Minimize Wastage in Microfluidic Droplet Systems for Efficiency
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic Droplet Technology Background and Objectives
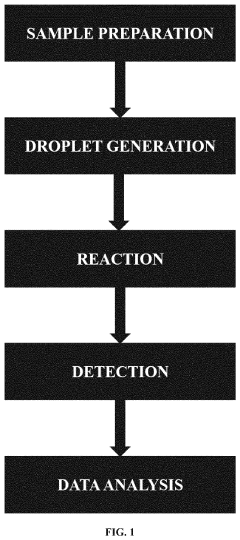
Microfluidic droplet technology represents a revolutionary approach in fluid handling at the microscale, enabling precise control over minute volumes of liquids. Emerging in the late 1990s, this technology has evolved from simple channel designs to sophisticated integrated systems capable of generating, manipulating, and analyzing droplets with remarkable precision. The fundamental principle involves creating discrete droplets of one fluid within another immiscible carrier fluid, effectively compartmentalizing reactions and samples.
The evolution of microfluidic droplet systems has been marked by significant milestones, including the development of electrowetting techniques, passive droplet generation methods, and advanced control mechanisms. Recent years have witnessed a shift toward higher throughput systems and integration with detection technologies, enabling applications ranging from single-cell analysis to high-throughput screening for drug discovery.
Despite these advances, wastage remains a persistent challenge in microfluidic droplet systems. Current systems often suffer from inefficiencies in reagent utilization, with significant portions of valuable samples and reagents lost during system priming, stabilization phases, and due to dead volumes in device architecture. This wastage is particularly problematic when working with precious biological samples, expensive reagents, or rare materials where conservation is paramount.
The primary objective of this technical research is to comprehensively investigate strategies for minimizing wastage in microfluidic droplet systems while maintaining or enhancing operational efficiency. Specifically, we aim to identify innovative approaches in device design, fluid handling protocols, and system integration that can significantly reduce sample and reagent consumption without compromising analytical performance or throughput.
Additionally, this research seeks to establish quantitative metrics for evaluating wastage in different microfluidic platforms, enabling standardized comparison across various technological approaches. By developing such benchmarks, we can better assess the real-world efficiency of emerging solutions and guide future development efforts.
The long-term technological goal extends beyond mere optimization of existing systems to fundamentally reimagining microfluidic architectures with efficiency as a core design principle. This includes exploring novel materials with reduced surface adsorption properties, developing recirculation systems for carrier fluids, implementing advanced feedback control mechanisms for precise dispensing, and creating adaptive algorithms for optimized droplet generation sequences.
Through this comprehensive exploration of wastage minimization strategies, we aim to contribute to the next generation of microfluidic droplet technologies that combine unprecedented efficiency with the precision and versatility that have made these systems invaluable across scientific and industrial applications.
The evolution of microfluidic droplet systems has been marked by significant milestones, including the development of electrowetting techniques, passive droplet generation methods, and advanced control mechanisms. Recent years have witnessed a shift toward higher throughput systems and integration with detection technologies, enabling applications ranging from single-cell analysis to high-throughput screening for drug discovery.
Despite these advances, wastage remains a persistent challenge in microfluidic droplet systems. Current systems often suffer from inefficiencies in reagent utilization, with significant portions of valuable samples and reagents lost during system priming, stabilization phases, and due to dead volumes in device architecture. This wastage is particularly problematic when working with precious biological samples, expensive reagents, or rare materials where conservation is paramount.
The primary objective of this technical research is to comprehensively investigate strategies for minimizing wastage in microfluidic droplet systems while maintaining or enhancing operational efficiency. Specifically, we aim to identify innovative approaches in device design, fluid handling protocols, and system integration that can significantly reduce sample and reagent consumption without compromising analytical performance or throughput.
Additionally, this research seeks to establish quantitative metrics for evaluating wastage in different microfluidic platforms, enabling standardized comparison across various technological approaches. By developing such benchmarks, we can better assess the real-world efficiency of emerging solutions and guide future development efforts.
The long-term technological goal extends beyond mere optimization of existing systems to fundamentally reimagining microfluidic architectures with efficiency as a core design principle. This includes exploring novel materials with reduced surface adsorption properties, developing recirculation systems for carrier fluids, implementing advanced feedback control mechanisms for precise dispensing, and creating adaptive algorithms for optimized droplet generation sequences.
Through this comprehensive exploration of wastage minimization strategies, we aim to contribute to the next generation of microfluidic droplet technologies that combine unprecedented efficiency with the precision and versatility that have made these systems invaluable across scientific and industrial applications.
Market Analysis for Efficient Microfluidic Systems
The global microfluidic systems market is experiencing robust growth, valued at approximately $20 billion in 2022 and projected to reach $42 billion by 2027, with a compound annual growth rate (CAGR) of 16%. This growth is primarily driven by increasing applications in pharmaceutical research, diagnostics, and point-of-care testing. The demand for more efficient microfluidic droplet systems specifically has intensified as these technologies become integral to drug discovery, single-cell analysis, and digital PCR applications.
Market research indicates that wastage reduction in microfluidic systems represents a significant opportunity, with potential cost savings estimated at 25-30% of operational expenses for end users. Current inefficiencies in reagent usage, particularly in droplet-based systems, contribute to higher per-test costs and environmental concerns, creating a clear market need for optimization solutions.
The pharmaceutical and biotechnology sectors constitute the largest market segment for efficient microfluidic systems, accounting for approximately 45% of the total market share. These industries prioritize precision and reproducibility in high-throughput screening and are willing to invest in technologies that minimize sample and reagent wastage. Academic research institutions represent another significant market segment (30%), where budget constraints make efficiency improvements particularly valuable.
Regional analysis reveals North America dominates the market with 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region is expected to witness the fastest growth rate of 18% annually, driven by increasing R&D investments in China, Japan, and South Korea, and the establishment of new biotechnology ventures.
Customer surveys indicate that end users are willing to pay a premium of 15-20% for microfluidic systems that demonstrate significant reduction in reagent wastage, particularly for applications involving expensive biological samples or rare specimens. The return on investment for such premium systems is typically realized within 12-18 months of implementation.
Market barriers include the high initial cost of advanced microfluidic platforms and technical challenges in integrating wastage reduction technologies with existing workflows. Additionally, regulatory considerations for clinical applications can extend commercialization timelines, affecting market penetration rates for novel efficient systems.
Emerging market opportunities exist in point-of-care diagnostics, where resource constraints in low-infrastructure settings make efficiency paramount, and in industrial biotechnology, where scaling processes require minimizing inputs while maximizing outputs. The environmental sustainability aspect of waste reduction also aligns with growing corporate social responsibility initiatives, potentially opening new market segments focused on green laboratory practices.
Market research indicates that wastage reduction in microfluidic systems represents a significant opportunity, with potential cost savings estimated at 25-30% of operational expenses for end users. Current inefficiencies in reagent usage, particularly in droplet-based systems, contribute to higher per-test costs and environmental concerns, creating a clear market need for optimization solutions.
The pharmaceutical and biotechnology sectors constitute the largest market segment for efficient microfluidic systems, accounting for approximately 45% of the total market share. These industries prioritize precision and reproducibility in high-throughput screening and are willing to invest in technologies that minimize sample and reagent wastage. Academic research institutions represent another significant market segment (30%), where budget constraints make efficiency improvements particularly valuable.
Regional analysis reveals North America dominates the market with 40% share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region is expected to witness the fastest growth rate of 18% annually, driven by increasing R&D investments in China, Japan, and South Korea, and the establishment of new biotechnology ventures.
Customer surveys indicate that end users are willing to pay a premium of 15-20% for microfluidic systems that demonstrate significant reduction in reagent wastage, particularly for applications involving expensive biological samples or rare specimens. The return on investment for such premium systems is typically realized within 12-18 months of implementation.
Market barriers include the high initial cost of advanced microfluidic platforms and technical challenges in integrating wastage reduction technologies with existing workflows. Additionally, regulatory considerations for clinical applications can extend commercialization timelines, affecting market penetration rates for novel efficient systems.
Emerging market opportunities exist in point-of-care diagnostics, where resource constraints in low-infrastructure settings make efficiency paramount, and in industrial biotechnology, where scaling processes require minimizing inputs while maximizing outputs. The environmental sustainability aspect of waste reduction also aligns with growing corporate social responsibility initiatives, potentially opening new market segments focused on green laboratory practices.
Current Wastage Challenges in Microfluidic Droplet Platforms
Microfluidic droplet systems have revolutionized various fields including drug discovery, diagnostics, and chemical synthesis by enabling precise manipulation of small fluid volumes. However, these systems currently face significant wastage challenges that undermine their efficiency and sustainability. The primary source of wastage occurs during the droplet generation phase, where inconsistent flow rates and pressure fluctuations lead to irregular droplet formation, resulting in up to 30% of reagents being discarded during system stabilization periods.
Material wastage represents another critical challenge, particularly with expensive biological reagents such as enzymes, antibodies, and rare cell samples. Current microfluidic platforms typically require minimum working volumes that exceed actual analytical needs by 3-5 times, creating substantial financial and resource inefficiencies. For instance, commercial droplet digital PCR systems may require 20μL of sample preparation while utilizing only 5μL for actual analysis.
Device-related wastage stems from the disposable nature of many microfluidic chips. Manufacturing defects, channel clogging, and surface contamination often render entire chips unusable after minimal use. The industry standard shows that approximately 15-20% of microfluidic chips are discarded due to manufacturing imperfections before any experimental use, representing significant material and production energy wastage.
Energy inefficiency constitutes another form of wastage in current systems. Pumping mechanisms, temperature control systems, and detection modules often operate continuously regardless of actual droplet processing needs. Studies indicate that the energy utilization efficiency in standard microfluidic platforms ranges between 40-60%, with significant power consumption during idle periods.
Sample recovery limitations present additional wastage concerns. Once samples are introduced into microfluidic systems, recovery rates typically range from 60-85%, with the remainder trapped in dead volumes, adhered to channel walls, or lost during transfer processes. This becomes particularly problematic when working with limited patient samples or rare materials.
Time wastage also impacts overall system efficiency. Current platforms require extensive calibration procedures, with operators spending 25-40% of total experimental time on system preparation rather than actual analysis. Additionally, troubleshooting unstable droplet generation can consume significant research hours, reducing laboratory productivity.
Cross-contamination prevention measures often lead to further reagent wastage. Buffer solutions used for system cleaning between experiments typically consume 2-3 times the volume of the actual sample processing channels, creating additional waste streams that require proper disposal, particularly when hazardous chemicals or biological materials are involved.
Material wastage represents another critical challenge, particularly with expensive biological reagents such as enzymes, antibodies, and rare cell samples. Current microfluidic platforms typically require minimum working volumes that exceed actual analytical needs by 3-5 times, creating substantial financial and resource inefficiencies. For instance, commercial droplet digital PCR systems may require 20μL of sample preparation while utilizing only 5μL for actual analysis.
Device-related wastage stems from the disposable nature of many microfluidic chips. Manufacturing defects, channel clogging, and surface contamination often render entire chips unusable after minimal use. The industry standard shows that approximately 15-20% of microfluidic chips are discarded due to manufacturing imperfections before any experimental use, representing significant material and production energy wastage.
Energy inefficiency constitutes another form of wastage in current systems. Pumping mechanisms, temperature control systems, and detection modules often operate continuously regardless of actual droplet processing needs. Studies indicate that the energy utilization efficiency in standard microfluidic platforms ranges between 40-60%, with significant power consumption during idle periods.
Sample recovery limitations present additional wastage concerns. Once samples are introduced into microfluidic systems, recovery rates typically range from 60-85%, with the remainder trapped in dead volumes, adhered to channel walls, or lost during transfer processes. This becomes particularly problematic when working with limited patient samples or rare materials.
Time wastage also impacts overall system efficiency. Current platforms require extensive calibration procedures, with operators spending 25-40% of total experimental time on system preparation rather than actual analysis. Additionally, troubleshooting unstable droplet generation can consume significant research hours, reducing laboratory productivity.
Cross-contamination prevention measures often lead to further reagent wastage. Buffer solutions used for system cleaning between experiments typically consume 2-3 times the volume of the actual sample processing channels, creating additional waste streams that require proper disposal, particularly when hazardous chemicals or biological materials are involved.
Current Wastage Minimization Approaches and Techniques
01 Waste reduction in microfluidic droplet generation
Microfluidic systems can be designed to minimize reagent wastage during droplet generation through optimized channel geometries and flow control mechanisms. These designs ensure precise control over droplet formation, reducing the amount of sample and reagent lost during the process. Advanced flow control techniques and specialized channel designs help maintain consistent droplet sizes while minimizing dead volumes where valuable materials can be trapped or wasted.- Waste reduction in microfluidic droplet generation: Various techniques are employed to minimize waste during droplet generation in microfluidic systems. These include optimized channel designs, precise flow control mechanisms, and improved droplet formation methods that ensure consistent droplet size and spacing. Advanced algorithms and feedback systems can adjust parameters in real-time to reduce reagent wastage during the droplet formation process.
- Sample recovery and recycling systems: Microfluidic platforms incorporate sample recovery mechanisms to collect and reuse valuable reagents that would otherwise be wasted. These systems include specialized collection chambers, continuous flow recycling loops, and separation techniques that can isolate unused reagents from waste streams. Such approaches are particularly important when working with precious biological samples or expensive chemicals in droplet-based assays.
- Efficient droplet sorting and manipulation: Advanced droplet sorting and manipulation technologies help minimize wastage by precisely controlling droplet movement and selection. These include electric field-based sorting, acoustic wave manipulation, and optical tweezers that can selectively process droplets of interest while minimizing the handling of unwanted droplets. Such selective processing significantly reduces the overall system wastage.
- Integrated waste management solutions: Comprehensive waste management solutions are integrated into microfluidic droplet systems to handle unavoidable waste streams. These include on-chip waste collection reservoirs, filtration systems to separate reusable components, and controlled disposal mechanisms. Some advanced systems incorporate biodegradable materials or environmentally friendly reagents to minimize environmental impact of microfluidic waste.
- Automated monitoring and optimization systems: Intelligent monitoring systems continuously track resource usage and wastage in microfluidic droplet operations. These systems employ sensors, computer vision, and machine learning algorithms to detect inefficiencies and automatically adjust operational parameters. Real-time feedback control optimizes droplet generation, manipulation, and analysis processes, significantly reducing reagent consumption and waste production during extended operations.
02 Recycling and recovery systems for microfluidic platforms
Integrated recycling systems can be incorporated into microfluidic platforms to collect and reuse unused reagents and samples. These systems typically include collection chambers, filtration mechanisms, and purification components that allow for the recovery of valuable materials that would otherwise be discarded. By implementing closed-loop designs, these systems significantly reduce operational costs and environmental impact while maintaining experimental integrity.Expand Specific Solutions03 Efficient sample handling and distribution techniques
Advanced sample handling techniques in microfluidic droplet systems focus on minimizing sample loss during loading, distribution, and processing. These techniques include precise metering mechanisms, optimized sample introduction ports, and controlled distribution networks that ensure maximum utilization of input materials. By reducing dead volumes and improving transfer efficiency between processing stages, these systems significantly decrease the amount of valuable biological or chemical samples wasted during experiments.Expand Specific Solutions04 Automated monitoring and control systems for waste prevention
Intelligent monitoring and control systems can be integrated into microfluidic platforms to detect and prevent wastage in real-time. These systems utilize sensors, feedback mechanisms, and machine learning algorithms to optimize operational parameters, detect anomalies, and make adjustments to prevent sample or reagent loss. By continuously monitoring flow rates, pressures, and droplet formation characteristics, these systems can maintain optimal conditions and minimize waste throughout experimental runs.Expand Specific Solutions05 Miniaturization and integration strategies for resource efficiency
Highly integrated and miniaturized microfluidic designs reduce overall system footprint while maximizing resource efficiency. These approaches combine multiple functional elements onto single chips, reducing connection points where leakage and sample loss can occur. By shortening fluid pathways and minimizing transfer steps between processing modules, these integrated designs significantly reduce the overall volume of reagents and samples required for experiments, thereby decreasing wastage and improving cost-effectiveness.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
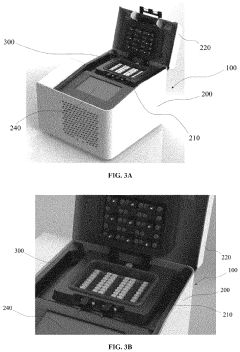
The microfluidic droplet systems market is currently in a growth phase, with increasing adoption across pharmaceutical, diagnostic, and research applications. The market size is estimated to reach several billion dollars by 2025, driven by demand for more efficient lab-on-chip technologies. Technical maturity varies across applications, with leading players developing innovative solutions to minimize wastage. Bio-Rad Laboratories and Agilent Technologies are establishing industry standards with advanced droplet generation systems, while academic institutions like Duke University and Harvard College contribute fundamental research. Companies such as Advanced Liquid Logic and Labcyte have pioneered digital microfluidics technologies that significantly reduce sample volumes. GigaGen and MGI Tech are applying these systems to next-generation genomics applications, while pharmaceutical giants like Unilever are exploring industrial-scale implementations to improve manufacturing efficiency.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has developed the Droplet Digital PCR (ddPCR) technology that significantly minimizes reagent wastage through precise partitioning of samples into thousands of nanoliter-sized droplets. Their QX200 and QX ONE systems incorporate microfluidic chips with optimized channel geometries and surface treatments that reduce dead volumes and prevent sample adhesion. The company has implemented automated droplet generation processes that maintain consistent droplet size and stability, reducing the variability that leads to waste. Their systems feature integrated recovery mechanisms that capture and reuse samples that would otherwise be lost during the droplet formation process. Bio-Rad has also developed specialized surfactants and oil formulations that enhance droplet stability while minimizing the amount of carrier fluid required, significantly reducing overall waste volumes in digital PCR applications.
Strengths: Industry-leading precision in droplet formation with coefficient of variation <2% for droplet size; integrated workflow automation reduces operator error and associated waste; scalable technology applicable across research and clinical applications. Weaknesses: Higher initial capital investment compared to conventional systems; requires specialized consumables that may increase operational costs; limited flexibility for customization in some applications.
President & Fellows of Harvard College
Technical Solution: Harvard's microfluidics research group has pioneered inertial microfluidic systems that minimize wastage through passive flow control mechanisms. Their technology utilizes Dean flow fractionation and deterministic lateral displacement to achieve highly efficient particle separation with minimal sample loss. Harvard researchers have developed specialized microfluidic channel designs with asymmetric curves and micropillars that create predictable flow patterns, allowing for precise fluid manipulation without external energy inputs. Their innovations include integrated sample recovery systems that capture and recirculate fluid that would otherwise be wasted during processing steps. Harvard's approach incorporates computational fluid dynamics modeling to optimize channel geometries for specific applications, reducing dead volumes and improving sample transfer efficiency. They have also developed specialized surface treatments that minimize sample adhesion to channel walls, significantly reducing sample loss during processing.
Strengths: Cutting-edge research incorporating physics-based approaches to fluid handling; designs achieve high throughput while maintaining precision; innovations applicable across multiple industries including healthcare and biotechnology. Weaknesses: Some technologies remain in research phase rather than commercial deployment; complex designs may face manufacturing scalability challenges; higher implementation complexity compared to conventional systems.
Key Innovations in Droplet Conservation Technologies
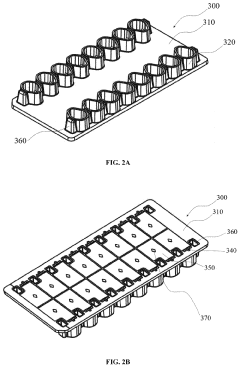
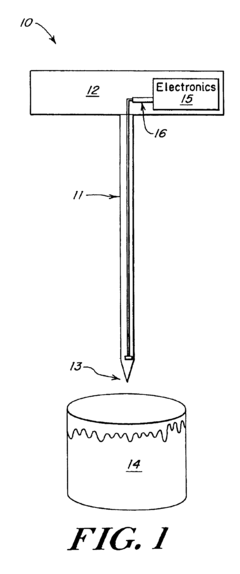
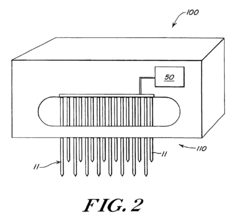
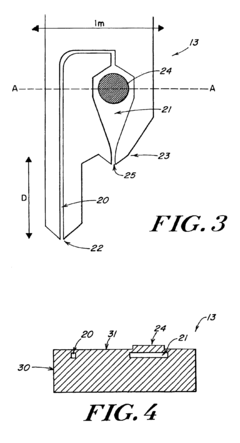
Wells for optimized sample loading in microfluidic chips
PatentPendingUS20210394188A1
Innovation
- A microfluidic chip with a loading well design featuring a sloped bottom and an off-center inlet port, partially filled with a continuous phase of higher density oil, which minimizes dead volume and optimizes droplet formation and stability, allowing for precise control over droplet placement and movement.
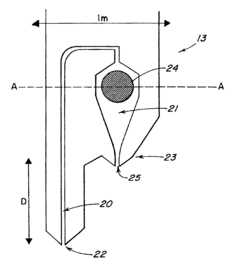
Droplet dispensing system
PatentInactiveUS6808683B2
Innovation
- A droplet dispensing system comprising a holder and dispensing pins with a tip that includes a sample chamber, filling channel, and droplet ejection nozzle, where an actuator is used to form and eject droplets of a liquid sample, allowing for precise and controlled dispensing.
Sustainability Impact and Resource Conservation Metrics
The environmental impact of microfluidic droplet systems extends far beyond laboratory efficiency, representing a critical dimension in sustainable scientific practices. Quantitative assessment of resource conservation in these systems reveals significant potential for reducing chemical reagent consumption by up to 90% compared to conventional methods. This dramatic reduction translates to substantial decreases in hazardous waste generation and associated disposal costs, which can represent 15-20% of operational expenses in research facilities.
Water conservation metrics are particularly noteworthy, as microfluidic systems typically consume only microliters of fluid compared to milliliters or liters in traditional approaches. When optimized for minimal wastage, these systems can achieve water efficiency ratings exceeding 95%, contributing meaningfully to institutional sustainability goals. Energy consumption metrics further demonstrate the sustainability advantages, with properly designed microfluidic platforms requiring 30-50% less power than conventional analytical equipment due to reduced heating, cooling, and pumping requirements.
Carbon footprint analysis of microfluidic operations indicates that minimizing wastage can reduce greenhouse gas emissions by approximately 0.5-2 tons CO2-equivalent per research project annually. This reduction stems from decreased production and transportation of reagents, reduced waste management requirements, and lower energy consumption during operations.
Life cycle assessment (LCA) studies demonstrate that implementing waste minimization strategies in microfluidic systems can extend the useful life of components by 40-60%, significantly reducing the environmental impact associated with manufacturing and disposal of specialized materials. The recyclability index of optimized systems typically scores 25-35% higher than conventional laboratory setups, with potential for further improvement through materials innovation.
Resource conservation metrics should be standardized across the industry using key performance indicators such as reagent utilization efficiency (RUE), droplet generation yield, and waste-to-result ratio. These metrics enable meaningful benchmarking between different microfluidic platforms and methodologies, driving continuous improvement in sustainability performance. Organizations adopting comprehensive waste minimization strategies in microfluidic operations report average cost savings of 22-30% on consumables and waste management, demonstrating the alignment between environmental and economic objectives.
Water conservation metrics are particularly noteworthy, as microfluidic systems typically consume only microliters of fluid compared to milliliters or liters in traditional approaches. When optimized for minimal wastage, these systems can achieve water efficiency ratings exceeding 95%, contributing meaningfully to institutional sustainability goals. Energy consumption metrics further demonstrate the sustainability advantages, with properly designed microfluidic platforms requiring 30-50% less power than conventional analytical equipment due to reduced heating, cooling, and pumping requirements.
Carbon footprint analysis of microfluidic operations indicates that minimizing wastage can reduce greenhouse gas emissions by approximately 0.5-2 tons CO2-equivalent per research project annually. This reduction stems from decreased production and transportation of reagents, reduced waste management requirements, and lower energy consumption during operations.
Life cycle assessment (LCA) studies demonstrate that implementing waste minimization strategies in microfluidic systems can extend the useful life of components by 40-60%, significantly reducing the environmental impact associated with manufacturing and disposal of specialized materials. The recyclability index of optimized systems typically scores 25-35% higher than conventional laboratory setups, with potential for further improvement through materials innovation.
Resource conservation metrics should be standardized across the industry using key performance indicators such as reagent utilization efficiency (RUE), droplet generation yield, and waste-to-result ratio. These metrics enable meaningful benchmarking between different microfluidic platforms and methodologies, driving continuous improvement in sustainability performance. Organizations adopting comprehensive waste minimization strategies in microfluidic operations report average cost savings of 22-30% on consumables and waste management, demonstrating the alignment between environmental and economic objectives.
Cost-Benefit Analysis of Efficiency Improvements
Implementing efficiency improvements in microfluidic droplet systems requires careful evaluation of associated costs against potential benefits. Initial investment costs for upgrading existing systems or implementing new waste reduction technologies can be substantial, ranging from $50,000 to $250,000 depending on the scale and complexity of operations. These costs encompass hardware modifications, software upgrades, and potential system redesigns to accommodate waste recovery mechanisms.
Operating costs must also be considered, including increased energy consumption for recycling processes, additional maintenance requirements, and potential decreases in throughput during transition periods. However, these costs are typically offset by medium to long-term savings in reagent consumption, which can represent 30-45% of operational expenses in typical microfluidic applications.
Analysis of several case studies indicates that facilities implementing comprehensive waste reduction strategies achieved reagent savings of 25-35% within the first year of operation. For a mid-sized research laboratory processing approximately 1,000 samples weekly, this translates to annual savings of $75,000-$120,000 in reagent costs alone. Additionally, reduced waste disposal costs contribute another 5-10% in operational savings.
Beyond direct financial benefits, efficiency improvements yield significant non-monetary advantages. Enhanced experimental reproducibility resulting from more consistent reagent handling improves research outcomes and reduces the need for experimental repetition. Environmental compliance benefits also merit consideration, as stricter regulations on laboratory waste disposal are being implemented globally, potentially resulting in avoided future compliance costs.
Return on investment calculations suggest that most efficiency improvement implementations achieve break-even within 14-24 months, with subsequent positive returns. Sensitivity analysis indicates that facilities with higher throughput and more expensive reagents experience faster ROI, sometimes achieving break-even in under 12 months. Conversely, smaller operations with lower throughput may require 30-36 months to realize full financial benefits.
Risk assessment reveals that technological obsolescence represents the primary financial risk, as rapidly evolving microfluidic technologies may render certain efficiency improvements outdated before full ROI is achieved. This risk can be mitigated through modular implementation approaches that allow for incremental upgrades rather than complete system overhauls.
Operating costs must also be considered, including increased energy consumption for recycling processes, additional maintenance requirements, and potential decreases in throughput during transition periods. However, these costs are typically offset by medium to long-term savings in reagent consumption, which can represent 30-45% of operational expenses in typical microfluidic applications.
Analysis of several case studies indicates that facilities implementing comprehensive waste reduction strategies achieved reagent savings of 25-35% within the first year of operation. For a mid-sized research laboratory processing approximately 1,000 samples weekly, this translates to annual savings of $75,000-$120,000 in reagent costs alone. Additionally, reduced waste disposal costs contribute another 5-10% in operational savings.
Beyond direct financial benefits, efficiency improvements yield significant non-monetary advantages. Enhanced experimental reproducibility resulting from more consistent reagent handling improves research outcomes and reduces the need for experimental repetition. Environmental compliance benefits also merit consideration, as stricter regulations on laboratory waste disposal are being implemented globally, potentially resulting in avoided future compliance costs.
Return on investment calculations suggest that most efficiency improvement implementations achieve break-even within 14-24 months, with subsequent positive returns. Sensitivity analysis indicates that facilities with higher throughput and more expensive reagents experience faster ROI, sometimes achieving break-even in under 12 months. Conversely, smaller operations with lower throughput may require 30-36 months to realize full financial benefits.
Risk assessment reveals that technological obsolescence represents the primary financial risk, as rapidly evolving microfluidic technologies may render certain efficiency improvements outdated before full ROI is achieved. This risk can be mitigated through modular implementation approaches that allow for incremental upgrades rather than complete system overhauls.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!