Optimizing Sample Pre-treatment for Isoelectric Focusing
SEP 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
IEF Sample Pre-treatment Background and Objectives
Isoelectric focusing (IEF) has evolved significantly since its introduction in the 1960s as a high-resolution technique for protein separation based on their isoelectric points (pI). This analytical method has become a cornerstone in proteomics research, biopharmaceutical development, and clinical diagnostics. The historical trajectory of IEF shows a continuous refinement of methodologies, from carrier ampholyte-based approaches to immobilized pH gradient (IPG) systems, which have dramatically improved reproducibility and resolution.
The optimization of sample pre-treatment for IEF represents a critical frontier in advancing this technology's capabilities. Current trends indicate a growing emphasis on developing standardized protocols that can accommodate diverse sample types while minimizing artifacts and maximizing protein recovery. The field is moving toward more sophisticated pre-treatment strategies that address specific challenges such as protein solubility, aggregation prevention, and removal of interfering substances.
Our technical objectives for this research focus on establishing comprehensive guidelines for sample pre-treatment that significantly enhance IEF performance across various applications. Specifically, we aim to develop optimized protocols for different sample matrices (serum, tissue extracts, cell lysates, etc.) that maximize protein solubilization while preserving native charge properties. Additionally, we seek to identify novel additives and buffer systems that minimize protein aggregation during the focusing process.
Another critical objective is to quantitatively assess the impact of various pre-treatment parameters on IEF resolution, reproducibility, and protein recovery rates. This includes evaluating the effects of chaotropic agents, detergents, reducing agents, and carrier ampholytes at different concentrations and combinations. We also aim to develop strategies for effectively removing contaminants that interfere with IEF, such as salts, lipids, nucleic acids, and polysaccharides.
Furthermore, we intend to explore automation possibilities for sample pre-treatment to enhance throughput and reproducibility, particularly for clinical and industrial applications. This includes investigating microfluidic approaches and robotic handling systems that can standardize the pre-treatment process while minimizing sample consumption.
The ultimate goal of this technical investigation is to establish a decision framework that guides researchers in selecting optimal pre-treatment conditions based on sample characteristics and analytical objectives. This framework will incorporate emerging technologies such as machine learning algorithms to predict optimal pre-treatment parameters based on sample composition data, potentially revolutionizing how IEF sample preparation is approached in both research and industrial settings.
The optimization of sample pre-treatment for IEF represents a critical frontier in advancing this technology's capabilities. Current trends indicate a growing emphasis on developing standardized protocols that can accommodate diverse sample types while minimizing artifacts and maximizing protein recovery. The field is moving toward more sophisticated pre-treatment strategies that address specific challenges such as protein solubility, aggregation prevention, and removal of interfering substances.
Our technical objectives for this research focus on establishing comprehensive guidelines for sample pre-treatment that significantly enhance IEF performance across various applications. Specifically, we aim to develop optimized protocols for different sample matrices (serum, tissue extracts, cell lysates, etc.) that maximize protein solubilization while preserving native charge properties. Additionally, we seek to identify novel additives and buffer systems that minimize protein aggregation during the focusing process.
Another critical objective is to quantitatively assess the impact of various pre-treatment parameters on IEF resolution, reproducibility, and protein recovery rates. This includes evaluating the effects of chaotropic agents, detergents, reducing agents, and carrier ampholytes at different concentrations and combinations. We also aim to develop strategies for effectively removing contaminants that interfere with IEF, such as salts, lipids, nucleic acids, and polysaccharides.
Furthermore, we intend to explore automation possibilities for sample pre-treatment to enhance throughput and reproducibility, particularly for clinical and industrial applications. This includes investigating microfluidic approaches and robotic handling systems that can standardize the pre-treatment process while minimizing sample consumption.
The ultimate goal of this technical investigation is to establish a decision framework that guides researchers in selecting optimal pre-treatment conditions based on sample characteristics and analytical objectives. This framework will incorporate emerging technologies such as machine learning algorithms to predict optimal pre-treatment parameters based on sample composition data, potentially revolutionizing how IEF sample preparation is approached in both research and industrial settings.
Market Analysis for Advanced Protein Separation Technologies
The protein separation technology market is experiencing robust growth, driven by increasing applications in proteomics research, biopharmaceutical development, and clinical diagnostics. The global market for protein separation technologies was valued at approximately $11.5 billion in 2022 and is projected to reach $25.7 billion by 2030, growing at a CAGR of 10.6% during the forecast period. Within this broader market, isoelectric focusing (IEF) technologies represent a significant segment due to their high resolution capabilities in separating proteins based on their isoelectric points.
The demand for advanced protein separation technologies is primarily fueled by the expanding biopharmaceutical industry, which requires precise protein characterization for drug development and quality control. The global biopharmaceutical market reached $325 billion in 2022, with monoclonal antibodies accounting for over 40% of sales, necessitating sophisticated separation techniques for purification and analysis.
Academic and research institutions constitute another major market segment, contributing approximately 35% of the total demand for protein separation technologies. The increasing focus on proteomics research, with global funding exceeding $2.3 billion annually, has significantly boosted the adoption of advanced separation methods including optimized IEF techniques.
Geographically, North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is witnessing the fastest growth rate of 12.8% annually, driven by expanding research infrastructure in China, India, and South Korea, and increasing investments in biotechnology sectors.
The clinical diagnostics segment represents a rapidly growing application area, expected to expand at 13.2% annually through 2030. This growth is attributed to the increasing adoption of protein biomarkers for disease diagnosis and monitoring, particularly in oncology and neurodegenerative disorders.
Sample pre-treatment optimization for IEF has emerged as a critical focus area, with the market for specialized sample preparation products growing at 11.5% annually. This sub-segment is valued at approximately $1.8 billion globally, reflecting the importance of proper sample handling to achieve accurate and reproducible protein separation results.
Key market trends include the integration of automation in sample preparation workflows, development of specialized reagents for complex biological samples, and increasing demand for standardized protocols that enhance reproducibility. The shift toward miniaturized and high-throughput systems is also driving innovation in sample pre-treatment technologies, with microfluidic-based solutions gaining significant traction in recent years.
The demand for advanced protein separation technologies is primarily fueled by the expanding biopharmaceutical industry, which requires precise protein characterization for drug development and quality control. The global biopharmaceutical market reached $325 billion in 2022, with monoclonal antibodies accounting for over 40% of sales, necessitating sophisticated separation techniques for purification and analysis.
Academic and research institutions constitute another major market segment, contributing approximately 35% of the total demand for protein separation technologies. The increasing focus on proteomics research, with global funding exceeding $2.3 billion annually, has significantly boosted the adoption of advanced separation methods including optimized IEF techniques.
Geographically, North America dominates the market with a 42% share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is witnessing the fastest growth rate of 12.8% annually, driven by expanding research infrastructure in China, India, and South Korea, and increasing investments in biotechnology sectors.
The clinical diagnostics segment represents a rapidly growing application area, expected to expand at 13.2% annually through 2030. This growth is attributed to the increasing adoption of protein biomarkers for disease diagnosis and monitoring, particularly in oncology and neurodegenerative disorders.
Sample pre-treatment optimization for IEF has emerged as a critical focus area, with the market for specialized sample preparation products growing at 11.5% annually. This sub-segment is valued at approximately $1.8 billion globally, reflecting the importance of proper sample handling to achieve accurate and reproducible protein separation results.
Key market trends include the integration of automation in sample preparation workflows, development of specialized reagents for complex biological samples, and increasing demand for standardized protocols that enhance reproducibility. The shift toward miniaturized and high-throughput systems is also driving innovation in sample pre-treatment technologies, with microfluidic-based solutions gaining significant traction in recent years.
Current Challenges in IEF Sample Preparation
Isoelectric focusing (IEF) faces significant sample preparation challenges that limit its analytical power and reproducibility. The primary obstacle remains protein solubility maintenance throughout the focusing process. Many proteins, particularly hydrophobic membrane proteins, tend to precipitate at their isoelectric points, causing streaking and poor resolution. This precipitation phenomenon is exacerbated when proteins reach regions where pH equals their pI, resulting in diminished solubility and aggregation.
Sample complexity presents another major hurdle, especially with biological specimens containing diverse protein populations with varying abundances. High-abundance proteins often mask the detection of low-abundance proteins, which are frequently the most biologically significant. Current depletion techniques for removing abundant proteins can inadvertently remove proteins of interest through non-specific binding interactions.
Protein modifications introduce additional complications. Post-translational modifications (PTMs) like phosphorylation, glycosylation, and acetylation alter protein charge profiles, creating multiple spots for a single protein. These modifications can shift the apparent pI, complicating identification and quantification efforts. Sample preparation methods must preserve these modifications while maintaining protein integrity.
Contaminant interference remains problematic despite advances in sample cleanup. Salts, lipids, nucleic acids, and small metabolites can disrupt the pH gradient formation and cause horizontal streaking. Current desalting methods like dialysis and precipitation often result in selective protein loss, particularly affecting low molecular weight proteins.
Detergent selection presents a particular challenge. While necessary for solubilizing membrane proteins, many detergents are incompatible with IEF as they disrupt the pH gradient. Non-ionic and zwitterionic detergents offer partial solutions but still compromise resolution in certain pH ranges. The recent development of cleavable detergents shows promise but introduces additional sample handling steps that may lead to protein losses.
Protein-protein interactions persist through conventional sample preparation, causing artificial spot patterns. Strong chaotropic agents like urea and thiourea help disrupt these interactions but can induce protein carbamylation when heated, altering the native pI values. The concentration balance between disrupting interactions and maintaining protein stability remains difficult to achieve consistently.
Time-dependent modifications during sample preparation further complicate analysis. Oxidation of cysteine and methionine residues, deamidation of asparagine and glutamine, and proteolytic degradation can occur during extended preparation procedures. These modifications alter protein charge and mass, creating artifacts that confound data interpretation and reduce reproducibility across experiments.
Sample complexity presents another major hurdle, especially with biological specimens containing diverse protein populations with varying abundances. High-abundance proteins often mask the detection of low-abundance proteins, which are frequently the most biologically significant. Current depletion techniques for removing abundant proteins can inadvertently remove proteins of interest through non-specific binding interactions.
Protein modifications introduce additional complications. Post-translational modifications (PTMs) like phosphorylation, glycosylation, and acetylation alter protein charge profiles, creating multiple spots for a single protein. These modifications can shift the apparent pI, complicating identification and quantification efforts. Sample preparation methods must preserve these modifications while maintaining protein integrity.
Contaminant interference remains problematic despite advances in sample cleanup. Salts, lipids, nucleic acids, and small metabolites can disrupt the pH gradient formation and cause horizontal streaking. Current desalting methods like dialysis and precipitation often result in selective protein loss, particularly affecting low molecular weight proteins.
Detergent selection presents a particular challenge. While necessary for solubilizing membrane proteins, many detergents are incompatible with IEF as they disrupt the pH gradient. Non-ionic and zwitterionic detergents offer partial solutions but still compromise resolution in certain pH ranges. The recent development of cleavable detergents shows promise but introduces additional sample handling steps that may lead to protein losses.
Protein-protein interactions persist through conventional sample preparation, causing artificial spot patterns. Strong chaotropic agents like urea and thiourea help disrupt these interactions but can induce protein carbamylation when heated, altering the native pI values. The concentration balance between disrupting interactions and maintaining protein stability remains difficult to achieve consistently.
Time-dependent modifications during sample preparation further complicate analysis. Oxidation of cysteine and methionine residues, deamidation of asparagine and glutamine, and proteolytic degradation can occur during extended preparation procedures. These modifications alter protein charge and mass, creating artifacts that confound data interpretation and reduce reproducibility across experiments.
State-of-the-Art Sample Pre-treatment Protocols
01 Sample preparation techniques for protein analysis
Proper sample preparation is crucial for successful isoelectric focusing of proteins. This includes techniques such as protein extraction, purification, and concentration to remove contaminants that might interfere with the focusing process. Methods like precipitation, dialysis, and ultrafiltration can be employed to prepare samples for optimal resolution in isoelectric focusing. These preparation steps help to maintain protein integrity while removing salts and other substances that could affect the pH gradient.- Sample preparation techniques for protein analysis: Proper sample preparation is crucial for successful isoelectric focusing of proteins. This involves techniques such as protein extraction, purification, and concentration to remove contaminants that might interfere with the focusing process. Methods include precipitation with acetone or trichloroacetic acid, dialysis to remove salts, and ultrafiltration to concentrate proteins while maintaining their native state. These preparation steps help to improve resolution and reproducibility in isoelectric focusing experiments.
- Buffer optimization for isoelectric focusing: The selection and optimization of buffer systems is essential for effective isoelectric focusing. This includes the use of ampholytes to create stable pH gradients and the addition of specific additives to improve protein solubility and prevent aggregation. Carrier ampholytes with appropriate pH ranges should be selected based on the isoelectric points of the target proteins. Optimizing buffer composition can significantly enhance the resolution and separation efficiency of the isoelectric focusing process.
- Denaturation and reduction strategies: Protein denaturation and reduction are often necessary pre-treatment steps to achieve optimal separation in isoelectric focusing. This involves the use of chaotropic agents such as urea or guanidine hydrochloride to unfold proteins, and reducing agents like dithiothreitol (DTT) or β-mercaptoethanol to break disulfide bonds. These treatments expose charged groups within proteins, allowing for more accurate determination of their isoelectric points and improving the resolution of complex protein mixtures.
- Removal of interfering substances: The removal of substances that can interfere with isoelectric focusing is a critical pre-treatment step. These substances include salts, lipids, nucleic acids, and certain detergents that can disrupt the electric field or cause protein precipitation. Techniques such as gel filtration, ion exchange chromatography, and specific precipitation methods can be employed to eliminate these interfering components. This pre-treatment ensures cleaner backgrounds and sharper protein bands during isoelectric focusing.
- Automation and microfluidic approaches: Advanced automation and microfluidic technologies have been developed to streamline sample pre-treatment for isoelectric focusing. These approaches include automated sample handling systems, microchip-based preparation methods, and integrated platforms that combine multiple pre-treatment steps. Such technologies offer advantages in terms of reproducibility, reduced sample consumption, and higher throughput. They are particularly valuable for processing multiple samples and for applications requiring standardized pre-treatment protocols.
02 Buffer and ampholyte optimization
The selection and optimization of buffer systems and carrier ampholytes significantly impact isoelectric focusing performance. Proper buffer composition helps maintain stable pH gradients during the separation process. Carrier ampholytes with appropriate pH ranges should be selected based on the isoelectric points of the target proteins. Optimization of ampholyte concentration and distribution can improve resolution and prevent protein precipitation at their isoelectric points.Expand Specific Solutions03 Denaturant and detergent usage
The addition of denaturants and detergents in sample preparation can improve protein solubility and prevent aggregation during isoelectric focusing. Common denaturants include urea and thiourea, which help unfold proteins and expose charged groups. Detergents like CHAPS or Triton X-100 aid in solubilizing hydrophobic proteins. The optimal concentration of these additives depends on the nature of the sample and should be carefully determined to maintain protein stability while enhancing separation efficiency.Expand Specific Solutions04 Reduction and alkylation protocols
Reduction and alkylation of protein samples prior to isoelectric focusing can improve separation by breaking disulfide bonds and preventing their reformation. Common reducing agents include dithiothreitol (DTT) and β-mercaptoethanol, while iodoacetamide is frequently used for alkylation. These treatments help to fully denature proteins, exposing all charged groups and allowing them to migrate to their true isoelectric points. The timing and concentration of these treatments are critical for optimal results without causing protein degradation.Expand Specific Solutions05 Sample loading and pre-focusing techniques
The method of sample application and pre-focusing can significantly impact isoelectric focusing results. Samples can be applied at various positions depending on their expected isoelectric points. Pre-focusing the pH gradient before sample application can improve resolution by establishing a stable gradient. Sequential loading techniques may be employed for complex samples to prevent overloading. The voltage and time parameters during the pre-focusing and focusing steps should be optimized based on sample characteristics to achieve the best separation while minimizing protein precipitation and drift.Expand Specific Solutions
Leading Companies and Research Institutions in IEF Technology
The isoelectric focusing (IEF) sample pre-treatment optimization market is currently in a growth phase, with increasing demand driven by proteomics research and biopharmaceutical development. The global market size is estimated at approximately $1.2 billion, expanding at a CAGR of 6-8%. Technology maturity varies across applications, with established players like Bio-Rad Laboratories, ProteinSimple, and Becton, Dickinson & Co. offering commercial solutions, while academic institutions such as MIT, University of Washington, and Texas A&M University continue to advance fundamental research. Research organizations like The Wistar Institute and Technion Research & Development Foundation are developing novel approaches, while pharmaceutical companies including Mitsubishi Tanabe Pharma and Octapharma are implementing optimized IEF techniques for therapeutic protein analysis. The competitive landscape is characterized by a mix of specialized instrumentation providers and reagent suppliers focusing on improving resolution, reproducibility, and automation.
Intabio LLC
Technical Solution: Intabio has developed the Blaze™ system, an innovative platform that integrates sample pre-treatment directly with isoelectric focusing analysis. Their technology employs a microchip-based approach that combines sample preparation, separation, and detection in a single automated workflow. The system utilizes proprietary microfluidic channels with integrated membranes that perform in-line buffer exchange and desalting, critical steps for successful IEF. Their approach incorporates a novel imaged capillary isoelectric focusing (iCIEF) technique that allows for real-time monitoring of protein focusing, enabling immediate assessment of sample preparation quality. The technology includes automated pH gradient formation with precise control over ampholyte distribution and electric field application. Intabio's system also features integrated UV absorption detection that provides quantitative analysis of separated proteins without additional sample handling. Their platform is particularly optimized for therapeutic protein characterization, with specialized protocols for monoclonal antibody charge variant analysis.
Strengths: Integration of sample preparation with analysis reduces handling steps and improves reproducibility; microfluidic approach minimizes sample consumption; real-time monitoring enables rapid method development. Weaknesses: Platform may have limited flexibility for diverse sample types; specialized consumables increase operational costs; primarily focused on biopharmaceutical applications rather than general research use.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has pioneered the ReadyPrep™ 2-D Cleanup Kit specifically designed for optimal sample preparation before isoelectric focusing. Their technology employs a precipitation-based approach that selectively removes interfering contaminants like salts, lipids, nucleic acids, and detergents while concentrating proteins. The process involves a specialized protein precipitation step followed by washing with proprietary buffers that maintain protein solubility during resuspension. Bio-Rad's approach includes the use of TGS (Tris-Glycine-SDS) buffer systems that enhance protein solubilization while preserving native charge properties critical for IEF. Their technology also incorporates specialized rehydration buffers containing optimized concentrations of chaotropes, ampholytes, and reducing agents to prevent protein aggregation and maintain sample integrity throughout the focusing process. The company has also developed specialized IPG (Immobilized pH Gradient) strips with enhanced sample loading capacity and improved resolution across various pH ranges.
Strengths: Highly effective at removing contaminants that interfere with IEF resolution; versatile application across various sample types; compatible with downstream proteomic workflows. Weaknesses: Multi-step process can lead to some protein loss; precipitation methods may not be optimal for all protein classes; requires significant hands-on time compared to automated systems.
Key Innovations in Sample Desalting and Protein Solubilization
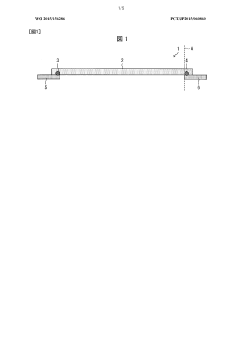
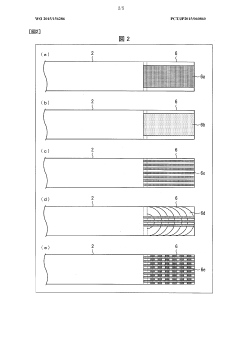
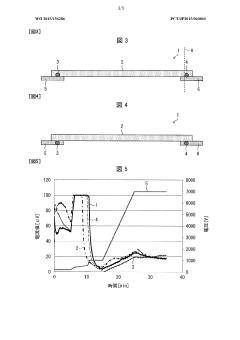
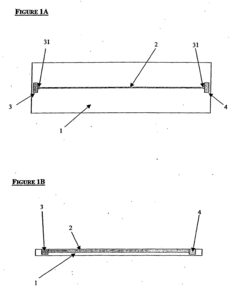
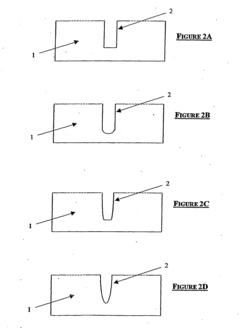
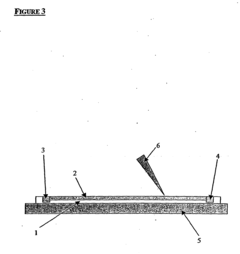
Isoelectric focusing apparatus and isoelectric focusing method
PatentWO2015156286A1
Innovation
- An isoelectric focusing instrument with a gel having a pH gradient and electrodes in contact with a solvent retention system that holds a solvent to prevent ionic contaminants from accumulating on the electrodes, using a solvent retention portion to diffuse contaminants into the solvent and maintain clear electrophoresis results.
Device for isoelectric focussing
PatentInactiveUS20050139470A1
Innovation
- An isoelectric focusing (IEF) module with an open channel, microfabricated on a planar substrate, allows for the exposure of the sample along its length, enabling easy access and analysis of proteins, coupled with a MALDI-MS module for efficient protein separation and identification, using a MALDI matrix to enhance ionization and reduce electroosmotic flow.
Regulatory Considerations for Analytical Method Validation
Regulatory frameworks governing analytical method validation are critical considerations when optimizing sample pre-treatment for isoelectric focusing (IEF). The validation of analytical methods for IEF must comply with guidelines established by regulatory bodies such as the FDA, EMA, and ICH, particularly ICH Q2(R1) which outlines validation parameters including specificity, linearity, accuracy, precision, detection limit, quantitation limit, range, and robustness.
For IEF sample pre-treatment optimization, method validation must address specific regulatory requirements related to protein analysis. The FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics emphasizes the importance of demonstrating that sample pre-treatment procedures do not introduce artifacts or alter the native state of proteins being analyzed.
Validation protocols must include comprehensive documentation of sample preparation steps, including buffer compositions, desalting procedures, and any concentration or dilution factors applied. These elements are particularly crucial for IEF, as sample ionic strength and protein concentration significantly impact focusing outcomes and reproducibility.
Risk assessment frameworks, as outlined in ICH Q9, should be incorporated into validation strategies for IEF sample pre-treatment methods. This involves identifying critical quality attributes that could be affected by sample handling and establishing appropriate control strategies to mitigate risks of protein modification, aggregation, or degradation during pre-treatment.
Regulatory bodies increasingly require lifecycle management approaches to analytical method validation, as described in USP <1220> and ICH Q12. This necessitates ongoing verification that optimized sample pre-treatment methods for IEF maintain their performance characteristics throughout routine use, with established criteria for revalidation when significant changes are implemented.
Transferability of validated methods between laboratories represents another regulatory consideration, particularly relevant for multi-site studies or technology transfer scenarios. Sample pre-treatment protocols must be sufficiently robust and well-documented to ensure consistent IEF results across different laboratory environments and analyst skill levels.
Compliance with GMP and GLP principles is mandatory for regulated environments, requiring traceability of reagents, calibration of instruments used in sample preparation, and appropriate training documentation for personnel performing pre-treatment procedures. These elements must be integrated into the overall validation strategy to ensure regulatory acceptance of IEF analytical methods.
For IEF sample pre-treatment optimization, method validation must address specific regulatory requirements related to protein analysis. The FDA's Guidance for Industry on Analytical Procedures and Methods Validation for Drugs and Biologics emphasizes the importance of demonstrating that sample pre-treatment procedures do not introduce artifacts or alter the native state of proteins being analyzed.
Validation protocols must include comprehensive documentation of sample preparation steps, including buffer compositions, desalting procedures, and any concentration or dilution factors applied. These elements are particularly crucial for IEF, as sample ionic strength and protein concentration significantly impact focusing outcomes and reproducibility.
Risk assessment frameworks, as outlined in ICH Q9, should be incorporated into validation strategies for IEF sample pre-treatment methods. This involves identifying critical quality attributes that could be affected by sample handling and establishing appropriate control strategies to mitigate risks of protein modification, aggregation, or degradation during pre-treatment.
Regulatory bodies increasingly require lifecycle management approaches to analytical method validation, as described in USP <1220> and ICH Q12. This necessitates ongoing verification that optimized sample pre-treatment methods for IEF maintain their performance characteristics throughout routine use, with established criteria for revalidation when significant changes are implemented.
Transferability of validated methods between laboratories represents another regulatory consideration, particularly relevant for multi-site studies or technology transfer scenarios. Sample pre-treatment protocols must be sufficiently robust and well-documented to ensure consistent IEF results across different laboratory environments and analyst skill levels.
Compliance with GMP and GLP principles is mandatory for regulated environments, requiring traceability of reagents, calibration of instruments used in sample preparation, and appropriate training documentation for personnel performing pre-treatment procedures. These elements must be integrated into the overall validation strategy to ensure regulatory acceptance of IEF analytical methods.
Cost-Benefit Analysis of Advanced Pre-treatment Techniques
When evaluating advanced pre-treatment techniques for isoelectric focusing (IEF), a comprehensive cost-benefit analysis reveals significant economic considerations that organizations must address. The initial investment in sophisticated pre-treatment equipment represents a substantial capital expenditure, with automated systems ranging from $50,000 to $150,000 depending on throughput capacity and level of automation. However, these systems typically demonstrate reduced per-sample costs over time through minimized reagent consumption and decreased manual handling requirements.
Operational expenses present another critical dimension, with advanced techniques generally requiring specialized reagents that cost 30-40% more than conventional alternatives. This premium is partially offset by the 25-35% reduction in sample volume requirements that advanced methods typically achieve. Labor costs show a notable shift pattern - while specialized personnel command higher salaries, the total labor hours per sample decrease by approximately 40-60% with automated pre-treatment systems.
Sample recovery rates significantly impact the economic equation, particularly when working with rare or expensive biological materials. Advanced techniques demonstrate recovery rates of 85-95% compared to 60-75% with traditional methods. For research involving costly antibodies or limited clinical samples, this improvement alone can justify the higher initial investment in advanced pre-treatment technology.
Throughput capabilities represent another crucial benefit, with modern systems processing 50-200 samples simultaneously compared to 10-30 samples with manual techniques. This scalability becomes particularly valuable in high-volume diagnostic settings or large-scale proteomics research, where time-to-result directly impacts operational efficiency and research productivity.
Quality-related costs must also factor into the analysis. Advanced pre-treatment methods reduce sample variability by 40-60%, resulting in more consistent IEF results. This improvement translates to fewer repeated experiments, decreased troubleshooting time, and more reliable data generation - benefits that, while difficult to quantify precisely, significantly impact overall research efficiency and validity.
Long-term considerations reveal that while advanced techniques require higher initial investment, their total cost of ownership over a 5-year period typically becomes favorable for laboratories processing more than 1,000 samples annually. Additionally, the enhanced resolution and reproducibility enable detection of protein variants that might otherwise remain unidentified, potentially opening new research and diagnostic opportunities that carry substantial scientific and commercial value.
Operational expenses present another critical dimension, with advanced techniques generally requiring specialized reagents that cost 30-40% more than conventional alternatives. This premium is partially offset by the 25-35% reduction in sample volume requirements that advanced methods typically achieve. Labor costs show a notable shift pattern - while specialized personnel command higher salaries, the total labor hours per sample decrease by approximately 40-60% with automated pre-treatment systems.
Sample recovery rates significantly impact the economic equation, particularly when working with rare or expensive biological materials. Advanced techniques demonstrate recovery rates of 85-95% compared to 60-75% with traditional methods. For research involving costly antibodies or limited clinical samples, this improvement alone can justify the higher initial investment in advanced pre-treatment technology.
Throughput capabilities represent another crucial benefit, with modern systems processing 50-200 samples simultaneously compared to 10-30 samples with manual techniques. This scalability becomes particularly valuable in high-volume diagnostic settings or large-scale proteomics research, where time-to-result directly impacts operational efficiency and research productivity.
Quality-related costs must also factor into the analysis. Advanced pre-treatment methods reduce sample variability by 40-60%, resulting in more consistent IEF results. This improvement translates to fewer repeated experiments, decreased troubleshooting time, and more reliable data generation - benefits that, while difficult to quantify precisely, significantly impact overall research efficiency and validity.
Long-term considerations reveal that while advanced techniques require higher initial investment, their total cost of ownership over a 5-year period typically becomes favorable for laboratories processing more than 1,000 samples annually. Additionally, the enhanced resolution and reproducibility enable detection of protein variants that might otherwise remain unidentified, potentially opening new research and diagnostic opportunities that carry substantial scientific and commercial value.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!