Microfluidics vs Traditional Techniques: Cost and Precision
SEP 10, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidics Evolution and Precision Goals
Microfluidics technology has evolved significantly since its inception in the early 1990s, transforming from a niche laboratory technique to a revolutionary approach in various scientific and industrial applications. The initial development focused primarily on miniaturizing traditional laboratory processes, with the first microfluidic devices being simple channel structures fabricated in silicon and glass. These early systems demonstrated basic fluid manipulation but lacked the sophistication and precision of modern platforms.
The mid-2000s marked a pivotal transition with the introduction of polydimethylsiloxane (PDMS) as a primary fabrication material, dramatically reducing production costs while increasing design flexibility. This material innovation catalyzed rapid advancement in microfluidic technologies, enabling more complex architectures and functionalities. Concurrently, integration with electronic components and sensing technologies enhanced control precision and expanded application possibilities.
Recent technological evolution has centered on achieving unprecedented levels of precision in fluid handling, with current systems capable of manipulating picoliters of liquid with nanometer-scale accuracy. This represents a thousand-fold improvement over traditional benchtop techniques, which typically operate at microliter volumes with significantly lower spatial resolution. The precision trajectory continues to advance, with research pushing toward femtoliter manipulation capabilities.
The primary precision goals in microfluidics development focus on several critical parameters. First is volumetric accuracy—the ability to consistently deliver exact fluid quantities regardless of viscosity or surface tension variations. Second is spatial precision—controlling exactly where fluids flow and interact within the device architecture. Third is temporal resolution—managing reaction timing with millisecond or microsecond accuracy. Finally, there is measurement sensitivity—detecting analytes at increasingly lower concentrations, approaching single-molecule detection limits.
Compared to traditional laboratory techniques, microfluidics aims to achieve superior precision while simultaneously reducing costs through miniaturization, reagent volume reduction, and process integration. Traditional methods often require expensive equipment, consume large volumes of costly reagents, and involve multiple discrete steps that introduce variability. The microfluidic approach consolidates these processes into single, automated platforms that minimize human intervention and maximize reproducibility.
The evolution trajectory suggests that future microfluidic systems will continue to push precision boundaries while becoming more accessible and affordable. Emerging fabrication techniques like 3D printing and roll-to-roll manufacturing promise to further reduce production costs, while advances in materials science enable new functionalities and improved performance characteristics. The ultimate goal is to develop platforms that combine nanoscale precision with robust, cost-effective designs suitable for widespread deployment across research, healthcare, and industrial applications.
The mid-2000s marked a pivotal transition with the introduction of polydimethylsiloxane (PDMS) as a primary fabrication material, dramatically reducing production costs while increasing design flexibility. This material innovation catalyzed rapid advancement in microfluidic technologies, enabling more complex architectures and functionalities. Concurrently, integration with electronic components and sensing technologies enhanced control precision and expanded application possibilities.
Recent technological evolution has centered on achieving unprecedented levels of precision in fluid handling, with current systems capable of manipulating picoliters of liquid with nanometer-scale accuracy. This represents a thousand-fold improvement over traditional benchtop techniques, which typically operate at microliter volumes with significantly lower spatial resolution. The precision trajectory continues to advance, with research pushing toward femtoliter manipulation capabilities.
The primary precision goals in microfluidics development focus on several critical parameters. First is volumetric accuracy—the ability to consistently deliver exact fluid quantities regardless of viscosity or surface tension variations. Second is spatial precision—controlling exactly where fluids flow and interact within the device architecture. Third is temporal resolution—managing reaction timing with millisecond or microsecond accuracy. Finally, there is measurement sensitivity—detecting analytes at increasingly lower concentrations, approaching single-molecule detection limits.
Compared to traditional laboratory techniques, microfluidics aims to achieve superior precision while simultaneously reducing costs through miniaturization, reagent volume reduction, and process integration. Traditional methods often require expensive equipment, consume large volumes of costly reagents, and involve multiple discrete steps that introduce variability. The microfluidic approach consolidates these processes into single, automated platforms that minimize human intervention and maximize reproducibility.
The evolution trajectory suggests that future microfluidic systems will continue to push precision boundaries while becoming more accessible and affordable. Emerging fabrication techniques like 3D printing and roll-to-roll manufacturing promise to further reduce production costs, while advances in materials science enable new functionalities and improved performance characteristics. The ultimate goal is to develop platforms that combine nanoscale precision with robust, cost-effective designs suitable for widespread deployment across research, healthcare, and industrial applications.
Market Analysis for Microfluidic Technologies
The global microfluidics market has demonstrated robust growth, valued at approximately $20 billion in 2022 and projected to reach $60 billion by 2030, with a compound annual growth rate (CAGR) of 14.5%. This significant expansion is driven by increasing applications across healthcare, pharmaceuticals, and life sciences sectors, where precision and cost-efficiency are paramount concerns.
Healthcare applications currently dominate the microfluidic technology market, accounting for nearly 45% of total market share. Point-of-care diagnostics represents the fastest-growing segment within healthcare applications, expanding at 16.8% CAGR due to the rising demand for rapid, accurate, and cost-effective diagnostic solutions. The pharmaceutical industry follows closely, utilizing microfluidic platforms for drug discovery and development processes, which has reduced research costs by up to 30% compared to traditional methods.
When comparing microfluidics to traditional techniques from a cost perspective, the initial capital investment for microfluidic systems remains higher, with average setup costs ranging from $50,000 to $200,000 depending on complexity and capabilities. However, the operational costs show significant advantages, with reagent consumption reduced by 70-90% and labor costs decreased by approximately 40% due to automation and miniaturization capabilities.
Precision metrics demonstrate microfluidics' superior performance, with sample volume requirements reduced to nanoliter or picoliter ranges compared to milliliter volumes in traditional methods. This translates to enhanced sensitivity, with detection limits improved by 2-3 orders of magnitude in many applications. Furthermore, microfluidic technologies have demonstrated coefficient of variation values below 5% in analytical applications, compared to 10-15% in conventional techniques.
Regional market analysis reveals North America leading with 38% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth at 16.7% CAGR, driven by increasing healthcare infrastructure investments and manufacturing capabilities in China, Japan, and South Korea.
Customer segmentation shows academic and research institutions as early adopters, while clinical laboratories and pharmaceutical companies represent the highest-value segments. The industrial adoption curve indicates microfluidics has moved beyond early adoption into early majority phase, with cost-benefit analysis increasingly favoring microfluidic solutions as manufacturing scales improve and technology matures.
Market barriers include standardization challenges, integration with existing workflows, and technical expertise requirements. However, these barriers are gradually diminishing as vendors develop more user-friendly systems and provide comprehensive support services, accelerating market penetration across various industries.
Healthcare applications currently dominate the microfluidic technology market, accounting for nearly 45% of total market share. Point-of-care diagnostics represents the fastest-growing segment within healthcare applications, expanding at 16.8% CAGR due to the rising demand for rapid, accurate, and cost-effective diagnostic solutions. The pharmaceutical industry follows closely, utilizing microfluidic platforms for drug discovery and development processes, which has reduced research costs by up to 30% compared to traditional methods.
When comparing microfluidics to traditional techniques from a cost perspective, the initial capital investment for microfluidic systems remains higher, with average setup costs ranging from $50,000 to $200,000 depending on complexity and capabilities. However, the operational costs show significant advantages, with reagent consumption reduced by 70-90% and labor costs decreased by approximately 40% due to automation and miniaturization capabilities.
Precision metrics demonstrate microfluidics' superior performance, with sample volume requirements reduced to nanoliter or picoliter ranges compared to milliliter volumes in traditional methods. This translates to enhanced sensitivity, with detection limits improved by 2-3 orders of magnitude in many applications. Furthermore, microfluidic technologies have demonstrated coefficient of variation values below 5% in analytical applications, compared to 10-15% in conventional techniques.
Regional market analysis reveals North America leading with 38% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is experiencing the fastest growth at 16.7% CAGR, driven by increasing healthcare infrastructure investments and manufacturing capabilities in China, Japan, and South Korea.
Customer segmentation shows academic and research institutions as early adopters, while clinical laboratories and pharmaceutical companies represent the highest-value segments. The industrial adoption curve indicates microfluidics has moved beyond early adoption into early majority phase, with cost-benefit analysis increasingly favoring microfluidic solutions as manufacturing scales improve and technology matures.
Market barriers include standardization challenges, integration with existing workflows, and technical expertise requirements. However, these barriers are gradually diminishing as vendors develop more user-friendly systems and provide comprehensive support services, accelerating market penetration across various industries.
Technical Challenges in Microfluidics vs Traditional Methods
Microfluidics technology faces several significant technical challenges when compared to traditional laboratory methods. One of the primary obstacles is the complex fabrication process required for microfluidic devices. While traditional methods often utilize standardized equipment with established manufacturing processes, microfluidic devices demand specialized microfabrication techniques including photolithography, soft lithography, and precision bonding. These processes require clean room facilities and specialized expertise, creating substantial barriers to entry.
Surface chemistry presents another critical challenge in microfluidics. The high surface-to-volume ratio in microchannels means that surface interactions dominate fluid behavior, unlike in traditional macro-scale systems. This necessitates precise surface modification techniques to control wetting properties, prevent non-specific adsorption, and maintain biocompatibility. Traditional methods typically don't require such meticulous surface engineering, making them more straightforward to implement.
Flow control and pumping mechanisms represent a significant technical hurdle in microfluidic systems. While traditional methods rely on well-established pumping technologies, microfluidics requires precise control of extremely small volumes, often in the nanoliter to picoliter range. This necessitates specialized micropumps, pressure controllers, or electrokinetic methods that add complexity and cost to the overall system.
Integration challenges are particularly pronounced in microfluidics. Creating fully functional lab-on-a-chip devices requires seamless integration of multiple components including sample preparation, separation, detection, and analysis modules. Traditional laboratory methods benefit from decades of standardization and modular design, while microfluidic integration often requires custom solutions for each application.
Detection sensitivity presents a paradoxical challenge. While microfluidics offers advantages in terms of reduced sample volumes, the extremely small quantities being analyzed require highly sensitive detection methods. Traditional analytical techniques often benefit from larger sample volumes that make detection more straightforward, whereas microfluidic systems frequently require integration with advanced optical, electrochemical, or mass spectrometry detection systems.
Scaling and parallelization also present unique challenges. Traditional methods have established protocols for scaling up processes, while microfluidic systems often face difficulties in maintaining performance when scaled to high-throughput applications. The physics of fluid behavior at the microscale introduces complexities that don't exist in traditional systems, requiring innovative design approaches to achieve effective parallelization.
Material compatibility issues further complicate microfluidic development. Many microfluidic devices are fabricated from polymers like PDMS that may have limitations regarding chemical compatibility, optical properties, or mechanical stability. Traditional laboratory equipment typically utilizes more robust materials with well-characterized properties and established performance parameters.
Surface chemistry presents another critical challenge in microfluidics. The high surface-to-volume ratio in microchannels means that surface interactions dominate fluid behavior, unlike in traditional macro-scale systems. This necessitates precise surface modification techniques to control wetting properties, prevent non-specific adsorption, and maintain biocompatibility. Traditional methods typically don't require such meticulous surface engineering, making them more straightforward to implement.
Flow control and pumping mechanisms represent a significant technical hurdle in microfluidic systems. While traditional methods rely on well-established pumping technologies, microfluidics requires precise control of extremely small volumes, often in the nanoliter to picoliter range. This necessitates specialized micropumps, pressure controllers, or electrokinetic methods that add complexity and cost to the overall system.
Integration challenges are particularly pronounced in microfluidics. Creating fully functional lab-on-a-chip devices requires seamless integration of multiple components including sample preparation, separation, detection, and analysis modules. Traditional laboratory methods benefit from decades of standardization and modular design, while microfluidic integration often requires custom solutions for each application.
Detection sensitivity presents a paradoxical challenge. While microfluidics offers advantages in terms of reduced sample volumes, the extremely small quantities being analyzed require highly sensitive detection methods. Traditional analytical techniques often benefit from larger sample volumes that make detection more straightforward, whereas microfluidic systems frequently require integration with advanced optical, electrochemical, or mass spectrometry detection systems.
Scaling and parallelization also present unique challenges. Traditional methods have established protocols for scaling up processes, while microfluidic systems often face difficulties in maintaining performance when scaled to high-throughput applications. The physics of fluid behavior at the microscale introduces complexities that don't exist in traditional systems, requiring innovative design approaches to achieve effective parallelization.
Material compatibility issues further complicate microfluidic development. Many microfluidic devices are fabricated from polymers like PDMS that may have limitations regarding chemical compatibility, optical properties, or mechanical stability. Traditional laboratory equipment typically utilizes more robust materials with well-characterized properties and established performance parameters.
Current Cost-Precision Solutions in Microfluidic Systems
01 Cost-effective microfluidic fabrication methods
Various fabrication methods have been developed to reduce the cost of microfluidic devices while maintaining precision. These include using alternative materials like polymers instead of glass or silicon, employing 3D printing technologies for rapid prototyping, and developing simplified manufacturing processes that require less specialized equipment. These approaches significantly lower the entry barrier for microfluidics technology adoption in various applications including point-of-care diagnostics and research settings.- Cost-effective microfluidic fabrication methods: Various fabrication techniques have been developed to reduce the cost of microfluidic devices while maintaining functionality. These include using alternative materials like polymers instead of glass or silicon, employing 3D printing for rapid prototyping, and developing simplified manufacturing processes that require less specialized equipment. These approaches significantly lower the entry barrier for microfluidics technology adoption in various applications including point-of-care diagnostics and research settings.
- High-precision fluid control systems: Advanced fluid control systems enable precise manipulation of small volumes in microfluidic devices. These systems incorporate precise pumps, valves, and flow sensors that can accurately control flow rates down to nanoliter or even picoliter volumes. The integration of electronic control systems with microfluidic components allows for automated operation with high reproducibility, which is essential for applications requiring exact fluid handling such as drug discovery and analytical chemistry.
- Integrated sensing and detection technologies: Microfluidic platforms incorporate various sensing technologies to enhance precision in detection and analysis. These include optical, electrochemical, and mechanical sensors integrated directly into the microfluidic channels. The miniaturization of these sensing elements allows for real-time monitoring of reactions and processes within the microfluidic device, improving both the sensitivity and accuracy of measurements while reducing the sample volume required for analysis.
- Scalable manufacturing for cost reduction: Scalable manufacturing techniques have been developed to reduce the per-unit cost of microfluidic devices. These include injection molding, hot embossing, and roll-to-roll processing that enable mass production of microfluidic components. By transitioning from laboratory-scale fabrication to industrial manufacturing processes, the cost of microfluidic devices can be significantly reduced while maintaining precision specifications, making the technology more accessible for commercial applications.
- Precision enhancement through digital microfluidics: Digital microfluidics technology manipulates discrete droplets rather than continuous fluid streams, offering enhanced precision in fluid handling. This approach uses electrowetting, acoustic waves, or magnetic forces to control individual droplets with high accuracy. Digital microfluidic systems enable precise volume control, reduce cross-contamination, and allow for complex protocols to be executed with minimal human intervention, thereby improving reproducibility while potentially reducing operational costs through reagent conservation.
02 High-precision fluid control systems
Advanced fluid control systems have been developed to enhance the precision of microfluidic operations. These systems incorporate precise pumping mechanisms, flow sensors, and feedback control algorithms to accurately manipulate small volumes of fluids. The integration of electronic components with microfluidic channels allows for real-time monitoring and adjustment of flow rates, pressure, and temperature, resulting in highly reproducible experimental conditions and analytical results.Expand Specific Solutions03 Integrated sensing and detection technologies
Microfluidic platforms incorporate various sensing and detection technologies to achieve high precision measurements while maintaining cost-effectiveness. These include optical detection methods, electrochemical sensors, and impedance-based detection systems that can be integrated directly into the microfluidic chip. This integration eliminates the need for expensive external equipment and reduces sample volume requirements, making precise analysis more accessible and affordable.Expand Specific Solutions04 Automated microfluidic systems for enhanced reproducibility
Automation technologies have been implemented in microfluidic systems to improve precision and reduce operational costs. These automated systems minimize human intervention, reducing variability and errors in experimental procedures. They incorporate robotics, programmable fluid handling, and software control systems that enable consistent operation over extended periods. This approach is particularly valuable for high-throughput applications where reproducibility is critical and labor costs would otherwise be significant.Expand Specific Solutions05 Novel microfluidic architectures balancing cost and precision
Innovative microfluidic device architectures have been designed to optimize the balance between manufacturing cost and operational precision. These designs include multiplexed channel configurations, droplet-based systems, and modular platforms that can be customized for specific applications. By carefully engineering channel geometries, surface properties, and interface connections, these architectures achieve high precision fluid manipulation while using cost-effective materials and fabrication methods.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
Microfluidics technology is currently in a growth phase, transitioning from early adoption to mainstream implementation across various industries. The global microfluidics market is expanding rapidly, projected to reach $50-60 billion by 2026, driven by applications in diagnostics, pharmaceuticals, and life sciences. While traditional techniques offer established reliability, microfluidics delivers superior precision at microscale volumes with significantly reduced sample requirements. Leading academic institutions (MIT, Tsinghua University, California Institute of Technology) are advancing fundamental research, while companies like Stokes Bio, miDIAGNOSTICS, and Truvian Sciences are commercializing applications. Industry giants including Agilent Technologies and Intel are investing in microfluidic platforms, indicating the technology's maturation from research-focused to commercially viable solutions that balance cost efficiency with unprecedented analytical precision.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered advanced microfluidic platforms that significantly reduce reagent consumption compared to traditional techniques. Their droplet-based microfluidic systems enable precise manipulation of fluid volumes in the picoliter to nanoliter range, representing a 100-1000x reduction in reagent usage compared to conventional methods[1]. MIT researchers have developed integrated microfluidic devices that combine sample preparation, reaction, and detection on a single chip, eliminating manual transfer steps that introduce variability. Their platforms incorporate novel surface treatments and channel geometries that prevent sample adsorption and cross-contamination, achieving coefficient of variation (CV) values below 2% for quantitative assays[3]. MIT's cost-effective fabrication approaches using soft lithography techniques have reduced manufacturing costs by approximately 60-80% compared to traditional microfabrication methods while maintaining precision specifications[5].
Strengths: Superior reagent efficiency (100-1000x reduction), exceptional precision (CV < 2%), and significantly lower manufacturing costs (60-80% reduction). Weaknesses: Higher initial development costs, requires specialized expertise for design optimization, and potential challenges in scaling to very high throughput applications.
The Charles Stark Draper Laboratory, Inc.
Technical Solution: Draper Laboratory has developed proprietary microfluidic technology that addresses both cost and precision challenges in analytical testing. Their platform utilizes precision-engineered microchannels with controlled flow dynamics that enable highly reproducible fluid handling with coefficient of variation below 3% across multiple test runs[2]. Draper's approach incorporates automated pressure control systems that maintain consistent flow rates regardless of sample viscosity variations, a significant improvement over traditional techniques that often suffer from sample-to-sample variability. Their microfluidic devices integrate multiple analytical functions including sample preparation, separation, and detection in a footprint approximately 75% smaller than conventional laboratory equipment[4]. The technology reduces reagent consumption by up to 95% compared to traditional methods while maintaining or improving analytical sensitivity through optimized reaction kinetics in the microenvironment. Draper has also pioneered manufacturing techniques that enable cost-effective mass production of complex microfluidic devices, reducing per-unit costs by approximately 60% compared to traditional analytical platforms[7].
Strengths: Exceptional reproducibility (CV < 3%), dramatic reagent reduction (up to 95%), and significant cost advantages in high-volume manufacturing. Weaknesses: Higher complexity in system integration, potential challenges with highly viscous or particulate-laden samples, and more limited compatibility with existing laboratory automation systems.
Key Patents and Innovations in Microfluidic Precision Engineering
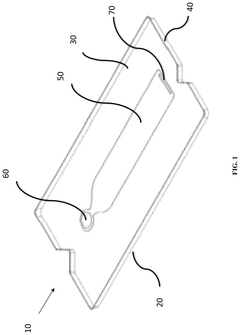
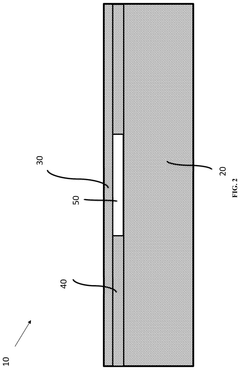
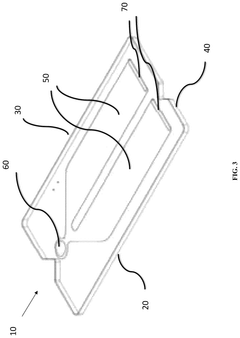
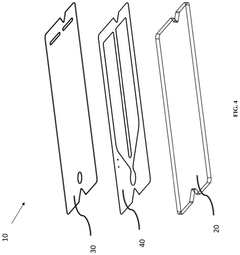
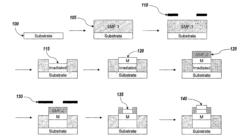
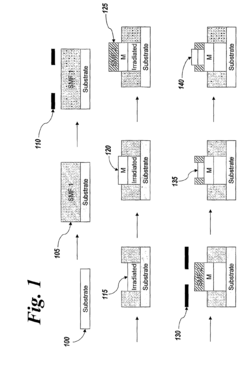
Microfluidic device and method of manufacturing the same
PatentActiveUS20250065323A1
Innovation
- A method of manufacturing a laminate microfluidic device involves providing a substrate layer, an adhesive layer with a curable adhesive, and a top layer, forming channels or recess features in the adhesive layer, laminating the layers together, and curing the composite laminate to achieve a stable and uniform thickness.
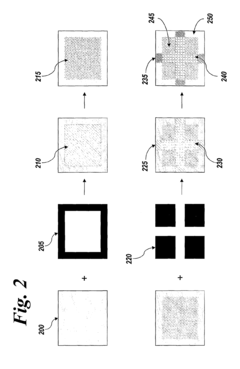
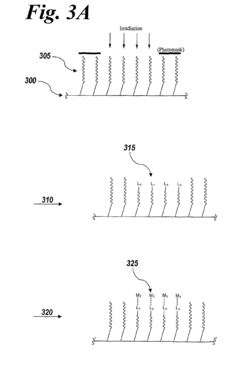
Imagewise patterning of films and devices comprising the same
PatentInactiveUS8076125B2
Innovation
- The development of methods for imagewise patterning of surfaces using photo-labile molecules and metal ions to create nanopatterned films for microfluidic devices, enabling control of fluid flow and analyte sensing without the need for complex circuitry or large power sources.
Manufacturing Scalability of Microfluidic Devices
The scalability of microfluidic device manufacturing represents a critical factor in determining the widespread adoption of this technology compared to traditional analytical techniques. Current manufacturing processes for microfluidic devices predominantly rely on cleanroom-based photolithography and soft lithography techniques, which present significant challenges for high-volume production. These methods typically involve labor-intensive manual steps and require specialized equipment, resulting in higher per-unit costs when compared to mass-produced traditional analytical instruments.
Several manufacturing approaches have emerged to address these scalability challenges. Injection molding offers promising potential for high-volume production of thermoplastic microfluidic devices, enabling the creation of thousands of identical units with relatively low per-unit costs once initial tooling investments are recovered. However, this approach requires substantial upfront capital expenditure and may limit design flexibility compared to prototyping methods.
Hot embossing represents another viable manufacturing route, offering a balance between production volume and initial investment. This technique allows for moderate-scale production with reasonable fidelity to original designs, though cycle times remain longer than injection molding, affecting overall throughput and scalability.
Roll-to-roll manufacturing has emerged as a particularly promising approach for continuous production of microfluidic devices on flexible substrates. This method enables significantly higher throughput compared to traditional batch processes, potentially reducing manufacturing costs by orders of magnitude. However, technical challenges remain in maintaining precise feature dimensions and ensuring consistent bonding across large surface areas.
The integration of microfluidic components with electronic elements presents additional manufacturing complexities. While traditional electronics benefit from decades of manufacturing optimization, the hybrid nature of microfluidic devices often necessitates custom assembly processes that are difficult to automate fully, creating bottlenecks in production scaling.
Recent advances in 3D printing technologies offer new possibilities for microfluidic device manufacturing, potentially enabling rapid prototyping and small-batch production without the overhead of traditional cleanroom processes. However, current limitations in resolution, material compatibility, and surface quality restrict the application of these techniques for high-precision microfluidic applications requiring sub-micron features or optical-quality surfaces.
For microfluidic technology to achieve cost parity with traditional analytical techniques, significant innovation in manufacturing processes is required. The development of standardized components, modular design approaches, and automated assembly systems will be crucial in bridging the current gap between laboratory prototypes and commercially viable products that can compete with established analytical platforms on both cost and performance metrics.
Several manufacturing approaches have emerged to address these scalability challenges. Injection molding offers promising potential for high-volume production of thermoplastic microfluidic devices, enabling the creation of thousands of identical units with relatively low per-unit costs once initial tooling investments are recovered. However, this approach requires substantial upfront capital expenditure and may limit design flexibility compared to prototyping methods.
Hot embossing represents another viable manufacturing route, offering a balance between production volume and initial investment. This technique allows for moderate-scale production with reasonable fidelity to original designs, though cycle times remain longer than injection molding, affecting overall throughput and scalability.
Roll-to-roll manufacturing has emerged as a particularly promising approach for continuous production of microfluidic devices on flexible substrates. This method enables significantly higher throughput compared to traditional batch processes, potentially reducing manufacturing costs by orders of magnitude. However, technical challenges remain in maintaining precise feature dimensions and ensuring consistent bonding across large surface areas.
The integration of microfluidic components with electronic elements presents additional manufacturing complexities. While traditional electronics benefit from decades of manufacturing optimization, the hybrid nature of microfluidic devices often necessitates custom assembly processes that are difficult to automate fully, creating bottlenecks in production scaling.
Recent advances in 3D printing technologies offer new possibilities for microfluidic device manufacturing, potentially enabling rapid prototyping and small-batch production without the overhead of traditional cleanroom processes. However, current limitations in resolution, material compatibility, and surface quality restrict the application of these techniques for high-precision microfluidic applications requiring sub-micron features or optical-quality surfaces.
For microfluidic technology to achieve cost parity with traditional analytical techniques, significant innovation in manufacturing processes is required. The development of standardized components, modular design approaches, and automated assembly systems will be crucial in bridging the current gap between laboratory prototypes and commercially viable products that can compete with established analytical platforms on both cost and performance metrics.
Regulatory Considerations for Microfluidic Applications
The regulatory landscape for microfluidic technologies presents a complex framework that significantly impacts their development, commercialization, and adoption compared to traditional analytical techniques. Microfluidic devices, particularly those intended for clinical diagnostics, must navigate stringent regulatory pathways established by agencies such as the FDA in the United States, the EMA in Europe, and similar bodies worldwide. These regulatory frameworks typically classify microfluidic devices based on their intended use and associated risks, with medical applications facing more rigorous scrutiny than research-use-only platforms.
A critical regulatory consideration is the validation of precision and accuracy claims. While microfluidics often demonstrates superior analytical performance compared to traditional techniques, regulatory bodies require comprehensive validation studies to substantiate these advantages. This validation process typically demands more extensive documentation for novel microfluidic approaches than for established traditional methods that benefit from historical regulatory precedents.
Cost implications of regulatory compliance create significant barriers to market entry for microfluidic technologies. The development of quality management systems, generation of validation data, and preparation of regulatory submissions can add substantial expenses to microfluidic product development. These regulatory costs may disproportionately impact smaller companies and startups that dominate microfluidic innovation but possess limited resources compared to established manufacturers of traditional analytical equipment.
Manufacturing consistency represents another key regulatory challenge. Microfluidic devices must demonstrate reproducible performance across production batches, which requires sophisticated quality control processes. Regulatory agencies increasingly scrutinize manufacturing processes, particularly for complex microfluidic chips with intricate channel geometries that may affect analytical precision.
International regulatory harmonization efforts are gradually addressing disparities between regional requirements, though significant differences persist. These variations complicate global commercialization strategies and may necessitate region-specific validation studies, further increasing costs compared to traditional techniques that often benefit from more standardized international regulatory pathways.
Emerging regulatory frameworks for novel applications, such as organ-on-chip technologies and point-of-care diagnostics, are still evolving. This regulatory uncertainty can delay commercialization timelines and increase development risks for innovative microfluidic approaches, while traditional techniques operate within well-established regulatory parameters.
A critical regulatory consideration is the validation of precision and accuracy claims. While microfluidics often demonstrates superior analytical performance compared to traditional techniques, regulatory bodies require comprehensive validation studies to substantiate these advantages. This validation process typically demands more extensive documentation for novel microfluidic approaches than for established traditional methods that benefit from historical regulatory precedents.
Cost implications of regulatory compliance create significant barriers to market entry for microfluidic technologies. The development of quality management systems, generation of validation data, and preparation of regulatory submissions can add substantial expenses to microfluidic product development. These regulatory costs may disproportionately impact smaller companies and startups that dominate microfluidic innovation but possess limited resources compared to established manufacturers of traditional analytical equipment.
Manufacturing consistency represents another key regulatory challenge. Microfluidic devices must demonstrate reproducible performance across production batches, which requires sophisticated quality control processes. Regulatory agencies increasingly scrutinize manufacturing processes, particularly for complex microfluidic chips with intricate channel geometries that may affect analytical precision.
International regulatory harmonization efforts are gradually addressing disparities between regional requirements, though significant differences persist. These variations complicate global commercialization strategies and may necessitate region-specific validation studies, further increasing costs compared to traditional techniques that often benefit from more standardized international regulatory pathways.
Emerging regulatory frameworks for novel applications, such as organ-on-chip technologies and point-of-care diagnostics, are still evolving. This regulatory uncertainty can delay commercialization timelines and increase development risks for innovative microfluidic approaches, while traditional techniques operate within well-established regulatory parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!