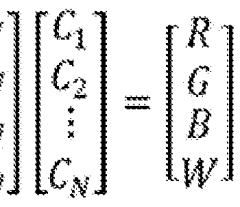
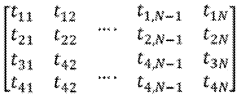Microfluidics vs Flow Cytometry: Parallel Testing Advantages
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics and Flow Cytometry Background and Objectives
Microfluidics and flow cytometry represent two pivotal technologies in biomedical research and clinical diagnostics, each with distinct historical trajectories and technological foundations. Flow cytometry emerged in the 1960s as a method to analyze multiple physical and chemical characteristics of single cells simultaneously, revolutionizing immunology, hematology, and cancer research. The technology evolved from simple cell counters to sophisticated multi-parameter systems capable of analyzing thousands of cells per second with high precision.
Microfluidics, conversely, developed primarily in the 1990s, leveraging semiconductor fabrication techniques to manipulate fluids at the microscale. This technology enables precise control over small volumes of liquids, facilitating complex biochemical reactions and cellular analyses in miniaturized systems. The field has expanded dramatically with applications ranging from point-of-care diagnostics to organ-on-chip platforms.
The convergence of these technologies presents significant opportunities for parallel testing applications. Traditional flow cytometry excels in high-throughput single-cell analysis but typically requires substantial sample volumes and expensive instrumentation. Microfluidic systems offer advantages in sample efficiency, reagent consumption, and potential for integration into portable devices, albeit often with lower throughput than conventional flow cytometers.
Recent technological advancements have begun bridging these limitations. Microfluidic flow cytometry systems combine the high-throughput capabilities of flow cytometry with the precise fluid handling of microfluidics, enabling multiplexed analysis of limited samples. This integration supports applications in rare cell detection, single-cell genomics, and personalized medicine where sample conservation is critical.
The primary objective of exploring the parallel testing advantages of microfluidics versus flow cytometry is to identify optimal technological approaches for different analytical scenarios. This includes evaluating throughput capabilities, sensitivity thresholds, sample requirements, and implementation costs across various applications. Additionally, this investigation aims to identify synergistic opportunities where combined approaches might overcome the limitations of each individual technology.
Understanding the complementary strengths of these technologies will guide future development of integrated systems that maximize analytical power while minimizing resource requirements. The ultimate goal is to advance diagnostic capabilities, research tools, and therapeutic monitoring systems that can operate with greater efficiency, accessibility, and clinical relevance than current standalone technologies.
Microfluidics, conversely, developed primarily in the 1990s, leveraging semiconductor fabrication techniques to manipulate fluids at the microscale. This technology enables precise control over small volumes of liquids, facilitating complex biochemical reactions and cellular analyses in miniaturized systems. The field has expanded dramatically with applications ranging from point-of-care diagnostics to organ-on-chip platforms.
The convergence of these technologies presents significant opportunities for parallel testing applications. Traditional flow cytometry excels in high-throughput single-cell analysis but typically requires substantial sample volumes and expensive instrumentation. Microfluidic systems offer advantages in sample efficiency, reagent consumption, and potential for integration into portable devices, albeit often with lower throughput than conventional flow cytometers.
Recent technological advancements have begun bridging these limitations. Microfluidic flow cytometry systems combine the high-throughput capabilities of flow cytometry with the precise fluid handling of microfluidics, enabling multiplexed analysis of limited samples. This integration supports applications in rare cell detection, single-cell genomics, and personalized medicine where sample conservation is critical.
The primary objective of exploring the parallel testing advantages of microfluidics versus flow cytometry is to identify optimal technological approaches for different analytical scenarios. This includes evaluating throughput capabilities, sensitivity thresholds, sample requirements, and implementation costs across various applications. Additionally, this investigation aims to identify synergistic opportunities where combined approaches might overcome the limitations of each individual technology.
Understanding the complementary strengths of these technologies will guide future development of integrated systems that maximize analytical power while minimizing resource requirements. The ultimate goal is to advance diagnostic capabilities, research tools, and therapeutic monitoring systems that can operate with greater efficiency, accessibility, and clinical relevance than current standalone technologies.
Market Demand Analysis for Parallel Testing Technologies
The global market for parallel testing technologies has witnessed substantial growth in recent years, driven by increasing demand for high-throughput screening in pharmaceutical research, clinical diagnostics, and academic research. The combined market for microfluidics and flow cytometry was valued at approximately $13.2 billion in 2022 and is projected to reach $21.5 billion by 2027, representing a compound annual growth rate (CAGR) of 10.3%.
Healthcare applications dominate the demand landscape, accounting for nearly 65% of the total market share. Within this segment, clinical diagnostics represents the largest application area, followed by drug discovery and development. The rising prevalence of chronic diseases, including cancer and infectious diseases, has significantly boosted the demand for rapid and accurate diagnostic tools capable of parallel testing capabilities.
Pharmaceutical and biotechnology companies are increasingly adopting parallel testing technologies to accelerate drug discovery processes and reduce time-to-market for new therapeutics. These industries value the ability to simultaneously analyze multiple parameters from limited sample volumes, a key advantage offered by both microfluidics and flow cytometry platforms.
Academic and research institutions constitute another significant market segment, driven by expanding research activities in cell biology, immunology, and genomics. Government funding for life sciences research, particularly in developed economies, has further stimulated market growth in this sector.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to increasing healthcare expenditure, expanding research infrastructure, and growing awareness about advanced diagnostic technologies in countries like China, Japan, and India.
Consumer preferences are increasingly shifting toward integrated systems that offer automation, ease of use, and compatibility with existing laboratory workflows. End-users are demanding solutions that provide higher sensitivity, specificity, and reproducibility while reducing sample volume requirements and analysis time.
The COVID-19 pandemic has accelerated market growth by highlighting the importance of rapid, high-throughput diagnostic capabilities. This has led to increased investments in parallel testing technologies across both public and private sectors. Post-pandemic, this momentum is expected to continue as healthcare systems worldwide prioritize preparedness for future outbreaks.
Cost considerations remain a significant factor influencing market adoption, particularly in resource-limited settings. While the initial investment for advanced parallel testing platforms can be substantial, the long-term economic benefits through improved efficiency and reduced reagent consumption are driving market penetration in cost-sensitive regions.
Healthcare applications dominate the demand landscape, accounting for nearly 65% of the total market share. Within this segment, clinical diagnostics represents the largest application area, followed by drug discovery and development. The rising prevalence of chronic diseases, including cancer and infectious diseases, has significantly boosted the demand for rapid and accurate diagnostic tools capable of parallel testing capabilities.
Pharmaceutical and biotechnology companies are increasingly adopting parallel testing technologies to accelerate drug discovery processes and reduce time-to-market for new therapeutics. These industries value the ability to simultaneously analyze multiple parameters from limited sample volumes, a key advantage offered by both microfluidics and flow cytometry platforms.
Academic and research institutions constitute another significant market segment, driven by expanding research activities in cell biology, immunology, and genomics. Government funding for life sciences research, particularly in developed economies, has further stimulated market growth in this sector.
Regionally, North America leads the market with approximately 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, primarily due to increasing healthcare expenditure, expanding research infrastructure, and growing awareness about advanced diagnostic technologies in countries like China, Japan, and India.
Consumer preferences are increasingly shifting toward integrated systems that offer automation, ease of use, and compatibility with existing laboratory workflows. End-users are demanding solutions that provide higher sensitivity, specificity, and reproducibility while reducing sample volume requirements and analysis time.
The COVID-19 pandemic has accelerated market growth by highlighting the importance of rapid, high-throughput diagnostic capabilities. This has led to increased investments in parallel testing technologies across both public and private sectors. Post-pandemic, this momentum is expected to continue as healthcare systems worldwide prioritize preparedness for future outbreaks.
Cost considerations remain a significant factor influencing market adoption, particularly in resource-limited settings. While the initial investment for advanced parallel testing platforms can be substantial, the long-term economic benefits through improved efficiency and reduced reagent consumption are driving market penetration in cost-sensitive regions.
Technical Challenges in Microfluidics and Flow Cytometry
Despite significant advancements in both microfluidics and flow cytometry technologies, several technical challenges persist that limit their full potential in parallel testing applications. Flow cytometry, while established and powerful, faces limitations in throughput capacity when handling complex sample matrices. The high-pressure fluidics systems required for traditional flow cytometry create technical constraints that restrict miniaturization efforts and integration with other analytical platforms.
Microfluidic systems encounter challenges related to channel clogging, particularly when processing biological samples with varying viscosities or those containing cellular debris. This issue becomes more pronounced in long-duration testing scenarios, where consistent flow rates must be maintained for accurate comparative analysis. Additionally, the fabrication of microfluidic chips with precise channel dimensions remains technically demanding and often requires specialized equipment and expertise.
Surface chemistry considerations present significant hurdles in both technologies. In microfluidics, protein adsorption to channel walls can alter flow characteristics and introduce analytical bias. Similarly, flow cytometry systems must contend with cell adhesion issues that can compromise detection accuracy and system reliability over extended operational periods.
Integration of detection systems poses another substantial challenge. While flow cytometry typically employs sophisticated optical detection methods, miniaturizing these components for incorporation into microfluidic platforms without sacrificing sensitivity remains difficult. The alignment precision required between microchannels and optical components demands nanometer-scale manufacturing tolerances that are challenging to achieve consistently in production environments.
Data processing and analysis represent increasingly significant bottlenecks as both technologies advance toward higher throughput capabilities. Flow cytometry generates massive datasets that require sophisticated algorithms for meaningful interpretation, particularly when multiplexed markers are employed. Microfluidic systems, especially those designed for parallel testing, face similar computational challenges when processing real-time data from multiple channels simultaneously.
Temperature control and thermal management present ongoing challenges, particularly for temperature-sensitive assays. Maintaining uniform temperature distributions across microfluidic chips or within flow cytometry sample chambers requires sophisticated engineering solutions that add complexity to system design and operation.
Power requirements for portable or point-of-care applications remain problematic, especially for flow cytometry systems that traditionally rely on high-powered lasers and complex fluidics. While microfluidics offers advantages in this regard, achieving the sensitivity of laboratory-grade flow cytometry in low-power configurations continues to challenge researchers and engineers.
Cross-contamination between samples represents a persistent concern in parallel testing applications, requiring innovative approaches to sample isolation and system cleaning protocols that don't compromise throughput or analytical performance.
Microfluidic systems encounter challenges related to channel clogging, particularly when processing biological samples with varying viscosities or those containing cellular debris. This issue becomes more pronounced in long-duration testing scenarios, where consistent flow rates must be maintained for accurate comparative analysis. Additionally, the fabrication of microfluidic chips with precise channel dimensions remains technically demanding and often requires specialized equipment and expertise.
Surface chemistry considerations present significant hurdles in both technologies. In microfluidics, protein adsorption to channel walls can alter flow characteristics and introduce analytical bias. Similarly, flow cytometry systems must contend with cell adhesion issues that can compromise detection accuracy and system reliability over extended operational periods.
Integration of detection systems poses another substantial challenge. While flow cytometry typically employs sophisticated optical detection methods, miniaturizing these components for incorporation into microfluidic platforms without sacrificing sensitivity remains difficult. The alignment precision required between microchannels and optical components demands nanometer-scale manufacturing tolerances that are challenging to achieve consistently in production environments.
Data processing and analysis represent increasingly significant bottlenecks as both technologies advance toward higher throughput capabilities. Flow cytometry generates massive datasets that require sophisticated algorithms for meaningful interpretation, particularly when multiplexed markers are employed. Microfluidic systems, especially those designed for parallel testing, face similar computational challenges when processing real-time data from multiple channels simultaneously.
Temperature control and thermal management present ongoing challenges, particularly for temperature-sensitive assays. Maintaining uniform temperature distributions across microfluidic chips or within flow cytometry sample chambers requires sophisticated engineering solutions that add complexity to system design and operation.
Power requirements for portable or point-of-care applications remain problematic, especially for flow cytometry systems that traditionally rely on high-powered lasers and complex fluidics. While microfluidics offers advantages in this regard, achieving the sensitivity of laboratory-grade flow cytometry in low-power configurations continues to challenge researchers and engineers.
Cross-contamination between samples represents a persistent concern in parallel testing applications, requiring innovative approaches to sample isolation and system cleaning protocols that don't compromise throughput or analytical performance.
Current Parallel Testing Implementation Approaches
01 Integrated microfluidic flow cytometry systems
Integration of microfluidic technology with flow cytometry enables parallel testing capabilities in a single platform. These systems combine the precise fluid handling of microfluidics with the high-throughput analysis capabilities of flow cytometry, allowing for simultaneous measurement of multiple parameters from small sample volumes. The integration reduces sample consumption, increases throughput, and enables more complex analyses than traditional methods.- Integration of microfluidics with flow cytometry systems: Microfluidic devices can be integrated with flow cytometry systems to enhance sample processing and analysis capabilities. This integration allows for precise control of fluid flow, enabling more accurate cell sorting and analysis. The combined systems can perform parallel testing of multiple samples simultaneously, increasing throughput while reducing sample volume requirements. These integrated platforms offer advantages in terms of efficiency, sensitivity, and reproducibility for various biomedical applications.
- Parallel sample processing in microfluidic flow cytometry: Microfluidic platforms enable parallel processing of multiple samples for flow cytometric analysis. These systems incorporate multiple channels or chambers that can simultaneously handle different samples or testing conditions. Parallel processing significantly increases throughput while maintaining the precision of individual sample analysis. This approach is particularly valuable for high-content screening applications, clinical diagnostics, and research settings where multiple parameters need to be assessed concurrently.
- Microfluidic flow control for cytometric applications: Advanced flow control mechanisms in microfluidic devices enable precise manipulation of samples for cytometric analysis. These systems utilize various techniques including pressure-driven flow, electrokinetic control, and acoustic focusing to achieve optimal sample presentation to detection systems. Precise flow control allows for better alignment of cells or particles, reducing signal variability and improving measurement accuracy. These technologies are critical for applications requiring high sensitivity and reproducibility in parallel testing environments.
- Detection systems for microfluidic flow cytometry: Specialized detection systems have been developed for microfluidic flow cytometry platforms to enable parallel testing. These include optical detection arrays, electrical impedance sensors, and integrated spectroscopic systems that can simultaneously monitor multiple parameters across different sample streams. Multi-parameter detection capabilities allow for comprehensive characterization of biological samples in a single analysis run. These detection systems often incorporate advanced signal processing algorithms to handle the complex data generated from parallel testing configurations.
- Sample preparation and handling in microfluidic cytometry: Innovative sample preparation and handling techniques have been developed specifically for microfluidic flow cytometry systems. These include on-chip sample dilution, filtration, and enrichment processes that prepare samples for parallel analysis. Automated sample handling reduces operator variability and contamination risks while improving reproducibility. Some systems incorporate cell concentration or sorting capabilities prior to cytometric analysis, enhancing the detection of rare cell populations. These integrated sample preparation approaches are essential for maximizing the efficiency and reliability of parallel testing applications.
02 Parallel sample processing in microfluidic devices
Microfluidic devices designed for parallel sample processing incorporate multiple channels or chambers that allow simultaneous testing of different samples or conditions. These designs feature parallel flow paths, multiplexed detection systems, and automated sample handling to increase throughput while maintaining precision. Such parallel architectures are particularly valuable for high-throughput screening applications and clinical diagnostics.Expand Specific Solutions03 Flow control and fluid manipulation techniques
Advanced flow control mechanisms in microfluidic cytometry systems enable precise manipulation of samples for parallel testing. These techniques include pressure-driven flow, electrokinetic control, acoustic focusing, and valve-based systems that allow for dynamic control of fluid movement. Proper flow control ensures accurate particle positioning, reduces clogging, and enables switching between multiple sample streams for parallel analysis.Expand Specific Solutions04 Detection systems for parallel cytometric analysis
Specialized detection systems for microfluidic flow cytometry enable parallel measurement of multiple parameters. These include optical detection arrays, multi-wavelength fluorescence detection, impedance-based sensing, and integrated spectroscopic techniques. Advanced detection systems can simultaneously analyze multiple physical and biochemical properties of cells or particles across multiple channels, increasing the information density obtained from each test.Expand Specific Solutions05 Sample preparation and handling for parallel testing
Efficient sample preparation and handling methods are crucial for successful parallel testing in microfluidic flow cytometry. These include on-chip sample dilution, filtration, cell concentration, and labeling techniques that prepare multiple samples simultaneously. Automated sample introduction systems, particle focusing techniques, and methods to prevent cross-contamination between parallel channels ensure reliable and reproducible results in high-throughput applications.Expand Specific Solutions
Key Industry Players and Competitive Landscape
Microfluidics and flow cytometry technologies are currently in a mature growth phase, with the global market expected to reach $25 billion by 2025. The competitive landscape features established players like Becton Dickinson, Agilent Technologies, and Abbott Laboratories dominating flow cytometry, while academic institutions (University of California, Vanderbilt University) drive microfluidics innovation. Emerging companies like NanoCellect Biomedical and Kinetic River are developing hybrid technologies that leverage advantages of both platforms. Technical maturity varies significantly—flow cytometry represents a well-established technology with standardized protocols, while microfluidics offers greater miniaturization potential but faces commercialization challenges. The convergence of these technologies is creating new opportunities for parallel testing applications in clinical diagnostics and research.
Becton, Dickinson & Co.
Technical Solution: BD的技术方案整合了微流控和流式细胞术的优势,开发了BD FACSymphony™系统,这是一种高参数流式细胞仪,能够同时分析超过50个参数。该系统采用了微流控芯片进行样本处理和细胞分选,显著提高了并行测试能力。BD还推出了BD Rhapsody™单细胞分析系统,结合微流控技术和流式细胞术原理,实现了高通量单细胞测序和多组学分析。该系统使用微流控芯片捕获单个细胞,然后进行基因表达分析,可同时处理数千个细胞,大大提高了测试效率和数据质量。BD的技术方案特别注重样本制备自动化,减少了人为误差,提高了结果的一致性和可重复性。
优势:BD的系统集成度高,自动化程度高,减少了人为操作误差;其高通量并行测试能力显著提高了实验效率;技术成熟度高,市场验证充分。劣势:设备成本较高,对操作人员技术要求高;系统复杂度增加了维护难度;某些应用场景下可能存在灵活性不足的问题。
Beckman Coulter, Inc.
Technical Solution: Beckman Coulter开发了CytoFLEX平台,这是一种结合微流控技术和流式细胞术的创新系统。该平台采用专利的微流控细胞聚焦技术,无需使用鞘液即可实现精确的细胞定位,大大提高了检测效率和准确性。CytoFLEX系统能够同时检测多达30个参数,支持高通量并行分析。Beckman Coulter还推出了Biomek系列自动化液体处理工作站,与微流控芯片和流式细胞仪无缝集成,实现了从样本制备到数据分析的全流程自动化。该公司的技术方案特别强调样本处理的标准化和一致性,通过微流控技术实现了精确的液体控制和细胞操作,显著减少了样本消耗和交叉污染风险。Beckman Coulter的系统还整合了先进的数据分析软件,支持高维数据的可视化和解释。
优势:微流控细胞聚焦技术减少了样本消耗和试剂使用量;系统集成度高,支持从样本制备到数据分析的全流程自动化;数据分析软件功能强大,便于高维数据解释。劣势:系统复杂度较高,初始设置和优化可能需要专业支持;某些应用场景下灵敏度可能不如传统流式细胞仪;设备投资成本较高。
Core Technical Innovations in Microfluidics vs Flow Cytometry
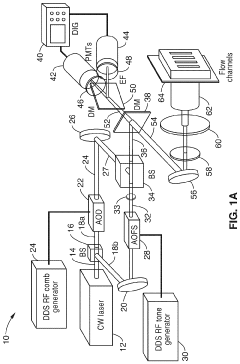
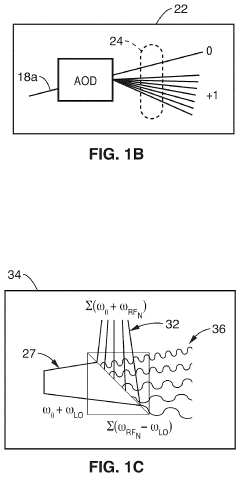
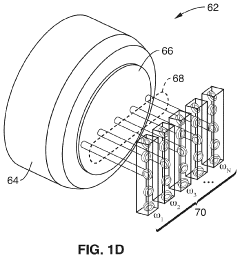
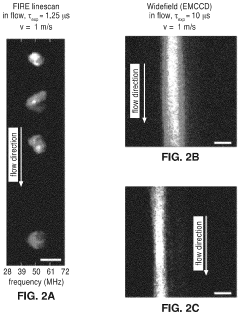
Parallel flow cytometer using radiofrequency multiplexing
PatentActiveUS11946851B2
Innovation
- A parallel flow cytometry system utilizing a FIRE optical engine with radio-frequency-tagged emission (FIRE) and a microfluidic device, enabling simultaneous probing of multiple flow channels with independent optical sensitivity and a single PMT detector for fluorescence analysis, allowing for higher throughput and cost-effective compound screening.
Fluidic flow cytometry devices and methods
PatentWO2014031900A1
Innovation
- The development of a flow cytometry device with a microfluidic channel system that incorporates a color filter with multiple zones, allowing specific spectral ranges of light to be transmitted and detected, and a piezoelectric actuator for precise cell sorting, enhancing the signal-to-noise ratio and cell viability.
Cost-Benefit Analysis of Competing Technologies
When comparing microfluidics and flow cytometry technologies for parallel testing applications, a comprehensive cost-benefit analysis reveals significant economic and operational differences that impact laboratory decision-making processes.
Initial capital investment represents a primary consideration, with traditional flow cytometry systems typically requiring investments ranging from $100,000 to $500,000 for high-end models. In contrast, microfluidic platforms generally present lower entry costs, with many systems available between $30,000 and $150,000, representing potential savings of 50-70% on equipment acquisition.
Operational expenses further differentiate these technologies. Flow cytometry demands specialized reagents and fluorescent antibodies that can cost $200-500 per test series, while microfluidic approaches often utilize smaller reagent volumes—typically 10-100 times less—translating to per-test costs of $20-150. This volumetric efficiency extends to sample requirements, with microfluidics typically requiring microliters compared to milliliters for conventional flow cytometry.
Maintenance economics also favor microfluidic systems, which generally require annual service costs of $5,000-10,000 versus $15,000-30,000 for flow cytometers. The simpler mechanical architecture of microfluidic devices contributes to fewer service interruptions and reduced downtime costs, estimated at 30-50% lower than flow cytometry systems.
Throughput capabilities present a more nuanced comparison. While high-end flow cytometers can process 10,000-100,000 cells per second, microfluidic parallel testing architectures enable simultaneous multi-parameter analysis across numerous samples, potentially increasing effective throughput for certain applications despite lower per-channel processing speeds.
Labor efficiency metrics demonstrate that microfluidic systems typically require 15-30% less technician time per test series due to increased automation and reduced sample preparation requirements. This translates to significant labor cost savings in high-volume testing environments.
Return on investment calculations indicate that microfluidic systems typically reach break-even points 30-40% faster than comparable flow cytometry installations, particularly in settings processing more than 50 samples daily. However, this advantage diminishes in specialized applications requiring the unique sensitivity and specificity characteristics of advanced flow cytometry.
The total cost of ownership analysis over a five-year operational period reveals that microfluidic systems generally demonstrate 25-45% lower lifetime costs compared to flow cytometry platforms with equivalent testing capabilities, making them increasingly attractive for budget-conscious laboratories seeking to expand parallel testing capabilities.
Initial capital investment represents a primary consideration, with traditional flow cytometry systems typically requiring investments ranging from $100,000 to $500,000 for high-end models. In contrast, microfluidic platforms generally present lower entry costs, with many systems available between $30,000 and $150,000, representing potential savings of 50-70% on equipment acquisition.
Operational expenses further differentiate these technologies. Flow cytometry demands specialized reagents and fluorescent antibodies that can cost $200-500 per test series, while microfluidic approaches often utilize smaller reagent volumes—typically 10-100 times less—translating to per-test costs of $20-150. This volumetric efficiency extends to sample requirements, with microfluidics typically requiring microliters compared to milliliters for conventional flow cytometry.
Maintenance economics also favor microfluidic systems, which generally require annual service costs of $5,000-10,000 versus $15,000-30,000 for flow cytometers. The simpler mechanical architecture of microfluidic devices contributes to fewer service interruptions and reduced downtime costs, estimated at 30-50% lower than flow cytometry systems.
Throughput capabilities present a more nuanced comparison. While high-end flow cytometers can process 10,000-100,000 cells per second, microfluidic parallel testing architectures enable simultaneous multi-parameter analysis across numerous samples, potentially increasing effective throughput for certain applications despite lower per-channel processing speeds.
Labor efficiency metrics demonstrate that microfluidic systems typically require 15-30% less technician time per test series due to increased automation and reduced sample preparation requirements. This translates to significant labor cost savings in high-volume testing environments.
Return on investment calculations indicate that microfluidic systems typically reach break-even points 30-40% faster than comparable flow cytometry installations, particularly in settings processing more than 50 samples daily. However, this advantage diminishes in specialized applications requiring the unique sensitivity and specificity characteristics of advanced flow cytometry.
The total cost of ownership analysis over a five-year operational period reveals that microfluidic systems generally demonstrate 25-45% lower lifetime costs compared to flow cytometry platforms with equivalent testing capabilities, making them increasingly attractive for budget-conscious laboratories seeking to expand parallel testing capabilities.
Standardization and Quality Control Considerations
Standardization and quality control represent critical dimensions when comparing microfluidics and flow cytometry for parallel testing applications. The establishment of robust standardization protocols ensures reproducibility across different laboratories and platforms, which remains a significant challenge in both technologies.
For flow cytometry, standardization has evolved considerably over decades of clinical and research applications. Organizations such as the International Society for Advancement of Cytometry (ISAC) have developed comprehensive guidelines including fluorescence calibration standards, instrument validation protocols, and data reporting formats. These established frameworks enable reliable inter-laboratory comparisons and facilitate regulatory compliance for clinical applications.
Microfluidic platforms, being relatively newer, face greater standardization challenges. The diversity of device designs, materials, and fabrication methods creates significant variability across systems. Current efforts focus on establishing standard operating procedures for chip fabrication, sample preparation, and data analysis. Organizations like the Microfluidics Association and ISO technical committees are working to develop industry-wide standards, though these remain less mature than flow cytometry equivalents.
Quality control considerations differ substantially between these technologies. Flow cytometry benefits from well-established quality control materials, including calibration beads and biological reference samples with known characteristics. Daily quality control runs using these materials ensure instrument performance remains within acceptable parameters. Software tools for automated quality assessment further enhance reliability in high-throughput environments.
Microfluidic systems require different quality control approaches due to their integrated nature. Channel dimensions, surface properties, and flow dynamics must be regularly verified to ensure consistent performance. Emerging quality control strategies include on-chip reference standards, integrated sensors for real-time monitoring, and automated image analysis tools to detect fabrication defects or operational anomalies.
The parallel testing advantages of both technologies are significantly enhanced when proper standardization and quality control measures are implemented. For microfluidics, standardized chip designs and materials can dramatically improve reproducibility across different manufacturing batches. Similarly, flow cytometry benefits from standardized antibody panels and gating strategies that enable consistent identification of cell populations across different instruments and operators.
Regulatory considerations also influence standardization requirements. Flow cytometry has established pathways for clinical validation under frameworks like CLIA and FDA guidelines. Microfluidic devices, particularly those intended for diagnostic applications, must navigate similar regulatory landscapes but with fewer precedents, creating additional challenges for developers seeking to commercialize parallel testing applications.
For flow cytometry, standardization has evolved considerably over decades of clinical and research applications. Organizations such as the International Society for Advancement of Cytometry (ISAC) have developed comprehensive guidelines including fluorescence calibration standards, instrument validation protocols, and data reporting formats. These established frameworks enable reliable inter-laboratory comparisons and facilitate regulatory compliance for clinical applications.
Microfluidic platforms, being relatively newer, face greater standardization challenges. The diversity of device designs, materials, and fabrication methods creates significant variability across systems. Current efforts focus on establishing standard operating procedures for chip fabrication, sample preparation, and data analysis. Organizations like the Microfluidics Association and ISO technical committees are working to develop industry-wide standards, though these remain less mature than flow cytometry equivalents.
Quality control considerations differ substantially between these technologies. Flow cytometry benefits from well-established quality control materials, including calibration beads and biological reference samples with known characteristics. Daily quality control runs using these materials ensure instrument performance remains within acceptable parameters. Software tools for automated quality assessment further enhance reliability in high-throughput environments.
Microfluidic systems require different quality control approaches due to their integrated nature. Channel dimensions, surface properties, and flow dynamics must be regularly verified to ensure consistent performance. Emerging quality control strategies include on-chip reference standards, integrated sensors for real-time monitoring, and automated image analysis tools to detect fabrication defects or operational anomalies.
The parallel testing advantages of both technologies are significantly enhanced when proper standardization and quality control measures are implemented. For microfluidics, standardized chip designs and materials can dramatically improve reproducibility across different manufacturing batches. Similarly, flow cytometry benefits from standardized antibody panels and gating strategies that enable consistent identification of cell populations across different instruments and operators.
Regulatory considerations also influence standardization requirements. Flow cytometry has established pathways for clinical validation under frameworks like CLIA and FDA guidelines. Microfluidic devices, particularly those intended for diagnostic applications, must navigate similar regulatory landscapes but with fewer precedents, creating additional challenges for developers seeking to commercialize parallel testing applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!