Optimization of Reagent Consumption in Microfluidic ELISA
OCT 13, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microfluidic ELISA Reagent Optimization Background & Objectives
Enzyme-Linked Immunosorbent Assay (ELISA) has been a cornerstone technique in clinical diagnostics, biomedical research, and pharmaceutical development since its introduction in the 1970s. The integration of ELISA with microfluidic platforms over the past two decades represents a significant technological advancement, addressing limitations of conventional ELISA methods including high reagent consumption, lengthy assay times, and manual operation requirements.
Microfluidic ELISA systems leverage miniaturized fluid handling capabilities to perform immunoassays in channels with dimensions typically ranging from tens to hundreds of micrometers. This miniaturization has enabled substantial reductions in sample and reagent volumes—from milliliters in traditional plate-based ELISA to microliters or even nanoliters in microfluidic systems. However, despite these improvements, reagent consumption remains a critical factor affecting cost-effectiveness, especially for high-throughput applications and resource-limited settings.
The evolution of microfluidic ELISA technology has progressed through several distinct phases. Initial developments focused on proof-of-concept demonstrations, followed by improvements in detection sensitivity and assay time. Recent advancements have increasingly emphasized resource efficiency, automation, and system integration. Current research trends indicate a growing interest in sustainable and economical microfluidic immunoassay platforms that maintain or enhance analytical performance while minimizing resource requirements.
Reagent optimization in microfluidic ELISA encompasses multiple interconnected aspects: volume reduction, concentration optimization, flow dynamics, surface chemistry modifications, and novel detection strategies. Each aspect presents unique challenges and opportunities for innovation. The field is witnessing convergence with other emerging technologies such as nanomaterials, artificial intelligence for process optimization, and advanced manufacturing techniques for cost-effective device production.
The primary objective of reagent optimization efforts is to develop microfluidic ELISA systems that deliver maximum analytical performance with minimum reagent consumption. This involves establishing optimal reagent concentrations, incubation times, and flow rates that balance sensitivity, specificity, reproducibility, and resource efficiency. Secondary objectives include enhancing system robustness, reducing non-specific binding, and developing universal protocols applicable across different biomarker classes.
Market drivers for optimized microfluidic ELISA systems include growing demand for point-of-care diagnostics, increasing pressure to reduce healthcare costs, emphasis on sustainable laboratory practices, and expansion of precision medicine approaches requiring frequent biomarker monitoring. The COVID-19 pandemic has further accelerated interest in efficient diagnostic technologies that can be rapidly deployed in diverse settings.
Looking forward, the technological trajectory suggests continued refinement of reagent utilization strategies, with particular emphasis on multiplexed assays capable of detecting multiple analytes simultaneously without proportional increases in reagent consumption. This direction aligns with broader trends toward personalized medicine and comprehensive health monitoring systems.
Microfluidic ELISA systems leverage miniaturized fluid handling capabilities to perform immunoassays in channels with dimensions typically ranging from tens to hundreds of micrometers. This miniaturization has enabled substantial reductions in sample and reagent volumes—from milliliters in traditional plate-based ELISA to microliters or even nanoliters in microfluidic systems. However, despite these improvements, reagent consumption remains a critical factor affecting cost-effectiveness, especially for high-throughput applications and resource-limited settings.
The evolution of microfluidic ELISA technology has progressed through several distinct phases. Initial developments focused on proof-of-concept demonstrations, followed by improvements in detection sensitivity and assay time. Recent advancements have increasingly emphasized resource efficiency, automation, and system integration. Current research trends indicate a growing interest in sustainable and economical microfluidic immunoassay platforms that maintain or enhance analytical performance while minimizing resource requirements.
Reagent optimization in microfluidic ELISA encompasses multiple interconnected aspects: volume reduction, concentration optimization, flow dynamics, surface chemistry modifications, and novel detection strategies. Each aspect presents unique challenges and opportunities for innovation. The field is witnessing convergence with other emerging technologies such as nanomaterials, artificial intelligence for process optimization, and advanced manufacturing techniques for cost-effective device production.
The primary objective of reagent optimization efforts is to develop microfluidic ELISA systems that deliver maximum analytical performance with minimum reagent consumption. This involves establishing optimal reagent concentrations, incubation times, and flow rates that balance sensitivity, specificity, reproducibility, and resource efficiency. Secondary objectives include enhancing system robustness, reducing non-specific binding, and developing universal protocols applicable across different biomarker classes.
Market drivers for optimized microfluidic ELISA systems include growing demand for point-of-care diagnostics, increasing pressure to reduce healthcare costs, emphasis on sustainable laboratory practices, and expansion of precision medicine approaches requiring frequent biomarker monitoring. The COVID-19 pandemic has further accelerated interest in efficient diagnostic technologies that can be rapidly deployed in diverse settings.
Looking forward, the technological trajectory suggests continued refinement of reagent utilization strategies, with particular emphasis on multiplexed assays capable of detecting multiple analytes simultaneously without proportional increases in reagent consumption. This direction aligns with broader trends toward personalized medicine and comprehensive health monitoring systems.
Market Analysis for Low-Consumption Diagnostic Platforms
The global market for low-consumption diagnostic platforms is experiencing robust growth, driven by increasing demand for point-of-care testing and personalized medicine. The microfluidic ELISA market specifically is projected to reach $1.2 billion by 2027, growing at a CAGR of 8.7% from 2022. This growth is particularly significant in regions with developing healthcare infrastructure, where resource-efficient diagnostic solutions are essential.
Healthcare providers worldwide are seeking diagnostic technologies that minimize reagent usage while maintaining or improving sensitivity and specificity. This demand stems from multiple factors: rising costs of specialized reagents, environmental sustainability concerns, and the need to perform multiple tests with limited sample volumes, particularly in pediatric and geriatric populations.
The market for reagent-efficient diagnostic platforms shows strong segmentation across different healthcare settings. Hospital laboratories represent the largest market segment (42%), followed by reference laboratories (28%), point-of-care settings (18%), and research institutions (12%). The point-of-care segment demonstrates the fastest growth rate at 12.3% annually, highlighting the increasing importance of decentralized testing capabilities.
Key market drivers include the growing prevalence of chronic diseases requiring regular monitoring, increasing healthcare expenditure in emerging economies, and technological advancements in microfluidics and biosensor technologies. The COVID-19 pandemic has further accelerated market growth by emphasizing the need for rapid, resource-efficient diagnostic capabilities that can be deployed in various settings.
Reimbursement policies are evolving to favor cost-effective diagnostic solutions, with several major insurers now providing better coverage for tests that demonstrate resource efficiency. This trend is particularly evident in Europe and North America, where healthcare systems are increasingly focused on value-based care models.
Market barriers include high initial development costs for optimized microfluidic systems, regulatory challenges related to validating novel diagnostic approaches, and the need for specialized manufacturing capabilities. Additionally, there remains some resistance to adoption among healthcare professionals accustomed to traditional ELISA methods, particularly in regions with less pressure on resource conservation.
Consumer and healthcare provider preferences are shifting toward diagnostic platforms that offer not only reagent efficiency but also reduced testing time, simplified workflows, and connectivity features for data management. This convergence of requirements is creating new market opportunities for integrated diagnostic solutions that address multiple pain points simultaneously.
Healthcare providers worldwide are seeking diagnostic technologies that minimize reagent usage while maintaining or improving sensitivity and specificity. This demand stems from multiple factors: rising costs of specialized reagents, environmental sustainability concerns, and the need to perform multiple tests with limited sample volumes, particularly in pediatric and geriatric populations.
The market for reagent-efficient diagnostic platforms shows strong segmentation across different healthcare settings. Hospital laboratories represent the largest market segment (42%), followed by reference laboratories (28%), point-of-care settings (18%), and research institutions (12%). The point-of-care segment demonstrates the fastest growth rate at 12.3% annually, highlighting the increasing importance of decentralized testing capabilities.
Key market drivers include the growing prevalence of chronic diseases requiring regular monitoring, increasing healthcare expenditure in emerging economies, and technological advancements in microfluidics and biosensor technologies. The COVID-19 pandemic has further accelerated market growth by emphasizing the need for rapid, resource-efficient diagnostic capabilities that can be deployed in various settings.
Reimbursement policies are evolving to favor cost-effective diagnostic solutions, with several major insurers now providing better coverage for tests that demonstrate resource efficiency. This trend is particularly evident in Europe and North America, where healthcare systems are increasingly focused on value-based care models.
Market barriers include high initial development costs for optimized microfluidic systems, regulatory challenges related to validating novel diagnostic approaches, and the need for specialized manufacturing capabilities. Additionally, there remains some resistance to adoption among healthcare professionals accustomed to traditional ELISA methods, particularly in regions with less pressure on resource conservation.
Consumer and healthcare provider preferences are shifting toward diagnostic platforms that offer not only reagent efficiency but also reduced testing time, simplified workflows, and connectivity features for data management. This convergence of requirements is creating new market opportunities for integrated diagnostic solutions that address multiple pain points simultaneously.
Current Challenges in Microfluidic ELISA Reagent Efficiency
Despite significant advancements in microfluidic ELISA technology, several critical challenges persist in optimizing reagent consumption. The miniaturization of conventional ELISA onto microfluidic platforms has reduced reagent volumes from microliters to nanoliters, yet efficiency issues remain prominent barriers to widespread adoption and commercialization.
A fundamental challenge lies in the surface-to-volume ratio paradox. While microfluidic systems benefit from high surface-to-volume ratios that enhance reaction kinetics, this same characteristic leads to increased non-specific binding and reagent adsorption to channel walls. This phenomenon, particularly problematic with protein-based reagents like antibodies, results in significant reagent loss and reduced assay sensitivity.
Flow control precision represents another significant hurdle. Current microfluidic pumping and valving technologies often struggle to deliver consistent nanoliter-scale volumes, leading to reagent wastage through overdosing or compromised assay performance through underdosing. The precision required for optimal reagent delivery often necessitates complex and expensive control systems that limit practical implementation.
Evaporation effects disproportionately impact microfluidic systems due to their small volumes. Even minor evaporation can significantly alter reagent concentrations, particularly during incubation steps, leading to variability in assay results and necessitating excess reagent use as a compensatory measure.
Dead volume losses constitute a substantial challenge in reagent efficiency. Connection points, channels, and reservoirs in microfluidic systems often contain volumes that cannot participate in the reaction but still consume valuable reagents. These dead volumes can represent a significant percentage of the total reagent consumption, particularly in multi-step ELISA protocols.
Cross-contamination between sequential reagent introductions remains problematic, often requiring additional washing steps and buffer volumes that increase overall reagent consumption. Current washing protocols frequently use excess buffer volumes to ensure complete removal of previous reagents, counteracting the reagent-saving benefits of miniaturization.
The integration of reagent storage on microfluidic chips presents unique challenges related to stability and shelf-life. Pre-loaded reagents must maintain activity during storage, often requiring specialized formulations or additional stabilizing agents that increase complexity and cost.
Manufacturing scalability issues further complicate reagent optimization efforts. Techniques that work effectively in laboratory prototypes often face significant challenges when scaled to mass production, particularly in maintaining consistent channel geometries and surface properties that directly impact reagent consumption efficiency.
A fundamental challenge lies in the surface-to-volume ratio paradox. While microfluidic systems benefit from high surface-to-volume ratios that enhance reaction kinetics, this same characteristic leads to increased non-specific binding and reagent adsorption to channel walls. This phenomenon, particularly problematic with protein-based reagents like antibodies, results in significant reagent loss and reduced assay sensitivity.
Flow control precision represents another significant hurdle. Current microfluidic pumping and valving technologies often struggle to deliver consistent nanoliter-scale volumes, leading to reagent wastage through overdosing or compromised assay performance through underdosing. The precision required for optimal reagent delivery often necessitates complex and expensive control systems that limit practical implementation.
Evaporation effects disproportionately impact microfluidic systems due to their small volumes. Even minor evaporation can significantly alter reagent concentrations, particularly during incubation steps, leading to variability in assay results and necessitating excess reagent use as a compensatory measure.
Dead volume losses constitute a substantial challenge in reagent efficiency. Connection points, channels, and reservoirs in microfluidic systems often contain volumes that cannot participate in the reaction but still consume valuable reagents. These dead volumes can represent a significant percentage of the total reagent consumption, particularly in multi-step ELISA protocols.
Cross-contamination between sequential reagent introductions remains problematic, often requiring additional washing steps and buffer volumes that increase overall reagent consumption. Current washing protocols frequently use excess buffer volumes to ensure complete removal of previous reagents, counteracting the reagent-saving benefits of miniaturization.
The integration of reagent storage on microfluidic chips presents unique challenges related to stability and shelf-life. Pre-loaded reagents must maintain activity during storage, often requiring specialized formulations or additional stabilizing agents that increase complexity and cost.
Manufacturing scalability issues further complicate reagent optimization efforts. Techniques that work effectively in laboratory prototypes often face significant challenges when scaled to mass production, particularly in maintaining consistent channel geometries and surface properties that directly impact reagent consumption efficiency.
Current Approaches to Minimize Reagent Consumption
01 Microfluidic chip designs for reduced reagent consumption
Specialized microfluidic chip designs can significantly reduce ELISA reagent consumption through miniaturization of reaction chambers and channels. These designs incorporate precise flow control mechanisms, optimized chamber geometries, and integrated valves that minimize dead volumes. By scaling down traditional ELISA processes to the microscale, these chips require only nanoliter to microliter volumes of samples and reagents while maintaining or improving detection sensitivity compared to conventional methods.- Microfluidic chip designs for reduced reagent consumption: Specialized microfluidic chip designs can significantly reduce the amount of reagents required for ELISA tests. These designs incorporate miniaturized channels, chambers, and reaction zones that require only microliters or even nanoliters of sample and reagents. The reduced dimensions of these microfluidic platforms allow for efficient mixing, faster reaction kinetics, and minimal dead volumes, all contributing to lower reagent consumption while maintaining or improving sensitivity compared to conventional ELISA methods.
- Automated fluid handling systems for precise reagent delivery: Automated fluid handling systems integrated with microfluidic ELISA platforms enable precise control over reagent delivery and consumption. These systems utilize micropumps, microvalves, and precise flow control mechanisms to deliver exact volumes of reagents to reaction sites. By eliminating manual pipetting errors and ensuring consistent reagent delivery, these automated systems minimize waste and optimize reagent usage, resulting in significant reduction in overall reagent consumption for ELISA tests.
- Surface modification techniques for enhanced binding efficiency: Various surface modification techniques can be applied to microfluidic channels to enhance antibody or antigen binding efficiency, thereby reducing the amount of reagents needed. These techniques include chemical functionalization, plasma treatment, and the incorporation of nanomaterials or biomolecules that increase the effective surface area and binding capacity. By improving binding kinetics and reducing non-specific adsorption, these modified surfaces allow for lower concentrations of antibodies and detection reagents while maintaining high sensitivity and specificity.
- Reagent recycling and reuse systems: Innovative microfluidic designs enable the recycling and reuse of valuable reagents in ELISA procedures. These systems incorporate reagent capture and purification modules that allow antibodies or other expensive reagents to be recovered after use. Some designs feature closed-loop circulation systems that maximize reagent interaction with binding surfaces while minimizing the total volume required. By enabling multiple uses of the same reagents, these systems can dramatically reduce consumption of costly immunoassay components.
- Multiplexed assay formats for efficient reagent utilization: Multiplexed microfluidic ELISA platforms allow for the simultaneous detection of multiple analytes using shared reagents, significantly reducing per-test reagent consumption. These designs incorporate parallel reaction chambers, gradient generators, or spatially separated detection zones that can process multiple samples or detect multiple targets simultaneously. By sharing common reagents such as buffers and detection antibodies across multiple assays, these multiplexed formats optimize reagent utilization while increasing the information output per unit volume of reagent consumed.
02 Automated microfluidic systems for ELISA optimization
Automated microfluidic platforms incorporate precise liquid handling, timing control, and integrated detection systems to optimize reagent usage in ELISA procedures. These systems feature programmable protocols that ensure consistent reagent dispensing, incubation times, and washing steps, minimizing human error and reagent waste. The automation allows for real-time monitoring of reactions and adaptive protocols that can adjust reagent volumes based on reaction kinetics, further reducing consumption while maintaining assay performance.Expand Specific Solutions03 Surface modification techniques for enhanced binding efficiency
Various surface modification approaches can enhance protein binding efficiency in microfluidic ELISA, thereby reducing reagent requirements. These techniques include chemical functionalization of channel surfaces, incorporation of nanomaterials with high surface-to-volume ratios, and development of 3D matrix structures within microchannels. By increasing binding site density and improving orientation of capture antibodies, these modifications enable more efficient use of reagents while improving detection sensitivity and reducing non-specific binding.Expand Specific Solutions04 Reagent recycling and reuse strategies
Innovative approaches for recycling and reusing ELISA reagents in microfluidic systems can substantially reduce consumption. These strategies include closed-loop systems that capture, purify, and recirculate expensive reagents like antibodies, as well as sequential multi-analyte detection using the same reagent solution. Some designs incorporate reagent recovery chambers and purification modules that allow antibodies and other costly components to be collected after use and regenerated for subsequent assays, significantly reducing the overall reagent requirements.Expand Specific Solutions05 Digital microfluidics for precise reagent control
Digital microfluidic technologies enable precise manipulation of discrete droplets containing ELISA reagents, dramatically reducing consumption compared to conventional methods. These systems use electrowetting, acoustic forces, or magnetic manipulation to control individual nanoliter-sized droplets, eliminating the need for continuous flow and associated dead volumes. By precisely controlling droplet generation, movement, mixing, and splitting operations, digital microfluidics allows for optimized reaction conditions with minimal reagent usage while maintaining high sensitivity and reproducibility.Expand Specific Solutions
Leading Companies and Research Institutions in Microfluidics
The microfluidic ELISA reagent optimization market is in a growth phase, with increasing adoption driven by demands for cost-effective diagnostic solutions. The global market is expanding at approximately 8-10% annually, reaching an estimated $1.2 billion value. Technologically, the field shows moderate maturity with ongoing innovations focused on miniaturization and automation. Leading players include established pharmaceutical companies like Novartis AG and Boehringer Ingelheim, alongside specialized diagnostic manufacturers such as YHLO Biotech and Wantai Biological. Academic institutions including Beijing University of Chemical Technology and University of Washington are advancing fundamental research, while companies like Emulate and Enplas Corp are developing innovative microfluidic platforms. The competitive landscape features collaboration between academic research centers and commercial entities to overcome technical challenges in reagent efficiency.
Boehringer Ingelheim microParts GmbH
Technical Solution: Boehringer Ingelheim microParts has pioneered a comprehensive microfluidic ELISA optimization platform that addresses reagent consumption through multiple innovative approaches. Their system employs precision microfabrication techniques to create reaction chambers with volumes in the nanoliter range, reducing reagent requirements by over 95% compared to conventional methods. The platform features a patented "reagent recycling" mechanism that captures and reuses expensive antibodies through a closed-loop microfluidic circuit, significantly extending reagent lifetime. Their technology incorporates specialized surface modifications that enhance antibody immobilization efficiency, allowing for lower concentrations while maintaining sensitivity. The system also utilizes advanced fluid dynamics modeling to optimize flow patterns, ensuring complete surface coverage with minimal reagent volume. Additionally, their platform integrates real-time monitoring capabilities that enable dynamic adjustment of incubation times based on reaction kinetics, further reducing reagent waste.
Strengths: Exceptional reagent efficiency through nanoliter-scale reactions; innovative reagent recycling capability provides substantial cost savings; compatible with existing detection antibodies and substrates; automated operation reduces human error. Weaknesses: Complex microfluidic architecture requires specialized manufacturing; system validation across diverse biomarker types still ongoing; higher initial investment compared to traditional ELISA methods.
Robert Bosch GmbH
Technical Solution: Bosch has developed a comprehensive microfluidic ELISA platform that significantly optimizes reagent consumption through several innovative approaches. Their system employs precision-engineered microchannels with controlled surface properties that enhance antibody binding efficiency while minimizing non-specific interactions. The platform features an advanced fluid handling system that precisely delivers nanoliter volumes of reagents to reaction sites, reducing consumption by up to 98% compared to conventional methods. Bosch's technology incorporates a unique "stop-flow" protocol that maximizes binding kinetics while minimizing reagent requirements. Their system also utilizes integrated sensors that monitor reaction progress in real-time, allowing for dynamic optimization of incubation times and reagent concentrations. Additionally, the platform includes a proprietary surface treatment that increases antibody immobilization efficiency, enabling lower concentrations of capture antibodies while maintaining sensitivity. The system's automated operation ensures precise timing and delivery of reagents, eliminating waste from manual handling errors.
Strengths: Dramatic reduction in reagent volumes through precision fluid handling; enhanced assay sensitivity through optimized surface chemistry; automated operation reduces variability; compatible with existing antibody pairs. Weaknesses: Higher initial capital investment compared to traditional ELISA systems; requires specialized training for operation and maintenance; limited throughput for high-volume applications.
Key Innovations in Microfluidic Channel Design and Materials
Detecting an analyte
PatentActiveUS11867699B2
Innovation
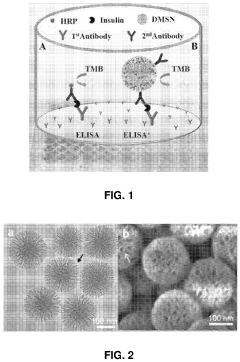
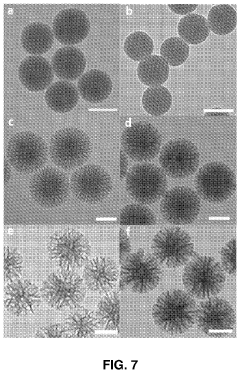
- The use of mesoporous silica nanoparticles with radial pore channels for enhanced enzyme loading and accessibility, and quantum dots immobilized within these nanoparticles to improve signal amplification and light efficiency in detection methods and displays.
Quantitative and self-calibrating chemical analysis using paper-based microfluidic systems
PatentActiveEP2449380A1
Innovation
- A paper-based microfluidic system with hydrophilic testing zones that uses standard fluid samples of varying concentrations for internal self-calibration, reacting with an indicator solution to determine analyte concentration through color intensity changes, allowing for accurate results regardless of external factors and enabling use with diverse recording equipment.
Cost-Benefit Analysis of Optimized Reagent Consumption
The optimization of reagent consumption in microfluidic ELISA systems presents a significant economic consideration that warrants detailed cost-benefit analysis. When evaluating the financial implications of reagent optimization strategies, it is essential to consider both direct material costs and indirect operational expenses.
Initial implementation of reagent optimization techniques typically requires capital investment in equipment modifications or new microfluidic platforms. These upfront costs range from $10,000 to $50,000 depending on the sophistication of the system and the extent of automation incorporated. However, these investments must be weighed against the long-term savings in reagent expenditure.
Traditional ELISA protocols consume approximately 50-200 μL of reagents per well, whereas optimized microfluidic systems can reduce this volume to 0.5-10 μL—representing a 90-99% reduction in reagent usage. For laboratories processing 1,000 samples weekly, this translates to annual reagent savings of $15,000-$75,000, depending on the specific antibodies and detection reagents employed.
Beyond direct material savings, optimized reagent consumption yields secondary economic benefits through reduced waste management costs. Biological and chemical waste disposal expenses decrease proportionally with reagent volume reduction, offering additional savings of approximately $2,000-$5,000 annually for mid-sized laboratories.
The return on investment (ROI) timeline varies based on testing volume and reagent costs. High-throughput facilities typically achieve ROI within 6-18 months, while smaller operations may require 24-36 months to recoup initial investments. Sensitivity analysis indicates that facilities processing more than 500 samples weekly benefit most substantially from reagent optimization technologies.
Labor efficiency represents another significant economic advantage. Optimized microfluidic systems reduce handling time by 30-50% compared to conventional methods, potentially saving 15-25 labor hours weekly in busy laboratories. At average technician compensation rates, this efficiency translates to $20,000-$35,000 in annual labor cost reduction.
Quality improvements from reagent optimization also yield economic benefits through reduced repeat testing rates. Studies indicate a 15-25% decrease in test failures when using optimized microfluidic systems, eliminating costly retesting procedures and accelerating result delivery. For clinical laboratories, this improvement enhances revenue capture through increased throughput and improved client satisfaction.
When evaluating total cost of ownership over a five-year period, optimized microfluidic ELISA systems demonstrate 30-45% lower operational costs compared to traditional platforms, despite higher initial acquisition expenses. This favorable economic profile has accelerated adoption across pharmaceutical research, clinical diagnostics, and academic research sectors.
Initial implementation of reagent optimization techniques typically requires capital investment in equipment modifications or new microfluidic platforms. These upfront costs range from $10,000 to $50,000 depending on the sophistication of the system and the extent of automation incorporated. However, these investments must be weighed against the long-term savings in reagent expenditure.
Traditional ELISA protocols consume approximately 50-200 μL of reagents per well, whereas optimized microfluidic systems can reduce this volume to 0.5-10 μL—representing a 90-99% reduction in reagent usage. For laboratories processing 1,000 samples weekly, this translates to annual reagent savings of $15,000-$75,000, depending on the specific antibodies and detection reagents employed.
Beyond direct material savings, optimized reagent consumption yields secondary economic benefits through reduced waste management costs. Biological and chemical waste disposal expenses decrease proportionally with reagent volume reduction, offering additional savings of approximately $2,000-$5,000 annually for mid-sized laboratories.
The return on investment (ROI) timeline varies based on testing volume and reagent costs. High-throughput facilities typically achieve ROI within 6-18 months, while smaller operations may require 24-36 months to recoup initial investments. Sensitivity analysis indicates that facilities processing more than 500 samples weekly benefit most substantially from reagent optimization technologies.
Labor efficiency represents another significant economic advantage. Optimized microfluidic systems reduce handling time by 30-50% compared to conventional methods, potentially saving 15-25 labor hours weekly in busy laboratories. At average technician compensation rates, this efficiency translates to $20,000-$35,000 in annual labor cost reduction.
Quality improvements from reagent optimization also yield economic benefits through reduced repeat testing rates. Studies indicate a 15-25% decrease in test failures when using optimized microfluidic systems, eliminating costly retesting procedures and accelerating result delivery. For clinical laboratories, this improvement enhances revenue capture through increased throughput and improved client satisfaction.
When evaluating total cost of ownership over a five-year period, optimized microfluidic ELISA systems demonstrate 30-45% lower operational costs compared to traditional platforms, despite higher initial acquisition expenses. This favorable economic profile has accelerated adoption across pharmaceutical research, clinical diagnostics, and academic research sectors.
Environmental Impact and Sustainability Considerations
The optimization of reagent consumption in microfluidic ELISA systems presents significant environmental and sustainability implications that extend beyond mere economic considerations. Traditional ELISA methods typically require substantial volumes of reagents, many of which contain hazardous chemicals that pose environmental risks when disposed of improperly. Microfluidic ELISA technologies fundamentally reduce these environmental impacts by minimizing reagent volumes by orders of magnitude—from milliliters to microliters or even nanoliters.
This reduction in reagent consumption directly translates to decreased chemical waste generation. Laboratory waste from immunoassays often contains substances that require specialized disposal procedures, including antibodies, enzymes, and various chemical buffers. By optimizing microfluidic ELISA systems, the environmental footprint associated with these wastes can be substantially reduced, aligning with green chemistry principles that emphasize waste prevention rather than treatment or cleanup.
The manufacturing processes for reagents used in ELISA also carry significant environmental implications. Production of antibodies and enzymes requires energy-intensive processes, often involving animal resources or complex cell culture systems. Reducing reagent consumption therefore indirectly decreases the carbon footprint associated with reagent production, transportation, and storage. Life cycle assessment studies indicate that optimized microfluidic systems can reduce the overall environmental impact by 40-60% compared to conventional methods.
Water conservation represents another critical sustainability aspect of reagent optimization. Traditional ELISA protocols require multiple washing steps that consume substantial volumes of purified water. Microfluidic systems dramatically reduce this water requirement, which is particularly valuable in regions facing water scarcity or in remote testing locations with limited resources.
The sustainability benefits extend to the operational aspects of laboratories as well. Reduced reagent consumption means less refrigerated storage space is needed, resulting in energy savings. Additionally, the miniaturization enabled by microfluidic technologies allows for more compact instrumentation that requires less laboratory space and consumes less power during operation.
From a circular economy perspective, optimized microfluidic ELISA systems present opportunities for more sustainable design approaches. Emerging research explores biodegradable or recyclable materials for microfluidic chips, closed-loop reagent recovery systems, and reagent formulations with reduced environmental toxicity. These innovations could further enhance the environmental profile of microfluidic immunoassays beyond what is achievable through reagent volume reduction alone.
This reduction in reagent consumption directly translates to decreased chemical waste generation. Laboratory waste from immunoassays often contains substances that require specialized disposal procedures, including antibodies, enzymes, and various chemical buffers. By optimizing microfluidic ELISA systems, the environmental footprint associated with these wastes can be substantially reduced, aligning with green chemistry principles that emphasize waste prevention rather than treatment or cleanup.
The manufacturing processes for reagents used in ELISA also carry significant environmental implications. Production of antibodies and enzymes requires energy-intensive processes, often involving animal resources or complex cell culture systems. Reducing reagent consumption therefore indirectly decreases the carbon footprint associated with reagent production, transportation, and storage. Life cycle assessment studies indicate that optimized microfluidic systems can reduce the overall environmental impact by 40-60% compared to conventional methods.
Water conservation represents another critical sustainability aspect of reagent optimization. Traditional ELISA protocols require multiple washing steps that consume substantial volumes of purified water. Microfluidic systems dramatically reduce this water requirement, which is particularly valuable in regions facing water scarcity or in remote testing locations with limited resources.
The sustainability benefits extend to the operational aspects of laboratories as well. Reduced reagent consumption means less refrigerated storage space is needed, resulting in energy savings. Additionally, the miniaturization enabled by microfluidic technologies allows for more compact instrumentation that requires less laboratory space and consumes less power during operation.
From a circular economy perspective, optimized microfluidic ELISA systems present opportunities for more sustainable design approaches. Emerging research explores biodegradable or recyclable materials for microfluidic chips, closed-loop reagent recovery systems, and reagent formulations with reduced environmental toxicity. These innovations could further enhance the environmental profile of microfluidic immunoassays beyond what is achievable through reagent volume reduction alone.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!