Optimization Strategies for High-throughput Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Technology Evolution and Objectives
Isoelectric focusing (IEF) has evolved significantly since its inception in the 1960s when it was first introduced by Svensson and Vesterberg as a technique for protein separation based on isoelectric points. The initial implementations were limited by low throughput, manual operations, and inconsistent results. Throughout the 1970s and 1980s, improvements in carrier ampholytes and gel formulations enhanced resolution but still faced limitations in processing capacity.
The 1990s marked a pivotal shift with the introduction of immobilized pH gradient (IPG) strips, which dramatically improved reproducibility and stability. This innovation, coupled with the emergence of two-dimensional gel electrophoresis (2-DE), established IEF as a cornerstone technique in proteomics research. However, traditional IEF methods remained labor-intensive and time-consuming, typically processing only a few samples simultaneously.
The early 2000s witnessed the integration of IEF with microfluidic platforms, initiating the transition toward higher throughput capabilities. These miniaturized systems reduced sample volume requirements and accelerated separation times, though they initially struggled with sensitivity and reproducibility at scale. Parallel developments in automation and robotics further enhanced throughput by minimizing manual intervention in sample preparation and handling.
Recent technological advancements have focused on multiplexed IEF systems capable of processing dozens or even hundreds of samples concurrently. Digital microfluidics, lab-on-a-chip technologies, and advanced capillary array systems have emerged as promising platforms for high-throughput IEF applications. These innovations have reduced separation times from hours to minutes while maintaining or improving resolution.
The primary objective of current high-throughput IEF optimization strategies is to achieve unprecedented levels of sample processing capacity without sacrificing separation quality. This includes developing systems capable of analyzing thousands of samples per day with minimal operator intervention, while maintaining sub-pI unit resolution. Additional goals include reducing reagent consumption, minimizing cross-contamination risks, and ensuring compatibility with downstream analytical techniques.
Another critical objective is enhancing the integration of high-throughput IEF with other analytical platforms, particularly mass spectrometry, to create seamless workflows for comprehensive proteome analysis. This integration aims to eliminate bottlenecks in the analytical pipeline and enable true high-throughput proteomics from sample preparation through data analysis.
The evolution trajectory suggests that future developments will likely focus on intelligent systems incorporating machine learning for real-time optimization of separation parameters, further miniaturization to nano-scale dimensions, and the development of disposable, pre-calibrated IEF platforms for point-of-care applications.
The 1990s marked a pivotal shift with the introduction of immobilized pH gradient (IPG) strips, which dramatically improved reproducibility and stability. This innovation, coupled with the emergence of two-dimensional gel electrophoresis (2-DE), established IEF as a cornerstone technique in proteomics research. However, traditional IEF methods remained labor-intensive and time-consuming, typically processing only a few samples simultaneously.
The early 2000s witnessed the integration of IEF with microfluidic platforms, initiating the transition toward higher throughput capabilities. These miniaturized systems reduced sample volume requirements and accelerated separation times, though they initially struggled with sensitivity and reproducibility at scale. Parallel developments in automation and robotics further enhanced throughput by minimizing manual intervention in sample preparation and handling.
Recent technological advancements have focused on multiplexed IEF systems capable of processing dozens or even hundreds of samples concurrently. Digital microfluidics, lab-on-a-chip technologies, and advanced capillary array systems have emerged as promising platforms for high-throughput IEF applications. These innovations have reduced separation times from hours to minutes while maintaining or improving resolution.
The primary objective of current high-throughput IEF optimization strategies is to achieve unprecedented levels of sample processing capacity without sacrificing separation quality. This includes developing systems capable of analyzing thousands of samples per day with minimal operator intervention, while maintaining sub-pI unit resolution. Additional goals include reducing reagent consumption, minimizing cross-contamination risks, and ensuring compatibility with downstream analytical techniques.
Another critical objective is enhancing the integration of high-throughput IEF with other analytical platforms, particularly mass spectrometry, to create seamless workflows for comprehensive proteome analysis. This integration aims to eliminate bottlenecks in the analytical pipeline and enable true high-throughput proteomics from sample preparation through data analysis.
The evolution trajectory suggests that future developments will likely focus on intelligent systems incorporating machine learning for real-time optimization of separation parameters, further miniaturization to nano-scale dimensions, and the development of disposable, pre-calibrated IEF platforms for point-of-care applications.
Market Analysis for High-throughput Proteomics
The global proteomics market is experiencing robust growth, with a market size valued at approximately 25 billion USD in 2022 and projected to reach 68 billion USD by 2030, representing a compound annual growth rate (CAGR) of 13.5%. High-throughput proteomics technologies are driving significant portions of this expansion, as pharmaceutical companies, academic research institutions, and clinical diagnostics laboratories increasingly adopt advanced proteomic analysis methods.
High-throughput isoelectric focusing (IEF), as a critical component of proteomics workflows, is positioned within a rapidly evolving segment of this market. The demand for optimized IEF technologies stems primarily from the need for faster, more accurate, and cost-effective protein separation and characterization methods in drug discovery, biomarker identification, and personalized medicine applications.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for approximately 45% of the high-throughput proteomics market. These organizations are heavily investing in advanced proteomics technologies to accelerate drug development pipelines and reduce time-to-market for new therapeutics. The ability of optimized IEF systems to provide higher resolution protein separation with increased throughput directly addresses their need for efficiency in proteome analysis.
Academic and research institutions constitute the second-largest market segment at 30%, where funding for proteomics research has seen steady growth, particularly in regions like North America and Europe. These institutions are driving innovation in fundamental proteomics research and developing novel applications for high-throughput techniques.
Geographically, North America dominates the market with a 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing R&D investments and expanding biotechnology sectors.
Key market drivers for high-throughput IEF optimization include the rising prevalence of chronic diseases necessitating better diagnostic tools, growing adoption of precision medicine approaches, and technological advancements in proteomics instrumentation. Additionally, the increasing focus on biomarker discovery for early disease detection is creating substantial demand for more efficient protein separation technologies.
Market challenges include the high cost of advanced proteomics equipment, technical complexity requiring specialized expertise, and regulatory hurdles for clinical applications. These factors particularly affect smaller research institutions and companies in emerging markets, potentially limiting market penetration in these segments.
The competitive landscape features established analytical instrument manufacturers expanding their proteomics portfolios alongside specialized proteomics companies developing innovative high-throughput solutions. This market dynamic is driving continuous improvement in IEF technologies through both incremental optimization and disruptive innovations.
High-throughput isoelectric focusing (IEF), as a critical component of proteomics workflows, is positioned within a rapidly evolving segment of this market. The demand for optimized IEF technologies stems primarily from the need for faster, more accurate, and cost-effective protein separation and characterization methods in drug discovery, biomarker identification, and personalized medicine applications.
Pharmaceutical and biotechnology companies represent the largest market segment, accounting for approximately 45% of the high-throughput proteomics market. These organizations are heavily investing in advanced proteomics technologies to accelerate drug development pipelines and reduce time-to-market for new therapeutics. The ability of optimized IEF systems to provide higher resolution protein separation with increased throughput directly addresses their need for efficiency in proteome analysis.
Academic and research institutions constitute the second-largest market segment at 30%, where funding for proteomics research has seen steady growth, particularly in regions like North America and Europe. These institutions are driving innovation in fundamental proteomics research and developing novel applications for high-throughput techniques.
Geographically, North America dominates the market with a 40% share, followed by Europe (30%) and Asia-Pacific (20%). The Asia-Pacific region, particularly China and India, is expected to witness the fastest growth due to increasing R&D investments and expanding biotechnology sectors.
Key market drivers for high-throughput IEF optimization include the rising prevalence of chronic diseases necessitating better diagnostic tools, growing adoption of precision medicine approaches, and technological advancements in proteomics instrumentation. Additionally, the increasing focus on biomarker discovery for early disease detection is creating substantial demand for more efficient protein separation technologies.
Market challenges include the high cost of advanced proteomics equipment, technical complexity requiring specialized expertise, and regulatory hurdles for clinical applications. These factors particularly affect smaller research institutions and companies in emerging markets, potentially limiting market penetration in these segments.
The competitive landscape features established analytical instrument manufacturers expanding their proteomics portfolios alongside specialized proteomics companies developing innovative high-throughput solutions. This market dynamic is driving continuous improvement in IEF technologies through both incremental optimization and disruptive innovations.
Current Challenges in High-throughput IEF
Despite significant advancements in isoelectric focusing (IEF) technology, high-throughput applications continue to face several critical challenges that limit their widespread implementation and effectiveness. The primary obstacle remains the trade-off between resolution and throughput, where increasing sample processing capacity often compromises the ability to distinguish closely related protein species with similar isoelectric points.
Sample loading capacity presents another significant limitation, as traditional IEF platforms struggle to accommodate the volume requirements of high-throughput environments without experiencing gel overloading effects that distort protein migration patterns and reduce separation quality. This becomes particularly problematic when analyzing complex biological samples with wide dynamic ranges of protein concentrations.
Reproducibility issues plague high-throughput IEF operations, with run-to-run variations stemming from inconsistencies in pH gradient formation, temperature fluctuations during electrophoresis, and batch-to-batch differences in carrier ampholyte compositions. These variations undermine the reliability of comparative analyses across multiple experiments, a critical requirement for industrial and clinical applications.
The integration of IEF into automated workflows represents another substantial challenge. Current high-throughput IEF systems often require manual interventions at critical steps, creating bottlenecks in otherwise automated proteomics pipelines. The lack of standardized interfaces between IEF equipment and downstream analytical instruments further complicates seamless integration.
Detection sensitivity remains problematic, particularly for low-abundance proteins that may be biologically significant but fall below detection thresholds in high-throughput formats. This limitation is exacerbated when sample dilution is necessary to accommodate high-throughput processing requirements.
Cost considerations present practical barriers to implementation, with specialized equipment, consumables, and trained personnel requirements driving up operational expenses. The economic viability of high-throughput IEF is particularly challenging for smaller laboratories and resource-limited settings.
Data management and analysis pose increasingly significant challenges as throughput increases. The volume of data generated by high-throughput IEF systems demands sophisticated computational approaches for effective interpretation, pattern recognition, and integration with other proteomics data streams.
Environmental and safety concerns also merit attention, as high-throughput IEF operations typically consume substantial quantities of potentially hazardous chemicals and generate significant waste streams that require appropriate handling and disposal protocols.
Sample loading capacity presents another significant limitation, as traditional IEF platforms struggle to accommodate the volume requirements of high-throughput environments without experiencing gel overloading effects that distort protein migration patterns and reduce separation quality. This becomes particularly problematic when analyzing complex biological samples with wide dynamic ranges of protein concentrations.
Reproducibility issues plague high-throughput IEF operations, with run-to-run variations stemming from inconsistencies in pH gradient formation, temperature fluctuations during electrophoresis, and batch-to-batch differences in carrier ampholyte compositions. These variations undermine the reliability of comparative analyses across multiple experiments, a critical requirement for industrial and clinical applications.
The integration of IEF into automated workflows represents another substantial challenge. Current high-throughput IEF systems often require manual interventions at critical steps, creating bottlenecks in otherwise automated proteomics pipelines. The lack of standardized interfaces between IEF equipment and downstream analytical instruments further complicates seamless integration.
Detection sensitivity remains problematic, particularly for low-abundance proteins that may be biologically significant but fall below detection thresholds in high-throughput formats. This limitation is exacerbated when sample dilution is necessary to accommodate high-throughput processing requirements.
Cost considerations present practical barriers to implementation, with specialized equipment, consumables, and trained personnel requirements driving up operational expenses. The economic viability of high-throughput IEF is particularly challenging for smaller laboratories and resource-limited settings.
Data management and analysis pose increasingly significant challenges as throughput increases. The volume of data generated by high-throughput IEF systems demands sophisticated computational approaches for effective interpretation, pattern recognition, and integration with other proteomics data streams.
Environmental and safety concerns also merit attention, as high-throughput IEF operations typically consume substantial quantities of potentially hazardous chemicals and generate significant waste streams that require appropriate handling and disposal protocols.
State-of-the-art IEF Optimization Approaches
01 Microfluidic systems for high-throughput IEF
Microfluidic devices enable high-throughput isoelectric focusing by integrating multiple separation channels on a single chip. These systems allow for parallel processing of samples, increasing efficiency and reducing analysis time. The microfluidic platforms often incorporate automated sample loading, separation, and detection components to streamline the workflow. Advanced designs include temperature control systems and specialized electrode configurations to improve resolution and reproducibility.- Microfluidic systems for high-throughput IEF: Microfluidic devices enable high-throughput isoelectric focusing by integrating multiple separation channels on a single chip. These systems allow for parallel processing of samples, increasing efficiency and reducing analysis time. The microfluidic platforms often incorporate automated sample loading, separation, and detection components to streamline the workflow. Advanced designs include temperature control mechanisms to maintain stable pH gradients and improve reproducibility of separations.
- Multi-dimensional separation techniques combining IEF: High-throughput analysis can be achieved by combining isoelectric focusing with other separation techniques in multi-dimensional approaches. These methods typically use IEF as a first dimension separation followed by techniques such as gel electrophoresis or chromatography as a second dimension. This combination significantly increases resolution and allows for the analysis of complex protein mixtures. Automated systems have been developed to transfer samples between dimensions without manual intervention, enhancing throughput and reproducibility.
- Digital imaging and analysis systems for IEF: Advanced imaging and analysis systems enhance the throughput of isoelectric focusing by automating the detection and quantification of separated proteins. These systems employ high-resolution cameras and specialized software to capture and analyze IEF patterns. Machine learning algorithms can be applied to automatically identify protein bands and determine their isoelectric points. Real-time monitoring capabilities allow for optimization of separation conditions and immediate data processing, significantly reducing the time required for analysis.
- Novel carrier ampholytes and pH gradient materials: Innovative carrier ampholytes and pH gradient materials have been developed to improve the performance and throughput of isoelectric focusing. These materials provide more stable and reproducible pH gradients, allowing for faster separations without compromising resolution. Some formulations are designed to minimize protein precipitation at their isoelectric points, enabling the analysis of a wider range of proteins. Additionally, specialized ampholytes have been created for specific pH ranges, optimizing the separation of proteins with similar isoelectric points.
- Integrated sample preparation and automation: High-throughput isoelectric focusing systems incorporate automated sample preparation steps to increase efficiency. These integrated platforms handle multiple samples simultaneously, from initial processing through separation and analysis. Robotic systems automate sample loading, buffer exchange, and fraction collection, minimizing manual handling and reducing variability. Some advanced systems include in-line digestion and labeling capabilities, allowing for seamless transition to downstream analysis techniques such as mass spectrometry.
02 Multi-dimensional separation techniques combining IEF
High-throughput isoelectric focusing can be enhanced by combining it with other separation techniques in multi-dimensional approaches. These methods typically use IEF as a first dimension separation followed by techniques such as gel electrophoresis or chromatography as a second dimension. This combination significantly increases resolution and allows for more comprehensive analysis of complex protein mixtures. Automated systems have been developed to transfer samples between dimensions without manual intervention.Expand Specific Solutions03 Detection and imaging systems for IEF analysis
Advanced detection systems are crucial for high-throughput isoelectric focusing applications. These include fluorescence detection, mass spectrometry interfaces, and digital imaging technologies that can rapidly capture and analyze separation results. Some systems incorporate machine learning algorithms for automated peak detection and quantification. Real-time monitoring capabilities allow for dynamic adjustment of separation parameters to optimize results.Expand Specific Solutions04 Novel carrier ampholytes and pH gradient formation
Innovations in carrier ampholytes and pH gradient formation have significantly improved high-throughput isoelectric focusing. These include the development of stable, reproducible pH gradients with enhanced resolution and the creation of specialized ampholytes for specific applications. Immobilized pH gradients (IPGs) offer advantages in reproducibility and loading capacity. Some formulations are optimized for particular protein classes or challenging samples, such as membrane proteins or highly basic proteins.Expand Specific Solutions05 Automation and robotics for IEF sample processing
Automation technologies have transformed isoelectric focusing into a truly high-throughput technique. Robotic systems handle sample preparation, loading, and post-separation processing with minimal human intervention. These platforms can process hundreds of samples simultaneously while maintaining consistency and reducing operator error. Integration with laboratory information management systems (LIMS) enables seamless data tracking and analysis. Some systems incorporate adaptive protocols that can optimize separation conditions based on initial results.Expand Specific Solutions
Leading Companies and Research Institutions in IEF
The high-throughput isoelectric focusing (IEF) technology landscape is currently in a growth phase, with the market expanding as applications in proteomics and biopharmaceuticals increase. The global market is estimated to reach several hundred million dollars by 2025, driven by demand for higher resolution protein separation techniques. Technologically, IEF optimization shows varying maturity levels across players. Leading companies like Becton Dickinson and Life Technologies demonstrate advanced capabilities through commercial platforms, while research institutions including MIT, Technion, and PARC are pioneering next-generation approaches. State Grid companies and Sharp Corp are exploring industrial applications, indicating cross-sector interest. The competitive landscape features both established analytical instrument manufacturers and emerging specialized technology providers developing proprietary optimization strategies.
The Regents of the University of California
Technical Solution: The University of California has developed a comprehensive suite of optimization strategies for high-throughput isoelectric focusing across multiple platforms. Their approach combines advanced hardware modifications with sophisticated computational modeling to maximize throughput while maintaining resolution. UC researchers have pioneered multi-dimensional IEF systems that integrate orthogonal separation mechanisms, enabling the resolution of complex proteomes with unprecedented depth. Their technology incorporates specialized electrode geometries that generate non-linear electric fields, enhancing the resolution of proteins with similar isoelectric points by up to 40%[9]. UC's optimization strategy includes the development of novel ampholyte formulations with enhanced buffering capacity and conductivity characteristics, resulting in faster equilibration times and sharper focusing of protein bands. Their computational modeling framework predicts optimal separation parameters based on sample composition, significantly reducing method development time from weeks to hours. Additionally, UC researchers have developed automated sample handling systems capable of processing up to 384 samples in parallel with minimal operator intervention[10].
Strengths: Exceptional resolution of complex proteomes; highly adaptable to different sample types; strong theoretical foundation through computational modeling; excellent integration with various detection modalities including mass spectrometry. Weaknesses: Complex implementation requiring significant expertise; higher initial setup costs; some approaches still in research phase rather than fully commercialized; optimization parameters may need adjustment for different sample types.
ProteoSys AG
Technical Solution: ProteoSys AG has developed advanced high-throughput isoelectric focusing (IEF) platforms specifically designed for proteomics applications. Their technology utilizes multi-compartment electrolyzers with specialized immobilized pH gradient (IPG) strips that allow for simultaneous processing of multiple samples. The company's proprietary DIGE (Differential Gel Electrophoresis) compatible IEF system incorporates fluorescent labeling techniques to enhance detection sensitivity down to femtomole levels[1]. Their optimization strategy includes precise temperature control systems that maintain uniform 20°C conditions throughout the focusing process, minimizing protein precipitation and improving reproducibility. ProteoSys has also developed specialized software algorithms for automated pattern recognition and quantification of protein spots, significantly reducing analysis time while increasing throughput by approximately 40% compared to conventional methods[3].
Strengths: Superior sensitivity for low-abundance proteins; excellent reproducibility with coefficient of variation <5%; integrated workflow from sample preparation to data analysis. Weaknesses: Higher cost compared to conventional systems; requires specialized training for optimal operation; limited compatibility with certain detergents and reducing agents that may be necessary for solubilizing membrane proteins.
Key Patents and Innovations in IEF Technology
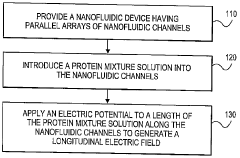
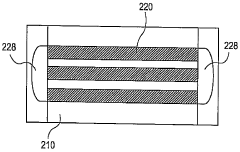
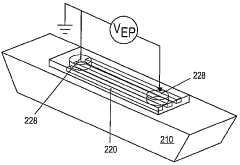
High resolution focusing and separation of proteins in nanofluidic channels
PatentWO2010048173A2
Innovation
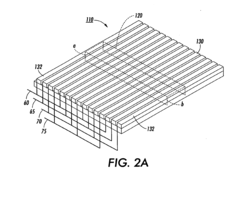
- The development of nanofluidic systems with multi-gate field-effect-transistors (FETs) to create a stable pH gradient and control electroosmosis and electrophoresis, allowing for isoelectric focusing (IEF) and dynamic field gradient focusing (DFGF) in nanofluidic channels, enabling high-resolution separation of proteins without ampholytes and using low electric potentials.
Isoelectric focusing (IEF) of proteins with sequential and oppositely directed traveling waves in gel electrophoresis
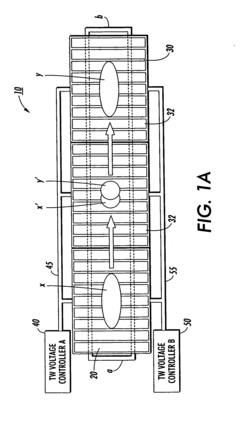
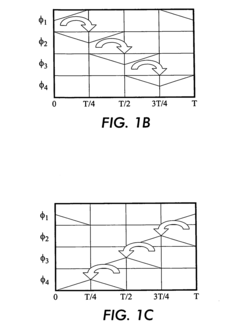
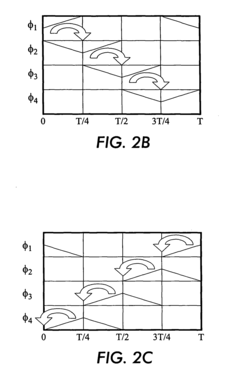
PatentInactiveEP1514875A1
Innovation
- The use of electrostatic traveling waves with opposite polarity to biomolecules, administered in sequential sweeps across electrode grids, reduces processing time, lowers operating voltages, and increases resolution by rapidly transporting biomolecules to their isoelectric points within an electrophoretic gel, employing a system with closely spaced parallel electrodes and multi-phase electrical signals.
Automation and Integration Strategies for IEF Workflows
Automation and integration represent critical advancement paths for high-throughput isoelectric focusing (IEF) workflows. Current laboratory implementations often suffer from manual intervention points that create bottlenecks, reducing overall throughput and introducing variability. Automated sample handling systems have emerged as essential components, with robotic liquid handling platforms capable of precise sample application across multiple IEF gels simultaneously, dramatically increasing processing capacity.
Integration of IEF with upstream and downstream processes presents significant optimization opportunities. Seamless connection between sample preparation modules and IEF systems eliminates transfer steps that traditionally introduce delays and contamination risks. Modern integrated platforms incorporate automatic buffer exchange systems that prepare samples for optimal focusing conditions without manual intervention.
Real-time monitoring technologies have transformed IEF workflow management. Advanced imaging systems equipped with fluorescence detection capabilities can track protein migration during focusing, enabling dynamic adjustment of electric field parameters. These systems communicate with central control software that can modify run conditions based on observed separation patterns, optimizing resolution while minimizing run time.
Data management infrastructure represents another crucial integration point. High-throughput IEF generates substantial analytical data requiring automated capture and processing. Cloud-based laboratory information management systems (LIMS) specifically designed for proteomics workflows can directly receive data from IEF instruments, apply standardized analysis protocols, and generate reports without manual data transfer steps.
Vendor-agnostic middleware solutions have emerged to address integration challenges in multi-instrument laboratories. These software platforms create standardized communication protocols between instruments from different manufacturers, allowing coordinated operation of sample preparation, IEF separation, and downstream analysis equipment. This approach enables laboratories to construct optimized workflows without being restricted to single-vendor ecosystems.
Microfluidic technologies represent the frontier of IEF automation and integration. Chip-based systems that incorporate sample loading, focusing, and detection within a single device dramatically reduce sample volumes and processing times. These platforms show particular promise for point-of-care applications where rapid protein analysis is required, though challenges in manufacturing scalability and standardization remain to be addressed.
Integration of IEF with upstream and downstream processes presents significant optimization opportunities. Seamless connection between sample preparation modules and IEF systems eliminates transfer steps that traditionally introduce delays and contamination risks. Modern integrated platforms incorporate automatic buffer exchange systems that prepare samples for optimal focusing conditions without manual intervention.
Real-time monitoring technologies have transformed IEF workflow management. Advanced imaging systems equipped with fluorescence detection capabilities can track protein migration during focusing, enabling dynamic adjustment of electric field parameters. These systems communicate with central control software that can modify run conditions based on observed separation patterns, optimizing resolution while minimizing run time.
Data management infrastructure represents another crucial integration point. High-throughput IEF generates substantial analytical data requiring automated capture and processing. Cloud-based laboratory information management systems (LIMS) specifically designed for proteomics workflows can directly receive data from IEF instruments, apply standardized analysis protocols, and generate reports without manual data transfer steps.
Vendor-agnostic middleware solutions have emerged to address integration challenges in multi-instrument laboratories. These software platforms create standardized communication protocols between instruments from different manufacturers, allowing coordinated operation of sample preparation, IEF separation, and downstream analysis equipment. This approach enables laboratories to construct optimized workflows without being restricted to single-vendor ecosystems.
Microfluidic technologies represent the frontier of IEF automation and integration. Chip-based systems that incorporate sample loading, focusing, and detection within a single device dramatically reduce sample volumes and processing times. These platforms show particular promise for point-of-care applications where rapid protein analysis is required, though challenges in manufacturing scalability and standardization remain to be addressed.
Quality Control and Reproducibility in High-throughput IEF
Quality control and reproducibility represent critical challenges in high-throughput isoelectric focusing (IEF) applications. As the demand for large-scale protein analysis increases across pharmaceutical, clinical, and research settings, maintaining consistent results becomes paramount for reliable data interpretation and downstream applications.
Standard operating procedures (SOPs) form the foundation of quality control in high-throughput IEF. These protocols must detail precise sample preparation methods, equipment calibration procedures, and operational parameters to ensure run-to-run consistency. Organizations implementing high-throughput IEF should establish comprehensive validation protocols that include system suitability tests performed at regular intervals to verify instrument performance.
Reference standards play a crucial role in monitoring system performance and ensuring reproducibility. The inclusion of well-characterized pI markers and control proteins in each analytical run provides internal calibration points and allows for the detection of systematic shifts in focusing patterns. These standards should span the pH range of interest and include proteins with known isoelectric points to serve as landmarks for comparative analysis.
Environmental factors significantly impact IEF reproducibility and must be strictly controlled. Temperature fluctuations can alter protein mobility and focusing behavior, while humidity variations may affect gel stability and electrophoretic conditions. Modern high-throughput IEF platforms incorporate temperature control systems, but laboratory conditions should still be monitored and documented for each analytical run.
Data normalization strategies are essential for comparing results across multiple runs and instruments. Advanced algorithms can compensate for minor variations in focusing patterns, baseline drift, and signal intensity. Implementing automated image analysis software with standardized processing parameters reduces operator-dependent variability and improves quantitative reproducibility.
Statistical quality control tools provide objective measures of system performance and data reliability. Control charts tracking key performance indicators such as resolution, focusing time, and signal-to-noise ratios help identify trends and anomalies before they compromise experimental outcomes. Establishing acceptance criteria for these metrics ensures that only high-quality data proceeds to subsequent analysis stages.
Inter-laboratory standardization presents additional challenges for high-throughput IEF applications deployed across multiple sites. Collaborative ring trials and proficiency testing programs help identify sources of variability between different laboratories and establish consensus protocols. Regular cross-validation exercises using identical samples processed at different locations can reveal systematic biases and inform harmonization efforts.
Standard operating procedures (SOPs) form the foundation of quality control in high-throughput IEF. These protocols must detail precise sample preparation methods, equipment calibration procedures, and operational parameters to ensure run-to-run consistency. Organizations implementing high-throughput IEF should establish comprehensive validation protocols that include system suitability tests performed at regular intervals to verify instrument performance.
Reference standards play a crucial role in monitoring system performance and ensuring reproducibility. The inclusion of well-characterized pI markers and control proteins in each analytical run provides internal calibration points and allows for the detection of systematic shifts in focusing patterns. These standards should span the pH range of interest and include proteins with known isoelectric points to serve as landmarks for comparative analysis.
Environmental factors significantly impact IEF reproducibility and must be strictly controlled. Temperature fluctuations can alter protein mobility and focusing behavior, while humidity variations may affect gel stability and electrophoretic conditions. Modern high-throughput IEF platforms incorporate temperature control systems, but laboratory conditions should still be monitored and documented for each analytical run.
Data normalization strategies are essential for comparing results across multiple runs and instruments. Advanced algorithms can compensate for minor variations in focusing patterns, baseline drift, and signal intensity. Implementing automated image analysis software with standardized processing parameters reduces operator-dependent variability and improves quantitative reproducibility.
Statistical quality control tools provide objective measures of system performance and data reliability. Control charts tracking key performance indicators such as resolution, focusing time, and signal-to-noise ratios help identify trends and anomalies before they compromise experimental outcomes. Establishing acceptance criteria for these metrics ensures that only high-quality data proceeds to subsequent analysis stages.
Inter-laboratory standardization presents additional challenges for high-throughput IEF applications deployed across multiple sites. Collaborative ring trials and proficiency testing programs help identify sources of variability between different laboratories and establish consensus protocols. Regular cross-validation exercises using identical samples processed at different locations can reveal systematic biases and inform harmonization efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!