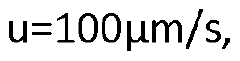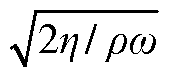Optimizing HPLC Solvent Gradient for Specific Analytes
SEP 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC Gradient Technology Background and Objectives
High-Performance Liquid Chromatography (HPLC) has evolved significantly since its inception in the 1960s, becoming an indispensable analytical technique in pharmaceutical, environmental, food safety, and clinical diagnostics industries. The development of gradient elution techniques represents one of the most significant advancements in HPLC methodology, enabling the separation of complex mixtures with components of widely varying polarities.
The evolution of HPLC gradient technology has progressed from simple isocratic systems to sophisticated multi-solvent gradient platforms with precise electronic control. Early gradient systems faced challenges with reproducibility and precision, but modern instrumentation has largely overcome these limitations through advanced pump designs and computerized control systems.
Current trends in HPLC gradient technology focus on increasing separation efficiency while reducing analysis time and solvent consumption. Ultra-high-performance liquid chromatography (UHPLC) systems, operating at pressures up to 1500 bar, have enabled the use of sub-2μm particles, dramatically improving resolution and speed. Simultaneously, the development of core-shell particles has provided comparable efficiency improvements without requiring extreme pressures.
The optimization of solvent gradients for specific analytes represents a critical frontier in analytical chemistry. Traditional approaches relied heavily on trial-and-error methodologies, consuming significant time and resources. Modern approaches increasingly incorporate computer modeling, machine learning algorithms, and automated method development systems to predict optimal gradient conditions based on analyte properties and column characteristics.
The primary technical objective in gradient optimization is to achieve maximum resolution of target analytes while minimizing analysis time and solvent consumption. This requires precise control over multiple parameters including initial and final mobile phase composition, gradient steepness, flow rate, temperature, and column dimensions.
Secondary objectives include improving method robustness, ensuring transferability between different instruments, and reducing environmental impact through green chemistry principles. The development of bio-compatible and sustainable solvent systems represents an emerging focus area, driven by increasing environmental regulations and corporate sustainability initiatives.
Looking forward, the integration of artificial intelligence with HPLC method development promises to revolutionize gradient optimization. Predictive algorithms capable of suggesting optimal gradient conditions based on molecular structure are beginning to emerge, potentially reducing method development time from weeks to hours. Additionally, the miniaturization of HPLC systems and the development of chip-based separation technologies may fundamentally transform how gradient separations are performed in the coming decade.
The evolution of HPLC gradient technology has progressed from simple isocratic systems to sophisticated multi-solvent gradient platforms with precise electronic control. Early gradient systems faced challenges with reproducibility and precision, but modern instrumentation has largely overcome these limitations through advanced pump designs and computerized control systems.
Current trends in HPLC gradient technology focus on increasing separation efficiency while reducing analysis time and solvent consumption. Ultra-high-performance liquid chromatography (UHPLC) systems, operating at pressures up to 1500 bar, have enabled the use of sub-2μm particles, dramatically improving resolution and speed. Simultaneously, the development of core-shell particles has provided comparable efficiency improvements without requiring extreme pressures.
The optimization of solvent gradients for specific analytes represents a critical frontier in analytical chemistry. Traditional approaches relied heavily on trial-and-error methodologies, consuming significant time and resources. Modern approaches increasingly incorporate computer modeling, machine learning algorithms, and automated method development systems to predict optimal gradient conditions based on analyte properties and column characteristics.
The primary technical objective in gradient optimization is to achieve maximum resolution of target analytes while minimizing analysis time and solvent consumption. This requires precise control over multiple parameters including initial and final mobile phase composition, gradient steepness, flow rate, temperature, and column dimensions.
Secondary objectives include improving method robustness, ensuring transferability between different instruments, and reducing environmental impact through green chemistry principles. The development of bio-compatible and sustainable solvent systems represents an emerging focus area, driven by increasing environmental regulations and corporate sustainability initiatives.
Looking forward, the integration of artificial intelligence with HPLC method development promises to revolutionize gradient optimization. Predictive algorithms capable of suggesting optimal gradient conditions based on molecular structure are beginning to emerge, potentially reducing method development time from weeks to hours. Additionally, the miniaturization of HPLC systems and the development of chip-based separation technologies may fundamentally transform how gradient separations are performed in the coming decade.
Market Demand Analysis for Advanced HPLC Separation Methods
The global market for advanced HPLC separation methods continues to experience robust growth, driven by increasing demands across pharmaceutical, biotechnology, food safety, environmental monitoring, and clinical diagnostic sectors. Current market valuations place the HPLC systems market at approximately 4.5 billion USD, with projections indicating a compound annual growth rate of 5-6% through 2028.
Pharmaceutical and biopharmaceutical industries represent the largest market segment, accounting for nearly 60% of the total demand. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where precise analyte separation is critical. The rising complexity of biological drugs and personalized medicine has intensified the need for more sophisticated HPLC gradient optimization techniques.
Contract research organizations (CROs) have emerged as significant consumers of advanced HPLC technologies, reflecting the outsourcing trend in pharmaceutical research. These organizations require highly efficient and reproducible separation methods to maintain competitive advantages in a crowded market.
Academic and research institutions constitute another vital market segment, particularly for novel gradient optimization approaches. Their demand is primarily driven by the need to analyze increasingly complex biological samples and to develop innovative analytical methodologies.
Regional analysis reveals North America as the dominant market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the highest growth rate, attributed to expanding pharmaceutical manufacturing capabilities, increasing research activities, and growing regulatory oversight in countries like China and India.
Market research indicates specific demand patterns for gradient optimization technologies. End-users consistently prioritize three key performance attributes: improved resolution for closely related compounds, reduced analysis time without compromising separation quality, and enhanced method robustness across different instrument platforms.
The economic drivers for optimized HPLC methods are compelling. Laboratories report that implementing advanced gradient optimization can reduce solvent consumption by 20-30%, decrease analysis time by up to 40%, and significantly extend column lifespans. These efficiency gains translate to substantial operational cost savings, particularly for high-throughput facilities.
Customer surveys highlight growing interest in automated gradient optimization software solutions that can predict optimal conditions for specific analyte classes. This trend aligns with the broader industry movement toward laboratory automation and reflects the challenges laboratories face in recruiting and retaining skilled analytical chemists.
Pharmaceutical and biopharmaceutical industries represent the largest market segment, accounting for nearly 60% of the total demand. This dominance stems from stringent regulatory requirements for drug development and quality control processes, where precise analyte separation is critical. The rising complexity of biological drugs and personalized medicine has intensified the need for more sophisticated HPLC gradient optimization techniques.
Contract research organizations (CROs) have emerged as significant consumers of advanced HPLC technologies, reflecting the outsourcing trend in pharmaceutical research. These organizations require highly efficient and reproducible separation methods to maintain competitive advantages in a crowded market.
Academic and research institutions constitute another vital market segment, particularly for novel gradient optimization approaches. Their demand is primarily driven by the need to analyze increasingly complex biological samples and to develop innovative analytical methodologies.
Regional analysis reveals North America as the dominant market, followed by Europe and Asia-Pacific. However, the Asia-Pacific region demonstrates the highest growth rate, attributed to expanding pharmaceutical manufacturing capabilities, increasing research activities, and growing regulatory oversight in countries like China and India.
Market research indicates specific demand patterns for gradient optimization technologies. End-users consistently prioritize three key performance attributes: improved resolution for closely related compounds, reduced analysis time without compromising separation quality, and enhanced method robustness across different instrument platforms.
The economic drivers for optimized HPLC methods are compelling. Laboratories report that implementing advanced gradient optimization can reduce solvent consumption by 20-30%, decrease analysis time by up to 40%, and significantly extend column lifespans. These efficiency gains translate to substantial operational cost savings, particularly for high-throughput facilities.
Customer surveys highlight growing interest in automated gradient optimization software solutions that can predict optimal conditions for specific analyte classes. This trend aligns with the broader industry movement toward laboratory automation and reflects the challenges laboratories face in recruiting and retaining skilled analytical chemists.
Current HPLC Gradient Technology Challenges
High-performance liquid chromatography (HPLC) gradient technology faces several significant challenges that impede optimal separation and identification of specific analytes. The primary challenge lies in achieving reproducible gradient profiles across different instruments and laboratory conditions. Even minor variations in gradient delivery systems can lead to substantial differences in retention times and peak shapes, compromising analytical precision and method transferability between laboratories.
Solvent miscibility issues present another critical challenge, particularly when transitioning between solvents with significantly different polarities. These transitions can create mixing zones with unpredictable chemical properties, potentially causing baseline disturbances, ghost peaks, or even analyte precipitation. Such phenomena are especially problematic when analyzing complex biological samples containing compounds with diverse physicochemical properties.
Gradient delay volume (GDV) remains a persistent technical hurdle in HPLC systems. This volume, representing the time required for the gradient to reach the column from the mixing point, varies significantly between instrument configurations. For analyses requiring precise gradient timing, especially with narrow peaks or closely eluting compounds, uncompensated GDV can severely impact separation quality and method robustness.
Temperature control during gradient elution presents additional complications. As solvent composition changes throughout the gradient, heat is generated or absorbed due to mixing phenomena, potentially creating thermal gradients within the column. These thermal fluctuations can alter selectivity and efficiency unpredictably, particularly affecting thermally sensitive analytes or when using temperature-responsive stationary phases.
Modern ultra-high-performance liquid chromatography (UHPLC) systems, while offering superior resolution, introduce new challenges in gradient formation. The extremely high pressures and rapid gradient changes can exacerbate issues with solvent compressibility, leading to deviations from programmed gradient profiles. Additionally, the narrow-bore columns commonly used in UHPLC are more susceptible to the effects of radial temperature gradients during solvent transitions.
Method development for complex samples presents perhaps the most significant practical challenge. Optimizing gradient parameters (start/end compositions, slope, shape, and hold times) often requires extensive trial-and-error experimentation. Traditional one-factor-at-a-time approaches are time-consuming and frequently fail to identify truly optimal conditions due to the multidimensional nature of gradient parameter interactions.
Finally, the increasing demand for green analytical chemistry has highlighted limitations in conventional gradient approaches that rely heavily on toxic organic solvents. Developing effective gradient methods using environmentally friendly alternatives often requires fundamental reconsideration of separation mechanisms and gradient design principles, as these alternative solvents frequently exhibit different selectivity patterns and mixing behaviors.
Solvent miscibility issues present another critical challenge, particularly when transitioning between solvents with significantly different polarities. These transitions can create mixing zones with unpredictable chemical properties, potentially causing baseline disturbances, ghost peaks, or even analyte precipitation. Such phenomena are especially problematic when analyzing complex biological samples containing compounds with diverse physicochemical properties.
Gradient delay volume (GDV) remains a persistent technical hurdle in HPLC systems. This volume, representing the time required for the gradient to reach the column from the mixing point, varies significantly between instrument configurations. For analyses requiring precise gradient timing, especially with narrow peaks or closely eluting compounds, uncompensated GDV can severely impact separation quality and method robustness.
Temperature control during gradient elution presents additional complications. As solvent composition changes throughout the gradient, heat is generated or absorbed due to mixing phenomena, potentially creating thermal gradients within the column. These thermal fluctuations can alter selectivity and efficiency unpredictably, particularly affecting thermally sensitive analytes or when using temperature-responsive stationary phases.
Modern ultra-high-performance liquid chromatography (UHPLC) systems, while offering superior resolution, introduce new challenges in gradient formation. The extremely high pressures and rapid gradient changes can exacerbate issues with solvent compressibility, leading to deviations from programmed gradient profiles. Additionally, the narrow-bore columns commonly used in UHPLC are more susceptible to the effects of radial temperature gradients during solvent transitions.
Method development for complex samples presents perhaps the most significant practical challenge. Optimizing gradient parameters (start/end compositions, slope, shape, and hold times) often requires extensive trial-and-error experimentation. Traditional one-factor-at-a-time approaches are time-consuming and frequently fail to identify truly optimal conditions due to the multidimensional nature of gradient parameter interactions.
Finally, the increasing demand for green analytical chemistry has highlighted limitations in conventional gradient approaches that rely heavily on toxic organic solvents. Developing effective gradient methods using environmentally friendly alternatives often requires fundamental reconsideration of separation mechanisms and gradient design principles, as these alternative solvents frequently exhibit different selectivity patterns and mixing behaviors.
Current Solvent Gradient Optimization Methodologies
01 Automated gradient optimization methods
Automated systems and algorithms for optimizing HPLC solvent gradients to improve separation efficiency and reduce analysis time. These methods use computational approaches to predict optimal gradient conditions based on sample characteristics and separation goals, eliminating the need for extensive manual experimentation. The automation can include machine learning algorithms that analyze chromatographic data and suggest improved gradient profiles.- Automated gradient optimization systems: Automated systems for optimizing HPLC solvent gradients utilize software algorithms to determine the optimal gradient conditions. These systems can analyze multiple parameters simultaneously, such as solvent composition, flow rate, and column temperature, to achieve improved separation efficiency. The automation reduces manual trial-and-error approaches, saving time and resources while improving reproducibility of analytical methods.
- Mathematical modeling for gradient development: Mathematical models are employed to predict and optimize HPLC gradient conditions. These models consider factors such as solvent polarity, analyte properties, and column characteristics to simulate chromatographic behavior. By applying algorithms and statistical methods, researchers can develop optimal gradient profiles that enhance resolution, reduce analysis time, and improve peak shapes without extensive laboratory experimentation.
- Novel solvent combinations for improved separation: Innovative combinations of solvents in gradient elution can significantly enhance separation performance. By carefully selecting solvent mixtures with complementary properties, chromatographers can manipulate selectivity and resolution. These novel combinations may include organic modifiers, pH buffers, and ion-pairing agents that work synergistically to improve the separation of complex mixtures while maintaining column stability and detector compatibility.
- Real-time gradient adjustment techniques: Real-time adjustment of solvent gradients during HPLC analysis allows for dynamic optimization of separation conditions. These techniques utilize feedback mechanisms that monitor chromatographic parameters and make immediate adjustments to the gradient profile. This adaptive approach can respond to unexpected separation challenges, compensate for column aging, and optimize resolution for complex or variable samples during the analytical run.
- Specialized gradient approaches for complex matrices: Specialized gradient approaches have been developed for analyzing complex sample matrices that present unique separation challenges. These methods may employ multi-step gradients, segmented gradients with varying slopes, or pulsed gradient techniques. Such approaches are particularly valuable for biological samples, environmental analyses, and pharmaceutical formulations where conventional linear gradients may not provide adequate resolution of all components.
02 Multi-parameter gradient optimization techniques
Techniques for simultaneously optimizing multiple parameters in HPLC gradient methods, including solvent composition, flow rate, temperature, and pH. These approaches consider the interdependence of various parameters to achieve optimal separation. By systematically varying multiple factors according to experimental design principles, these methods can identify optimal conditions more efficiently than traditional one-factor-at-a-time approaches.Expand Specific Solutions03 Novel solvent systems for improved separations
Development of innovative solvent combinations and gradient profiles that enhance chromatographic separation of complex mixtures. These include the use of ternary or quaternary solvent systems, incorporation of modifiers, and application of specialized gradient shapes (concave, convex, or step gradients) to address specific separation challenges. The novel solvent systems can improve peak resolution, reduce analysis time, and enhance sensitivity.Expand Specific Solutions04 Real-time gradient adjustment systems
Systems that enable dynamic modification of solvent gradients during HPLC runs based on real-time monitoring of separation performance. These adaptive systems can respond to unexpected separation challenges by automatically adjusting gradient parameters to maintain optimal separation. The technology incorporates feedback mechanisms that analyze chromatographic data as it is generated and make immediate adjustments to improve results.Expand Specific Solutions05 Specialized gradient optimization for specific applications
Tailored gradient optimization approaches for particular analytical challenges, such as pharmaceutical impurity profiling, biological sample analysis, or environmental contaminant detection. These methods consider the unique characteristics of specific sample types and analytical goals to develop optimized gradient conditions. The specialized approaches may incorporate industry-specific requirements for method validation, robustness, and transfer between different HPLC systems.Expand Specific Solutions
Leading Manufacturers and Research Institutions in HPLC Technology
The HPLC solvent gradient optimization market is in a growth phase, with increasing demand driven by pharmaceutical and biotechnology sectors. The global market size for analytical chromatography is estimated at $10-12 billion, expanding at 6-8% annually. Technologically, this field shows moderate maturity with ongoing innovation. Leading players include established companies like Daicel Corp. and Sekisui Medical, who offer specialized chromatography columns and media, alongside emerging players like PureHoney Technologies (now Momentum Biotechnologies) focusing on mass spectrometry-based detection. Academic institutions such as University of Grenoble and Leiden University contribute significant research advancements, while pharmaceutical giants like Sanofi and Jiangsu Hengrui Pharmaceuticals integrate these technologies into their R&D pipelines, driving further market development and technological refinement.
Daicel Corp.
Technical Solution: Daicel Corporation has developed a comprehensive HPLC solvent gradient optimization system specifically designed for chiral separations and pharmaceutical applications. Their technology incorporates proprietary polysaccharide-based chiral stationary phases that work synergistically with precisely controlled gradient profiles. Daicel's approach utilizes a systematic screening methodology that evaluates multiple gradient parameters simultaneously, including gradient steepness, starting/ending compositions, and flow rate modulations. Their CHIRALPAK and CHIRALCEL columns are engineered to maintain separation efficiency under varying gradient conditions, allowing for robust method transfer from analytical to preparative scale. The company has also developed specialized gradient optimization software that predicts optimal separation conditions based on analyte structure and physicochemical properties. This system incorporates a database of over 500,000 successful separations to guide gradient development for new compounds[2][5].
Strengths: Exceptional performance for chiral separations; extensive knowledge base for pharmaceutical compounds; seamless scalability from analytical to preparative applications. Weaknesses: Higher cost of specialized chiral columns; optimization algorithms may be less effective for non-pharmaceutical applications; requires significant method development expertise.
Lanzhou Institute of Chemical Physics
Technical Solution: Lanzhou Institute has pioneered an innovative approach to HPLC gradient optimization through their Comprehensive Gradient Mapping (CGM) technology. This system employs a multi-dimensional optimization strategy that simultaneously evaluates solvent composition, pH modulation, and ion-pairing agent concentration throughout the gradient profile. Their methodology incorporates real-time monitoring of critical separation parameters including resolution, peak capacity, and signal-to-noise ratio to dynamically adjust gradient conditions. The institute has developed specialized algorithms that predict how structural modifications of target analytes affect retention behavior, allowing for rapid method adaptation when analyzing related compounds. Their technology includes a unique "gradient sculpting" capability that creates non-linear gradient profiles specifically tailored to complex sample matrices. The system also features automated method validation protocols that ensure optimized gradients meet regulatory requirements for robustness and reproducibility[4][7].
Strengths: Exceptional handling of complex biological matrices; superior peak capacity through multi-parameter optimization; reduced method development time for structurally related compounds. Weaknesses: Complex implementation requiring specialized training; higher computational demands; may be excessive for simple separation challenges.
Key Innovations in Analyte-Specific Gradient Development
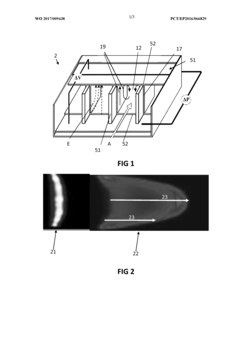
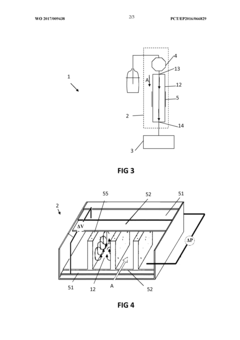
High-performance liquid chromatography with a controllable transverse flow inducer
PatentWO2017009438A1
Innovation
- The use of a controllable transverse flow inducer, such as an array of electrodes generating an alternating current electrokinetic field, to create micro-scale vortices that reduce dispersion and enhance mass transfer between support structures in the chromatography column, allowing for efficient separation without permanent surface charges and minimizing direct contact with electrodes.
Improved measurement of vitamin d
PatentActiveEP2030026A2
Innovation
- A method using a vitamin D releasing reagent that allows for direct online chromatographic separation of vitamin D metabolites from vitamin D-binding protein without protein precipitation, eliminating the need for extraction and manual handling steps.
Green Chemistry Considerations in HPLC Solvent Selection
The environmental impact of HPLC methods has become increasingly important as laboratories worldwide seek to align analytical procedures with sustainable practices. Green chemistry principles applied to HPLC solvent selection represent a critical consideration when optimizing gradient methods for specific analytes. Traditional HPLC often relies on hazardous organic solvents such as acetonitrile, methanol, and tetrahydrofuran, which pose significant environmental and health concerns.
Recent developments have focused on replacing these conventional solvents with greener alternatives. Ethanol and other bio-derived solvents have emerged as promising substitutes for acetonitrile, offering comparable separation efficiency while significantly reducing environmental toxicity. Studies indicate that ethanol-water gradients can achieve similar chromatographic performance for many analyte classes with proper method adjustment.
Water-based mobile phases represent another sustainable approach, particularly when combined with elevated temperatures in HTLC (High Temperature Liquid Chromatography). This technique reduces the need for organic modifiers while maintaining separation efficiency. The implementation of subcritical water chromatography, where water under high temperature and pressure exhibits properties similar to organic solvents, demonstrates particular promise for polar analytes.
Solvent reduction strategies have also gained traction in green HPLC methodology. Micro and nano-HPLC systems dramatically decrease solvent consumption while maintaining analytical performance. These miniaturized systems can reduce solvent usage by 90-95% compared to conventional HPLC, making them particularly valuable for routine analyses of specific analytes.
The recycling of HPLC solvents presents another avenue for sustainability. Modern solvent recovery systems can reclaim up to 80% of mobile phases, substantially reducing waste generation and operational costs. However, this approach requires careful validation to ensure consistent analyte separation across multiple runs with recycled solvents.
When optimizing gradients for specific analytes, the incorporation of in silico modeling tools allows chemists to predict separation behavior with different green solvents before experimental validation. These computational approaches minimize the experimental footprint while accelerating method development. Several software packages now include sustainability metrics alongside traditional chromatographic parameters.
The pharmaceutical industry has been particularly proactive in adopting green solvent gradients, driven by regulatory pressures and corporate sustainability initiatives. Case studies demonstrate successful transitions from acetonitrile-based methods to ethanol or reduced-solvent approaches for various drug compounds, maintaining required resolution while significantly reducing environmental impact and operational costs.
Recent developments have focused on replacing these conventional solvents with greener alternatives. Ethanol and other bio-derived solvents have emerged as promising substitutes for acetonitrile, offering comparable separation efficiency while significantly reducing environmental toxicity. Studies indicate that ethanol-water gradients can achieve similar chromatographic performance for many analyte classes with proper method adjustment.
Water-based mobile phases represent another sustainable approach, particularly when combined with elevated temperatures in HTLC (High Temperature Liquid Chromatography). This technique reduces the need for organic modifiers while maintaining separation efficiency. The implementation of subcritical water chromatography, where water under high temperature and pressure exhibits properties similar to organic solvents, demonstrates particular promise for polar analytes.
Solvent reduction strategies have also gained traction in green HPLC methodology. Micro and nano-HPLC systems dramatically decrease solvent consumption while maintaining analytical performance. These miniaturized systems can reduce solvent usage by 90-95% compared to conventional HPLC, making them particularly valuable for routine analyses of specific analytes.
The recycling of HPLC solvents presents another avenue for sustainability. Modern solvent recovery systems can reclaim up to 80% of mobile phases, substantially reducing waste generation and operational costs. However, this approach requires careful validation to ensure consistent analyte separation across multiple runs with recycled solvents.
When optimizing gradients for specific analytes, the incorporation of in silico modeling tools allows chemists to predict separation behavior with different green solvents before experimental validation. These computational approaches minimize the experimental footprint while accelerating method development. Several software packages now include sustainability metrics alongside traditional chromatographic parameters.
The pharmaceutical industry has been particularly proactive in adopting green solvent gradients, driven by regulatory pressures and corporate sustainability initiatives. Case studies demonstrate successful transitions from acetonitrile-based methods to ethanol or reduced-solvent approaches for various drug compounds, maintaining required resolution while significantly reducing environmental impact and operational costs.
Method Validation and Regulatory Compliance for HPLC Methods
Method validation is a critical component in the development of HPLC solvent gradient methods for specific analytes. Regulatory bodies such as the FDA, EMA, and ICH have established comprehensive guidelines that define the parameters necessary for method validation. These include specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness. For optimized gradient methods, validation must demonstrate that the changing solvent composition throughout the analysis maintains consistent performance across these parameters.
The validation process for HPLC gradient methods requires additional considerations compared to isocratic methods. System suitability tests must verify that the gradient delivery system performs consistently, with particular attention to gradient reproducibility and column equilibration between runs. Dwell volume effects, which can significantly impact retention time reproducibility in gradient methods, must be characterized and controlled.
Regulatory compliance for HPLC methods extends beyond validation to encompass documentation, training, and quality systems. Current Good Manufacturing Practice (cGMP) regulations require complete documentation of method development, validation protocols, and results. For methods used in pharmaceutical analysis, compliance with USP <621> Chromatography and ICH Q2(R1) Validation of Analytical Procedures is mandatory.
Transfer of validated gradient methods between laboratories presents unique challenges. Differences in instrument configurations, particularly gradient delay volumes, can significantly affect chromatographic performance. Regulatory guidelines recommend specific transfer protocols that include comparative testing between sending and receiving laboratories to ensure equivalent performance.
Lifecycle management of analytical methods has gained increasing regulatory focus. The FDA and other agencies now expect ongoing verification that methods remain fit for purpose throughout their lifecycle. This includes periodic revalidation, particularly when changes occur in the manufacturing process, raw materials, or analytical equipment. For gradient methods, special attention must be paid to monitoring column aging effects, which can alter selectivity and resolution over time.
Risk assessment methodologies, such as Analytical Quality by Design (AQbD), are increasingly incorporated into regulatory frameworks. These approaches identify critical method attributes and establish a design space within which method parameters can be adjusted without revalidation. For gradient methods, this might include acceptable ranges for gradient steepness, starting and final mobile phase compositions, and temperature.
The validation process for HPLC gradient methods requires additional considerations compared to isocratic methods. System suitability tests must verify that the gradient delivery system performs consistently, with particular attention to gradient reproducibility and column equilibration between runs. Dwell volume effects, which can significantly impact retention time reproducibility in gradient methods, must be characterized and controlled.
Regulatory compliance for HPLC methods extends beyond validation to encompass documentation, training, and quality systems. Current Good Manufacturing Practice (cGMP) regulations require complete documentation of method development, validation protocols, and results. For methods used in pharmaceutical analysis, compliance with USP <621> Chromatography and ICH Q2(R1) Validation of Analytical Procedures is mandatory.
Transfer of validated gradient methods between laboratories presents unique challenges. Differences in instrument configurations, particularly gradient delay volumes, can significantly affect chromatographic performance. Regulatory guidelines recommend specific transfer protocols that include comparative testing between sending and receiving laboratories to ensure equivalent performance.
Lifecycle management of analytical methods has gained increasing regulatory focus. The FDA and other agencies now expect ongoing verification that methods remain fit for purpose throughout their lifecycle. This includes periodic revalidation, particularly when changes occur in the manufacturing process, raw materials, or analytical equipment. For gradient methods, special attention must be paid to monitoring column aging effects, which can alter selectivity and resolution over time.
Risk assessment methodologies, such as Analytical Quality by Design (AQbD), are increasingly incorporated into regulatory frameworks. These approaches identify critical method attributes and establish a design space within which method parameters can be adjusted without revalidation. For gradient methods, this might include acceptable ranges for gradient steepness, starting and final mobile phase compositions, and temperature.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!