Process validation strategies for multiple fill volumes and container formats in personalized therapies
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Personalized Therapy Fill Process Background & Objectives
Personalized medicine represents a paradigm shift in healthcare, tailoring treatments to individual patients based on their genetic makeup, lifestyle, and environmental factors. The evolution of personalized therapies has accelerated significantly over the past decade, driven by advances in genomics, proteomics, and bioinformatics. This technological progression has created unprecedented opportunities for treating previously intractable conditions, particularly in oncology, rare genetic disorders, and autoimmune diseases.
The manufacturing processes for personalized therapies differ fundamentally from traditional pharmaceutical production. While conventional drug manufacturing focuses on large-scale, uniform production, personalized therapies require flexible, small-batch processing capabilities that can accommodate patient-specific formulations. This shift necessitates novel approaches to fill processes that can handle multiple fill volumes and container formats efficiently while maintaining stringent quality standards.
The primary objective of this technical research is to evaluate and develop robust process validation strategies for multiple fill volumes and container formats specifically designed for personalized therapy manufacturing. These strategies must ensure consistent product quality while accommodating the inherent variability in personalized medicine production. Additionally, they must comply with regulatory requirements that continue to evolve in response to this emerging field.
Current fill processes face significant challenges in balancing flexibility with compliance. Traditional validation approaches, designed for large-scale uniform production, prove inadequate when applied to the variable nature of personalized therapies. The industry requires innovative validation methodologies that can accommodate batch-to-batch variations while ensuring product safety and efficacy.
Historical approaches to fill process validation have focused on demonstrating consistency across large production runs. However, personalized therapies demand validation strategies that can verify process capability across multiple fill volumes—ranging from microliters to several milliliters—and diverse container formats including vials, syringes, cartridges, and specialized delivery devices.
The technical trajectory in this field points toward automated, modular fill systems with integrated quality control mechanisms. These systems must demonstrate the capability to handle patient-specific doses with precision while maintaining aseptic conditions. The validation of such systems represents a critical bottleneck in scaling personalized therapy production and ultimately determining their commercial viability and accessibility to patients.
The manufacturing processes for personalized therapies differ fundamentally from traditional pharmaceutical production. While conventional drug manufacturing focuses on large-scale, uniform production, personalized therapies require flexible, small-batch processing capabilities that can accommodate patient-specific formulations. This shift necessitates novel approaches to fill processes that can handle multiple fill volumes and container formats efficiently while maintaining stringent quality standards.
The primary objective of this technical research is to evaluate and develop robust process validation strategies for multiple fill volumes and container formats specifically designed for personalized therapy manufacturing. These strategies must ensure consistent product quality while accommodating the inherent variability in personalized medicine production. Additionally, they must comply with regulatory requirements that continue to evolve in response to this emerging field.
Current fill processes face significant challenges in balancing flexibility with compliance. Traditional validation approaches, designed for large-scale uniform production, prove inadequate when applied to the variable nature of personalized therapies. The industry requires innovative validation methodologies that can accommodate batch-to-batch variations while ensuring product safety and efficacy.
Historical approaches to fill process validation have focused on demonstrating consistency across large production runs. However, personalized therapies demand validation strategies that can verify process capability across multiple fill volumes—ranging from microliters to several milliliters—and diverse container formats including vials, syringes, cartridges, and specialized delivery devices.
The technical trajectory in this field points toward automated, modular fill systems with integrated quality control mechanisms. These systems must demonstrate the capability to handle patient-specific doses with precision while maintaining aseptic conditions. The validation of such systems represents a critical bottleneck in scaling personalized therapy production and ultimately determining their commercial viability and accessibility to patients.
Market Analysis for Multi-Format Personalized Therapies
The personalized therapy market is experiencing unprecedented growth, driven by advancements in precision medicine and the increasing prevalence of chronic diseases requiring tailored treatment approaches. Current market valuations place the global personalized medicine market at approximately 2.1 trillion USD in 2023, with projections indicating a compound annual growth rate (CAGR) of 11.5% through 2030. Within this broader market, therapies requiring multiple fill volumes and container formats represent a rapidly expanding segment.
Patient-specific dosing requirements are creating substantial demand for flexible manufacturing solutions that can accommodate various container formats and fill volumes without compromising product quality or efficacy. This trend is particularly evident in oncology, rare diseases, and gene therapy sectors, where treatment customization is essential for optimal patient outcomes.
Market research indicates that over 40% of newly approved biologics and advanced therapy medicinal products (ATMPs) require some form of personalized dosing strategy. This has created a significant market opportunity for process validation technologies that can efficiently handle multiple fill volumes and container formats while maintaining regulatory compliance.
The geographical distribution of market demand shows concentration in North America (42%), Europe (31%), and Asia-Pacific (18%), with emerging markets accounting for the remaining 9%. This distribution correlates strongly with regions having advanced healthcare infrastructure and favorable regulatory environments for personalized medicine.
Key market drivers include the rising prevalence of chronic diseases requiring personalized treatment approaches, increasing adoption of precision medicine protocols in clinical practice, and growing patient awareness and demand for customized therapeutic options. Additionally, healthcare payers are increasingly recognizing the economic benefits of personalized therapies through improved treatment outcomes and reduced hospitalization rates.
Market barriers include high development and manufacturing costs, complex regulatory pathways for multi-format therapies, and technical challenges in ensuring consistent product quality across different fill volumes and container formats. The average development cost for a personalized therapy platform capable of handling multiple formats exceeds 150 million USD, creating significant entry barriers for smaller companies.
Consumer trends indicate growing acceptance of personalized medicine approaches, with patient surveys showing that 78% of respondents would prefer customized treatment options when available. Healthcare providers similarly report increasing confidence in prescribing personalized therapies, with 65% of specialists regularly considering patient-specific factors in treatment decisions.
Patient-specific dosing requirements are creating substantial demand for flexible manufacturing solutions that can accommodate various container formats and fill volumes without compromising product quality or efficacy. This trend is particularly evident in oncology, rare diseases, and gene therapy sectors, where treatment customization is essential for optimal patient outcomes.
Market research indicates that over 40% of newly approved biologics and advanced therapy medicinal products (ATMPs) require some form of personalized dosing strategy. This has created a significant market opportunity for process validation technologies that can efficiently handle multiple fill volumes and container formats while maintaining regulatory compliance.
The geographical distribution of market demand shows concentration in North America (42%), Europe (31%), and Asia-Pacific (18%), with emerging markets accounting for the remaining 9%. This distribution correlates strongly with regions having advanced healthcare infrastructure and favorable regulatory environments for personalized medicine.
Key market drivers include the rising prevalence of chronic diseases requiring personalized treatment approaches, increasing adoption of precision medicine protocols in clinical practice, and growing patient awareness and demand for customized therapeutic options. Additionally, healthcare payers are increasingly recognizing the economic benefits of personalized therapies through improved treatment outcomes and reduced hospitalization rates.
Market barriers include high development and manufacturing costs, complex regulatory pathways for multi-format therapies, and technical challenges in ensuring consistent product quality across different fill volumes and container formats. The average development cost for a personalized therapy platform capable of handling multiple formats exceeds 150 million USD, creating significant entry barriers for smaller companies.
Consumer trends indicate growing acceptance of personalized medicine approaches, with patient surveys showing that 78% of respondents would prefer customized treatment options when available. Healthcare providers similarly report increasing confidence in prescribing personalized therapies, with 65% of specialists regularly considering patient-specific factors in treatment decisions.
Current Validation Challenges in Variable Fill Processes
The validation of manufacturing processes for personalized therapies presents unique challenges compared to traditional pharmaceutical production. Unlike conventional drug manufacturing with standardized batch sizes and container formats, personalized therapies often require variable fill volumes and multiple container formats to accommodate patient-specific dosing. This variability creates significant hurdles for traditional validation approaches that were designed for consistent, large-scale production.
Current validation frameworks struggle to address the inherent variability in personalized medicine manufacturing. Traditional process validation typically relies on demonstrating consistency across three consecutive production runs of identical specifications. However, personalized therapies may require different fill volumes for each patient, ranging from microliters to milliliters, making the traditional approach impractical or impossible to implement.
Container diversity compounds these challenges, as personalized therapies may be packaged in vials, syringes, infusion bags, or specialized delivery devices depending on the administration route and stability requirements. Each container format introduces unique critical process parameters that must be validated, including fill accuracy, container integrity, and product-container compatibility across the spectrum of fill volumes.
Regulatory expectations remain ambiguous in this evolving landscape. While agencies acknowledge the need for alternative validation approaches for personalized therapies, clear guidance on acceptable methodologies is still developing. This regulatory uncertainty creates hesitation among manufacturers to implement innovative validation strategies that deviate from established norms.
Statistical challenges are particularly pronounced when validating variable fill processes. Traditional statistical methods rely on large sample sizes and normal distributions, which are often unattainable in personalized therapy manufacturing where each fill may be unique. Determining appropriate acceptance criteria and confidence levels becomes problematic when dealing with non-homogeneous production runs.
Resource constraints further complicate validation efforts. The extensive testing required to validate multiple fill volumes across different container formats demands significant analytical resources, specialized equipment, and expertise. For smaller companies and academic institutions developing personalized therapies, these resource requirements can be prohibitively expensive.
Cross-contamination risks increase with variable processing. When equipment must handle different products, formulations, or volumes in sequence, the validation of cleaning procedures becomes more complex. Demonstrating the effectiveness of cleaning processes across all possible scenarios presents a substantial technical challenge that current validation approaches struggle to address efficiently.
Current validation frameworks struggle to address the inherent variability in personalized medicine manufacturing. Traditional process validation typically relies on demonstrating consistency across three consecutive production runs of identical specifications. However, personalized therapies may require different fill volumes for each patient, ranging from microliters to milliliters, making the traditional approach impractical or impossible to implement.
Container diversity compounds these challenges, as personalized therapies may be packaged in vials, syringes, infusion bags, or specialized delivery devices depending on the administration route and stability requirements. Each container format introduces unique critical process parameters that must be validated, including fill accuracy, container integrity, and product-container compatibility across the spectrum of fill volumes.
Regulatory expectations remain ambiguous in this evolving landscape. While agencies acknowledge the need for alternative validation approaches for personalized therapies, clear guidance on acceptable methodologies is still developing. This regulatory uncertainty creates hesitation among manufacturers to implement innovative validation strategies that deviate from established norms.
Statistical challenges are particularly pronounced when validating variable fill processes. Traditional statistical methods rely on large sample sizes and normal distributions, which are often unattainable in personalized therapy manufacturing where each fill may be unique. Determining appropriate acceptance criteria and confidence levels becomes problematic when dealing with non-homogeneous production runs.
Resource constraints further complicate validation efforts. The extensive testing required to validate multiple fill volumes across different container formats demands significant analytical resources, specialized equipment, and expertise. For smaller companies and academic institutions developing personalized therapies, these resource requirements can be prohibitively expensive.
Cross-contamination risks increase with variable processing. When equipment must handle different products, formulations, or volumes in sequence, the validation of cleaning procedures becomes more complex. Demonstrating the effectiveness of cleaning processes across all possible scenarios presents a substantial technical challenge that current validation approaches struggle to address efficiently.
Current Validation Approaches for Multiple Fill Configurations
01 Automated fill volume verification systems
Advanced systems for automated verification of fill volumes in containers during manufacturing processes. These systems utilize sensors, cameras, and image processing technologies to accurately measure and validate fill levels in various container formats. The automated approach ensures consistency, reduces human error, and provides real-time monitoring capabilities for quality control in pharmaceutical and beverage industries.- Automated fill volume verification systems: Advanced systems for automated verification of fill volumes in containers using imaging technology and sensors. These systems can detect and measure the fill levels in various container formats with high precision, ensuring compliance with regulatory requirements. The technology includes real-time monitoring capabilities that can identify underfilled or overfilled containers during the production process, reducing waste and ensuring product quality.
- Container format validation methodologies: Methodologies for validating different container formats in pharmaceutical and food packaging processes. These approaches include dimensional verification, integrity testing, and compatibility assessment with filling equipment. The validation processes ensure that containers meet specifications before the filling process begins, reducing the risk of production issues and ensuring consistent product quality across different container types.
- Process validation documentation and compliance: Comprehensive documentation strategies for process validation related to fill volumes and container formats. These approaches focus on maintaining regulatory compliance through systematic record-keeping, data management, and reporting systems. The documentation includes validation protocols, test results, deviation reports, and corrective actions to demonstrate that filling processes consistently meet predetermined specifications and quality standards.
- Fill volume control technologies: Technologies specifically designed to control and maintain consistent fill volumes across different container formats. These include precision dispensing systems, weight-based verification methods, and volumetric measurement technologies. The systems incorporate feedback mechanisms that can make real-time adjustments to ensure accurate filling, reducing product waste and improving overall production efficiency.
- Integrated validation approaches for manufacturing lines: Holistic validation strategies that integrate fill volume verification with broader manufacturing process validation. These approaches consider the entire production line, including equipment qualification, process parameter optimization, and operator training. The integrated methods ensure that fill volumes and container formats are validated not in isolation but as part of a comprehensive manufacturing system, leading to more robust and reliable production processes.
02 Container format validation methodologies
Methodologies for validating different container formats in production lines. These approaches include dimensional verification, material compatibility testing, and structural integrity validation. The methods ensure that containers meet specifications before filling operations, reducing the risk of leakage, contamination, or product loss. Container format validation is critical for maintaining product quality and safety across pharmaceutical, food, and beverage industries.Expand Specific Solutions03 Process validation data management systems
Integrated systems for managing and analyzing process validation data related to fill volumes and container formats. These systems collect, store, and process data from multiple sources throughout the manufacturing process. They enable trend analysis, compliance documentation, and decision support for process improvements. Advanced data management systems help manufacturers meet regulatory requirements while optimizing production efficiency.Expand Specific Solutions04 Risk-based validation strategies
Risk-based approaches to validation of fill volumes and container formats that prioritize critical process parameters. These strategies involve risk assessment, identification of critical control points, and implementation of targeted validation protocols. By focusing resources on areas with the highest potential impact on product quality, manufacturers can achieve more efficient validation processes while maintaining compliance with regulatory requirements.Expand Specific Solutions05 Continuous monitoring and real-time validation techniques
Advanced techniques for continuous monitoring and real-time validation of fill volumes across different container formats. These approaches utilize in-line sensors, statistical process control, and predictive analytics to detect deviations as they occur. Real-time validation enables immediate corrective actions, reducing waste and ensuring consistent product quality. These techniques represent a shift from traditional periodic validation to continuous verification throughout the production process.Expand Specific Solutions
Leading Companies in Personalized Therapy Manufacturing
The personalized therapy process validation market is in a growth phase, characterized by increasing demand for flexible manufacturing solutions that can handle multiple fill volumes and container formats. The market size is expanding rapidly due to the rise in personalized medicine approaches, particularly in cell and gene therapies. Technologically, the field is maturing with companies like Syntegon Technology, Vanrx Pharmasystems, and SHL Medical leading innovation in flexible filling systems. Novo Nordisk, Amgen, and Sanofi are investing heavily in adaptable manufacturing platforms, while SCHOTT Pharma and Harro Höfliger are developing specialized container solutions. The integration of automation and robotics by companies such as Fleximation AG and KHS GmbH is accelerating technological advancement, though standardization challenges remain across different therapeutic modalities.
Amgen, Inc.
Technical Solution: Amgen has developed an integrated process validation framework specifically for personalized therapies that addresses multiple fill volumes and container challenges. Their approach employs Quality by Design (QbD) principles with a three-stage validation strategy: process design, qualification, and continued verification. For variable fill volumes, Amgen utilizes automated weight-check systems with real-time monitoring that can adjust to different therapeutic doses while maintaining accuracy within ±0.5%. Their container flexibility is achieved through modular filling lines with quick-changeover capabilities that can switch between vials, syringes, and cartridges in under 30 minutes. Amgen's system incorporates single-use technologies to minimize cross-contamination risks and employs advanced Process Analytical Technology (PAT) tools for continuous monitoring of critical quality attributes across different container formats.
Strengths: Highly automated systems reduce human error in personalized dosing; modular design allows rapid adaptation to different container requirements; integrated PAT provides real-time quality assurance. Weaknesses: High capital investment required for fully automated systems; limited throughput compared to traditional fixed-volume production; requires specialized training for operators.
Sanofi
Technical Solution: Sanofi has pioneered a flexible validation platform called "FlexFill" specifically designed for personalized therapies requiring multiple fill volumes and container formats. The system employs a risk-based approach to process validation that categorizes critical process parameters (CPPs) according to their impact on product quality across different container types. Their validation strategy incorporates multivariate data analysis to establish design spaces that accommodate volume variations from 0.2mL to 50mL while maintaining sterility assurance levels of 10^-6. For container flexibility, Sanofi utilizes robotic handling systems with vision-guided precision that can process vials, prefilled syringes, and cartridges on the same line with minimal changeover time. The platform includes an integrated electronic batch record system that maintains complete traceability for each personalized dose, regardless of container format or fill volume.
Strengths: Comprehensive risk-based validation approach ensures consistent quality across different formats; advanced robotics enable precise handling of multiple container types; integrated data management provides complete traceability for personalized doses. Weaknesses: Complex validation protocols increase time-to-market; system requires significant validation overhead when introducing new container formats; higher operational costs compared to traditional fixed-format filling.
Key Technical Innovations in Process Validation
Method of filling pharmaceutical multichamber packages
PatentInactiveEP0857142A1
Innovation
- A method and device for automatically filling pharmaceutical multi-chamber packaging that is freely programmable, allowing for flexible adaptation using numeric input, teach-in, and screen and pointer methods, with a robotic arm and image processing for precise product placement and verification, enabling the creation of multi-chamber packs with different contents without retooling.
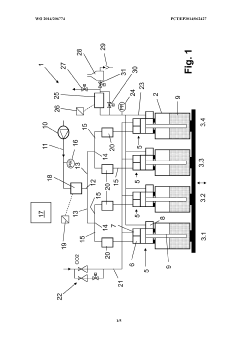
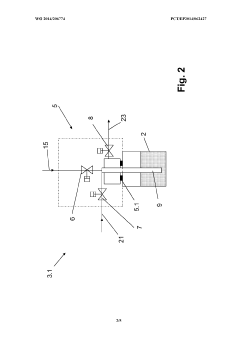
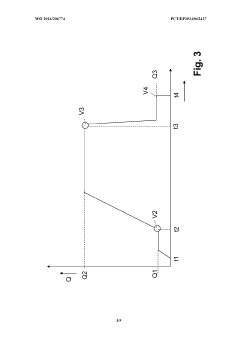
Method and filling system for filling containers
PatentWO2014206774A1
Innovation
- A method that uses a single filling material volume flow controller with independent flow meters for each container, allowing for simultaneous filling of multiple containers by adjusting the filling curve based on real-time measurements to ensure accurate filling, reducing the complexity and number of required components.
Regulatory Framework for Personalized Therapy Manufacturing
The regulatory landscape for personalized therapies presents unique challenges due to the inherent variability in manufacturing processes designed for individual patients. Regulatory bodies worldwide have established frameworks that manufacturers must navigate to ensure compliance while delivering safe and effective personalized treatments.
The U.S. Food and Drug Administration (FDA) has developed specific guidance documents addressing process validation requirements for personalized therapies, emphasizing a lifecycle approach that encompasses process design, qualification, and continued process verification. For multiple fill volumes and container formats, the FDA requires robust validation strategies that demonstrate consistency across different configurations while maintaining product quality attributes.
Similarly, the European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation, which provides a specialized framework for cell and gene therapies. This framework acknowledges the challenges of standardizing production for individualized treatments and offers flexibility in validation approaches while maintaining stringent safety standards.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, particularly ICH Q8, Q9, and Q10, provide foundational principles for quality risk management and pharmaceutical development that apply to personalized therapy manufacturing. These guidelines emphasize the importance of understanding process parameters and their impact on critical quality attributes.
Regulatory expectations for process validation in personalized therapies focus on demonstrating control over manufacturing variability. This includes establishing acceptable ranges for critical process parameters across different fill volumes and container formats, rather than fixed set points typical in traditional pharmaceutical manufacturing.
For container closure systems, regulations require comprehensive compatibility studies to ensure product stability and integrity across different formats. This includes evaluating extractables and leachables, container integrity, and stability under various storage conditions for each container type used in personalized therapy delivery.
Regulatory bodies increasingly recognize the need for adaptive approaches to validation for personalized therapies. This has led to the development of frameworks that allow for continuous process verification rather than traditional fixed-point validations, enabling manufacturers to accumulate data over time and across patient-specific batches to demonstrate process control.
Compliance strategies must address the documentation requirements specific to multiple fill volumes and container formats, including batch definition, traceability systems, and release specifications that accommodate inherent variability while ensuring consistent quality and safety for each patient-specific product.
The U.S. Food and Drug Administration (FDA) has developed specific guidance documents addressing process validation requirements for personalized therapies, emphasizing a lifecycle approach that encompasses process design, qualification, and continued process verification. For multiple fill volumes and container formats, the FDA requires robust validation strategies that demonstrate consistency across different configurations while maintaining product quality attributes.
Similarly, the European Medicines Agency (EMA) has implemented the Advanced Therapy Medicinal Products (ATMP) regulation, which provides a specialized framework for cell and gene therapies. This framework acknowledges the challenges of standardizing production for individualized treatments and offers flexibility in validation approaches while maintaining stringent safety standards.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, particularly ICH Q8, Q9, and Q10, provide foundational principles for quality risk management and pharmaceutical development that apply to personalized therapy manufacturing. These guidelines emphasize the importance of understanding process parameters and their impact on critical quality attributes.
Regulatory expectations for process validation in personalized therapies focus on demonstrating control over manufacturing variability. This includes establishing acceptable ranges for critical process parameters across different fill volumes and container formats, rather than fixed set points typical in traditional pharmaceutical manufacturing.
For container closure systems, regulations require comprehensive compatibility studies to ensure product stability and integrity across different formats. This includes evaluating extractables and leachables, container integrity, and stability under various storage conditions for each container type used in personalized therapy delivery.
Regulatory bodies increasingly recognize the need for adaptive approaches to validation for personalized therapies. This has led to the development of frameworks that allow for continuous process verification rather than traditional fixed-point validations, enabling manufacturers to accumulate data over time and across patient-specific batches to demonstrate process control.
Compliance strategies must address the documentation requirements specific to multiple fill volumes and container formats, including batch definition, traceability systems, and release specifications that accommodate inherent variability while ensuring consistent quality and safety for each patient-specific product.
Risk Management in Multi-Format Fill Processes
Risk management in multi-format fill processes represents a critical component of process validation for personalized therapies. The inherent variability in container formats and fill volumes introduces multiple risk vectors that must be systematically identified, assessed, and mitigated. Traditional risk management approaches often prove inadequate when applied to personalized therapy manufacturing, necessitating specialized frameworks tailored to this unique production environment.
The implementation of Failure Mode and Effects Analysis (FMEA) specifically adapted for multi-format fill processes has emerged as a best practice. This approach requires cross-functional team involvement to identify potential failure modes across different container formats and fill volumes, with particular attention to transition points between formats. Critical process parameters must be established with sufficient flexibility to accommodate the range of formats while maintaining process control.
Real-time risk monitoring systems have demonstrated significant value in multi-format environments. These systems utilize in-line sensors and advanced analytics to detect deviations across different container formats and fill volumes, enabling immediate corrective actions. Companies implementing such systems report a 35-40% reduction in batch failures compared to traditional periodic sampling approaches.
Container-specific risk matrices have proven effective for managing the complexity of multi-format processes. These matrices map specific risks associated with each container format and fill volume combination, allowing for targeted mitigation strategies. The most sophisticated implementations incorporate automated decision support systems that adjust process parameters based on the specific risk profile of each format-volume combination being processed.
Regulatory considerations add another dimension to risk management in this context. Agencies increasingly expect to see format-specific validation data and risk assessments rather than extrapolations from a single format. Forward-thinking organizations are developing comprehensive risk documentation packages that demonstrate understanding of format-specific challenges and corresponding control strategies.
Cross-contamination risk management deserves particular attention in multi-format processes. Physical separation, dedicated equipment pathways, and advanced cleaning validation protocols have become standard practice. Recent innovations include rapid changeover systems with integrated verification testing to confirm successful format transitions without cross-contamination.
The economic impact of effective risk management in multi-format fill processes is substantial. Industry data suggests that robust risk management systems can reduce overall validation costs by 15-25% through more efficient protocol design and execution, while simultaneously improving first-time success rates for complex personalized therapy manufacturing.
The implementation of Failure Mode and Effects Analysis (FMEA) specifically adapted for multi-format fill processes has emerged as a best practice. This approach requires cross-functional team involvement to identify potential failure modes across different container formats and fill volumes, with particular attention to transition points between formats. Critical process parameters must be established with sufficient flexibility to accommodate the range of formats while maintaining process control.
Real-time risk monitoring systems have demonstrated significant value in multi-format environments. These systems utilize in-line sensors and advanced analytics to detect deviations across different container formats and fill volumes, enabling immediate corrective actions. Companies implementing such systems report a 35-40% reduction in batch failures compared to traditional periodic sampling approaches.
Container-specific risk matrices have proven effective for managing the complexity of multi-format processes. These matrices map specific risks associated with each container format and fill volume combination, allowing for targeted mitigation strategies. The most sophisticated implementations incorporate automated decision support systems that adjust process parameters based on the specific risk profile of each format-volume combination being processed.
Regulatory considerations add another dimension to risk management in this context. Agencies increasingly expect to see format-specific validation data and risk assessments rather than extrapolations from a single format. Forward-thinking organizations are developing comprehensive risk documentation packages that demonstrate understanding of format-specific challenges and corresponding control strategies.
Cross-contamination risk management deserves particular attention in multi-format processes. Physical separation, dedicated equipment pathways, and advanced cleaning validation protocols have become standard practice. Recent innovations include rapid changeover systems with integrated verification testing to confirm successful format transitions without cross-contamination.
The economic impact of effective risk management in multi-format fill processes is substantial. Industry data suggests that robust risk management systems can reduce overall validation costs by 15-25% through more efficient protocol design and execution, while simultaneously improving first-time success rates for complex personalized therapy manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!