantial, necessitating detailed records of carryover evaluation protocols, acceptance criteria, results, and corrective actions when specifications are not met. This documentation forms part of the analytical method validation package that undergoes regulatory review during drug approval processes.
For pharmaceutical laboratories, implementing a risk-based approach to carryover management aligns with modern regulatory expectations. This involves categorizing analytes based on their potency, toxicity, and therapeutic index, then establishing appropriate carryover limits and control strategies proportionate to the identified risks.
The FDA's Guidance for Industry on Analytical Procedures and Methods Validation specifically addresses carryover as a critical performance characteristic that must be evaluated during method validation. According to 21 CFR Part 211, pharmaceutical manufacturers must establish and follow appropriate written procedures designed to prevent cross-contamination, which directly relates to carryover management in HPLC systems.
Similarly, the European Medicines Agency (EMA) guideline on bioanalytical method validation explicitly requires carryover evaluation, stating that carryover should not exceed 20% of the lower limit of quantification (LLOQ). This threshold has become an industry standard benchmark for acceptable carryover levels in many applications.
The International Conference on Harmonisation (ICH) guidelines, particularly ICH Q2(R1) on Validation of Analytical Procedures, while not explicitly mentioning carryover, establish principles for method specificity that inherently require carryover control. These guidelines emphasize the importance of demonstrating that the analytical procedure is unaffected by interfering substances, which includes residual analytes from previous injections.
Compliance with USP <621> Chromatography standards requires system suitability testing, which may include carryover assessment depending on the specific application. For certain pharmaceutical analyses, especially those involving highly potent compounds or compounds with significant clinical implications, regulatory bodies may require more stringent carryover limits than the general 0.1-0.5% typically considered acceptable in routine analyses.
Documentation requirements for carryover studies are substantial, necessitating detailed records of carryover evaluation protocols, acceptance criteria, results, and corrective actions when specifications are not met. This documentation forms part of the analytical method validation package that undergoes regulatory review during drug approval processes.
For pharmaceutical laboratories, implementing a risk-based approach to carryover management aligns with modern regulatory expectations. This involves categorizing analytes based on their potency, toxicity, and therapeutic index, then establishing appropriate carryover limits and control strategies proportionate to the identified risks.
Cost-Benefit Analysis of Carryover Reduction Strategies
When evaluating strategies to reduce carryover in HPLC systems, a comprehensive cost-benefit analysis is essential for making informed decisions. Initial investments in advanced hardware solutions such as specialized autosampler designs or dedicated wash systems typically range from $5,000 to $20,000, representing significant capital expenditure. However, these investments often yield substantial long-term returns through improved data quality and reduced reanalysis requirements.
Procedural modifications, including optimized needle wash protocols and extended rinse cycles, present a more economical approach with minimal direct costs. The primary expense associated with these strategies is the increased analysis time, which may reduce laboratory throughput by 5-15% depending on implementation specifics. This throughput reduction translates to approximately $10-30 per sample batch in opportunity costs for high-volume laboratories.
Mobile phase modification strategies occupy a middle ground in the cost spectrum. Incorporating specialized additives or adjusting organic solvent ratios typically adds $2-5 per liter to mobile phase preparation costs. While modest in isolation, these expenses can accumulate significantly in high-throughput environments processing thousands of samples monthly.
The benefits side of the equation must consider both quantifiable and qualitative factors. Quantifiable benefits include reduced reanalysis rates (typically 10-30% improvement), decreased investigation time for anomalous results (saving 2-5 hours per incident), and extended column lifespans (15-25% increase). For a medium-sized analytical laboratory, these improvements can translate to annual savings of $30,000-75,000.
Risk mitigation represents a critical yet often undervalued benefit. Carryover-induced false positives in regulated environments may trigger extensive investigations, regulatory reporting, and potential compliance issues. The average cost of a single major carryover-related investigation exceeds $15,000 when accounting for personnel time, documentation requirements, and potential production delays.
Return on investment calculations indicate that hardware solutions typically achieve breakeven within 12-18 months, while procedural and mobile phase modifications often deliver positive returns within 3-6 months. The optimal strategy frequently involves a tiered approach, implementing low-cost procedural changes immediately while planning for strategic hardware investments based on carryover reduction requirements and laboratory workflow demands.
Procedural modifications, including optimized needle wash protocols and extended rinse cycles, present a more economical approach with minimal direct costs. The primary expense associated with these strategies is the increased analysis time, which may reduce laboratory throughput by 5-15% depending on implementation specifics. This throughput reduction translates to approximately $10-30 per sample batch in opportunity costs for high-volume laboratories.
Mobile phase modification strategies occupy a middle ground in the cost spectrum. Incorporating specialized additives or adjusting organic solvent ratios typically adds $2-5 per liter to mobile phase preparation costs. While modest in isolation, these expenses can accumulate significantly in high-throughput environments processing thousands of samples monthly.
The benefits side of the equation must consider both quantifiable and qualitative factors. Quantifiable benefits include reduced reanalysis rates (typically 10-30% improvement), decreased investigation time for anomalous results (saving 2-5 hours per incident), and extended column lifespans (15-25% increase). For a medium-sized analytical laboratory, these improvements can translate to annual savings of $30,000-75,000.
Risk mitigation represents a critical yet often undervalued benefit. Carryover-induced false positives in regulated environments may trigger extensive investigations, regulatory reporting, and potential compliance issues. The average cost of a single major carryover-related investigation exceeds $15,000 when accounting for personnel time, documentation requirements, and potential production delays.
Return on investment calculations indicate that hardware solutions typically achieve breakeven within 12-18 months, while procedural and mobile phase modifications often deliver positive returns within 3-6 months. The optimal strategy frequently involves a tiered approach, implementing low-cost procedural changes immediately while planning for strategic hardware investments based on carryover reduction requirements and laboratory workflow demands.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!